Abstract
The concept of ‘cirrhosis’ is evolving and it is now clear that compensated and decompensated cirrhosis are completely different in terms of prognosis. Furthermore, the term ‘advanced chronic liver disease (ACLD)’ better reflects the continuum of histological changes occurring in the liver, which continue to progress even after cirrhosis has developed, and might regress after removing the etiological factor causing the liver disease. In compensated ACLD, portal hypertension marks the progression to a stage with higher risk of clinical complication and requires an appropriate evaluation and treatment. Invasive tests to diagnose cirrhosis (liver biopsy) and portal hypertension (hepatic venous pressure gradient measurement and endoscopy) remain of crucial importance in several difficult clinical scenarios, but their need can be reduced by using different non-invasive tests in standard cases. Among non-invasive tests, the accepted use, major limitations and major benefits of serum markers of fibrosis, elastography and imaging methods are summarized in the present review.
Keywords: compensated advanced chronic liver disease, hepatic venous pressure gradient, elastography, ultrasound
Introduction
Classically, cirrhosis is defined by its histological hallmark findings on liver biopsy (regenerative nodules surrounded by fibrotic tissue) and is considered as the final evolution stage of any progressive liver disease, irrespective of its etiology. The advances in diagnostic methods allow now early diagnosis, even before the development of complications, which are mostly related to development of portal hypertension [1]. Recently, with the development of new and very effective treatments, especially in the viral-related cirrhosis scenario, there is increasing evidence that cirrhosis can regress and that histological improvement is associated with better prognosis [2]. However, in the particular case of direct acting antiviral (DAA) treatment of hepatitis C virus (HCV), some data suggest that the complications of portal hypertension can occur even after sustained virological response (SVR) and the risk of hepatocellular carcinoma (HCC) is not abolished [3,4]. Therefore, the international expert consensus currently suggests continuing screening and surveillance of these patients according to the standard guidelines used for portal hypertension and HCC, and it is still unknown whether these patients should be managed and followed according to different schemes.
The natural history of cirrhosis is marked by the transition from the compensated stage (with good prognosis) to the occurrence of decompensation events, such as ascites, variceal bleeding, jaundice and hepatic encephalopathy. If the diagnosis of cirrhosis is relatively straightforward during the decompensated stage when the treatment may be problematic, on the contrary, diagnosing cirrhosis while it is still in the compensated stage is more challenging. The progression of fibrosis parallels the increase in portal pressure and, frequently, patients with severe fibrosis in the pre-cirrhotic stage have a hepatic venous pressure gradient (HVPG) >5 mmHg [5]. Since chronic liver disease is a continuum, and due to the inhomogeneity of fibrosis within the liver [6], the border between severe fibrosis and compensated cirrhosis is often unclear and, recently, the Baveno VI consensus recommended that this clinical scenario including severe fibrosis and initial cirrhosis should be named compensated advanced chronic liver disease (cACLD) [7].
Moreover, the concept of diagnosis of cirrhosis is changing from the documentation of histological F4 fibrosis to the identification of patients truly at risk of developing complications. It has been clearly demonstrated that the onset of clinically significant portal hypertension (defined as HVPG ≥10 mmHg) marks the progression to a stage at risk of clinical complications. In this scenario, non-invasive methods able to mirror the haemodynamic threshold play an important role. For instance, according to the recommendations of the last Baveno consensus conference, liver stiffness (measured by transient elastography) over 21 kPa is accurate enough to identify patients with clinically significant portal hypertension, so allowing a simple and readily available risk stratification when more sophisticated and exact methods are not available [7]. The natural history of chronic liver disease eventually leading to cACLD and complications of cirrhosis is represented in Figure 1, together with the main tests used for its diagnosis, staging and risk stratification.
Figure 1.
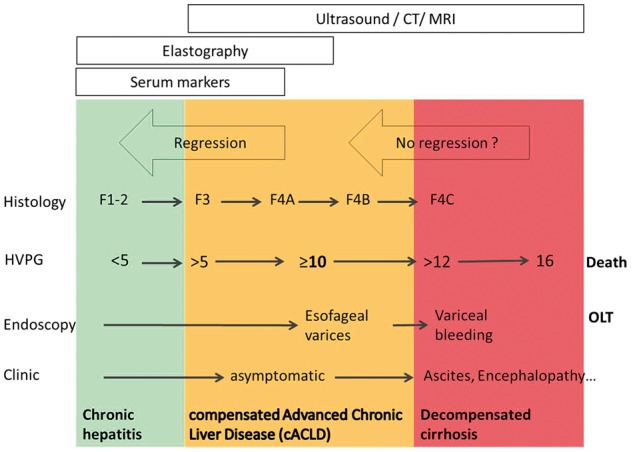
Natural history of diagnostic on-invasive diagnostic tests in compensated advanced chronic liver disease. The most appropriate timeframe for using different techniques in order to maximize the information for clinical use is given. Combination of different non-invasive unrelated tests can further improve the amount of information retrieved and reduce the risk of false-positive and false-negative results.
In this manuscript, we review the diagnostic performance of gold-standard invasive methods and surrogate non-invasive methods used in this setting in light of the new concept of cACLD.
Gold-standard diagnostic methods for cirrhosis and portal hypertension
Liver biopsy is still considered the gold-standard diagnostic method to identify the typical features of cirrhosis. Alternative diagnostic methods have been validated in comparison to liver biopsy and have a good diagnostic accuracy for the diagnosis of cirrhosis. As a consequence, the use of liver biopsy has dramatically decreased in the last 10 years; nonetheless, it remains a crucial diagnostic tool when concomitant potential etiological factors for liver disease coexist and when the identification of features other than fibrosis leads to changes in the clinical management of patients, such as in the case of acute or chronic liver injury. Currently, the most important and frequent scenario that requires a mandatory liver biopsy is the differentiation between severe alcoholic hepatitis and decompensated alcoholic cirrhosis, because by now there are no specific clinical signs or non-invasive methods to differentiate between the two conditions [8]. Liver biopsy is largely used in patients with suspected liver cirrhosis of unknown aetiology in order to confirm the diagnosis and expand on its possible cause (e.g. indicating the distribution of fibrosis in the liver). It remains key also in the case of suspected non-alcoholic steatohepatitis (NASH)-related ACLD and in cholestatic and autoimmune chronic liver disease for which data regarding the diagnostic accuracy of non-invasive methods are scarce.
Liver biopsy can be carried out from a percutaneous or a transjugular route. Percutaneous liver biopsy is done through a right intercostal space after or under ultrasound control, on local anaesthesia, using Menghini core-aspiration or Tru-cut automatic 16-gauge needles. Before the procedure, coagulation parameters should be checked (including platelet count and prothrombin time/international normalized ratio). The 50/50 rule (prothrombin time over 50% and platelet count over 50 × 109/L) is frequently used to consider the coagulation and platelet status acceptable. The contraindications for percutaneous liver biopsy include severe coagulopathy, biliary ducts dilatation, sepsis, ascites, suspicion of vascular lesions, hydatid disease or uncooperative patient [9]. Some of the contraindications (especially coagulopathy and ascites) are overcome by using a transjugular approach that carries lower haemorrhagic risk. The most frequent indications for liver biopsy are presented in Table 1 [9,10].
Table 1.
The main indications for performing liver biopsy
| Percutaneous liver biopsy | Transjugular liver biopsy |
|---|---|
| Diffuse liver diseases with multiple aetiologies | Need for parallel measurement of hepatic venous pressure gradient (HVPG) |
| Abnormal liver test from unknown origin | Contraindications to percutaneous access (note that dilatation of the biliary tree is a contraindication for any liver biopsy) |
| Non-alcoholic fatty liver disease | Suspicion of severe alcoholic hepatitis |
| Autoimmune hepatitis | Acute liver failure of unknown aetiology |
| Focal lesions | Suspicion of non-cirrhotic portal hypertension |
| Abnormal liver test in haematological patients |
Regarding the diagnostic performance of both approaches, although previously transjugular liver biopsy was considered inferior because of the use of thinner needles (18G), there is strong evidence suggesting that the two techniques are similar in terms of sample length and the number of complete portal spaces [11]. The greatest advantage of the transjugular route is that it allows concomitant HVPG measurement and multiple passes without increasing the risk of complications.
Liver fibrosis and its patterns remain of paramount importance in risk stratification of patients, even in those who have fully established liver cirrhosis. Four main patterns of fibrosis development according to different aetiologies are described: (i) portal-to-central fibrosis distribution (characteristic to viral and autoimmune hepatitis); (ii) portal-to-portal distribution (specific for biliary diseases); (iii) perisinusoidal and pericellular distribution (metabolic diseases, alcoholic and non-alcoholic liver diseases) and (iv) central-to-central fibrosis distribution (for venous outflow obstruction such as Budd-Chiari syndrome) [12]. According to the different types of fibrosis distribution, the portal hypertension occurs earlier, as in the case of viral, autoimmune or Budd-Chiari syndrome, or later in the course of the disease, as in the case of metabolic diseases. Interestingly, even if sinusoidal portal hypertension develops later in the case of biliary diseases, due to the portal-to-portal distribution of fibrosis and development of porto-portal septa, there is an increased presinusoidal resistance that will increase portal pressure, so that the HVPG underestimates the value of the portal pressure gradient in patients with cholestatic liver disease.
As the disease progress, the amount of fibrosis increases and in parallel the portal pressure rises [13] corresponding to worsening in the prognosis [14,15]. The Laennec sub-classification of cirrhosis [16] in three subclasses of stage 4: 4A—mild cirrhosis with thin septa and large nodules; 4B—at least two broad septa, but no very broad septa and less than half of biopsy length composed of minute nodules; 4C—very broad septum or more than half of biopsy length composed of minute nodules (micronodular cirrhosis) offers additional prognosis relevance [17,18]. Moreover, histological markers of fibrosis regression under therapy, named ‘hepatic repair complex’, can be observed, and consist of delicate and perforated fibrous septa; isolated and thick collagen fibres; delicate periportal fibrous spikes; portal tract remnants; hepatic vein remnants with prolapsed hepatocytes; hepatocytes within portal tracts or splitting septa; minute regenerative nodules; and aberrant parenchymal veins [19,20].
Because the majority of complications are conditioned by portal hypertension occurrence, the measurement of HVPG has probably an important prognostic relevance that might exceed that of histological modifications. HVPG is through internal jugular vein, femoral vein or cubital vein access under local anaesthesia [21,22]. One of the hepatic veins is catheterized with a balloon catheter under fluoroscopic control. By the inflation of the balloon, the hepatic venous outflow is blocked and, at the end of 1–2 minutes, the pressure at the tip of the catheter equals that of the hepatic sinusoidal pressure and portal pressure, respectively. That represents the wedge hepatic venous pressure (WHVP). Free hepatic venous pressure (FHVP) is measured by deflating the balloon at 2–3 cm from the hepatic vein ostium, and usually its value is very close to the inferior vena cava. HVPG is the difference between WHVP and FHVP, and represents the pressure gradient that the portal blood flow has to exceed to pass through the liver. While some authors consider that FHVP should be substituted by the pressure in the inferior vena cava or right atrium pressure [23], HVPG loses its prognostic value if it is calculated to any other vessel except the liver vein, so that FHVP should be mandatorily used [24,25].
Due to the relative invasiveness and the lack of wide diffusion of the method, HVPG is considered by many as only a research method to assess portal pressure but, in the authors’ opinion, it should be considered a crucial and readily available, mature method to achieve clinically important information. In clinical practice is a useful technique to make the differential diagnosis in case of clinical signs of portal hypertension, especially if cirrhosis is not obvious on non-invasive techniques such as ultrasound or transient elastography. HVPG is a safe technique that has no absolute contraindications [26]. In patients requiring transjugular liver biopsy, the measurement of HVPG adds only a few minutes to the procedure but provides very relevant haemodynamic information. In our practice, whenever liver biopsy is indicated in patients with cACLD, we prefer the transjugular approach to obtain both histological findings and HVPG measurement.
HVPG is probably the most validated tool for assessing prognosis in cACLD. In Table 2 are presented the most relevant clinical end-points with which HVPG was associated [1,5,24,27–33]. All this body of evidence indicates HVPG to be the best tool for assessing the prognostics of patients with cACLD.
Table 2.
Different thresholds of hepatic venous pressure gradient (HVPG) correlated with clinical end-points in compensated advanced chronic liver disease (cACLD)
| HVPG | Clinical end-points | |
|---|---|---|
| <5 mmHg | Normal | |
| 5–10 mmHg | Mild portal hypertension | |
| >6 mmHg | Progression of chronic viral hepatitis [5] | |
| High risk of recurrence after liver transplantation [27] | ||
| >10 mmHg | Clinically significant portal hypertension | |
| >10 mmHg | Oesophageal varices development [28,29] | |
| Ascites [1] | ||
| Decompensation [1] | ||
| Hepatocellular occurrence [30] | ||
| Decompensation after hepatic resection [31] | ||
| >12 mmHg | Oesofageal varices bleeding | |
| >16 mmHg | High mortality [24] | |
| >20 mmHg | Failure to control bleeding [32] | |
| >22 mmHg | High mortality in severe alcoholic hepatitis [33] | |
An ideal diagnostic and prognostic method would reflect also the changes under therapy. A decrease in HVPG <10 mmHg under non-selective beta-blockers prevents the development of varices in patients with clinically significant hypertension [29]; in patients with high-risk varices, a decrease to < 12 mmHg or with 20% from the baseline will prevent first variceal bleeding; and, in patients who have already suffered variceal bleeding, the reduction of HVPG below this threshold will prevent rebleeding [34–37], these patients being considered ‘responders’. Moreover, in patients with clinically significant hypertension (HVPG ≥10 mmHg), without any previous decompensation and high-risk varices under primary prophylaxis with nadolol, a ≥ 10% decrease in HVPG prevents the development of ascites [38]. In ‘non-responders’, further decrease in HVPG could be obtained by adding isosorbid-5-mononitrate [39] or shifting from propranolol to carvedilol [40].
As for the histological modification, portal pressure is increasing over time in parallel to the worsening of liver function, and patients that were considered good ‘responders’ may lose their response to beta-blockers [41]. However, with effective etiological treatment, portal pressure can improve [42]. At the moment, it is unknown whether clinically significant hypertension should be seen as a point of ‘no return’ [42,43]. In this particular context, where liver-stiffness measurement is at the moment of unclear value, more data about fibrosis and portal pressure dynamics are needed to better understand how patients who have improved under treatment should be further managed and, therefore, liver biopsy and HVPG measurement should be encouraged.
Due to its extensive validation against strong clinical end-points and since changes in HVPG well reflect changes in prognosis (in terms both of improvement when HVPG decreases and of worsening when HVPG increases), HVPG is probably the best surrogate marker of clinical events in patients with cACLD.
Endoscopy
Bleeding from oesophageal varices remains one of the most severe complications of cirrhosis despite the advances in its management. In order to prevent bleeding from occurring, an appropriate early diagnosis of oesophageal varices at risk of bleeding should be achieved. Upper digestive tract endoscopy is the gold-standard method for diagnosing the presence of gastroesophageal varices and identifying signs of risk of bleeding (large size; red signs). Universal endoscopic screening of oesophageal varices was recommended in all patients newly diagnosed with cirrhosis until 2015 [44]. However, due to the increase in the proportion of patients with early cirrhosis/cACLD achieved by non-invasive diagnostic methods, this strategy proved to lead to a large number of unnecessary endoscopies [45] that would eventually decrease the patient’s compliance and increase the health system costs. In the last 10 years, increasing evidence regarding non-invasive methods (especially transient elastography) accumulated and proved useful for stratifying the risk of carrying varices and varices requiring treatment in patients with cACLD. Based on an acceptable risk of 5% of missed varices requiring treatment, the 2015 Baveno consensus conference recommended that patients with normal platelet count and liver-stiffness measurement by transient elastography <20 kPa could safely avoid screening endoscopy [7]. Papers published in full and several abstracts confirmed that this strategy is safe and allows the sparing of 15–25% of unnecessary endoscopies. Patients exceeding at least one of these criteria should undergo screening endoscopy in order to detect high-risk varices that would benefit from prophylactic treatment. It is important to note that this strategy applies to well-compensated patients, while patients with decompensated cirrhosis should undergo endoscopy irrespective of their platelet count and liver-stiffness value, due to the much higher risk of varices requiring treatment in this population [7].
Endoscopy remains needed to identify other signs of portal hypertension such as hypertensive gastropathy that is often the cause of minor bleeding in patients with cirrhosis.
Non-invasive serum markers of fibrosis
Since a long time ago, simple blood tests were used in the diagnosis and prognostication of patients with advanced liver diseases. The most largely used is a combination of markers of liver synthetic function (albumin, bilirubin and prothrombin time) that, together with two clinical variables (presence and severity of ascites and encephalopathy), constitute the Child-Pugh score [46]. The possibility of achieving a diagnosis with blood tests is appealing, since the approach would have an applicability close to 100%. Hence, several serum tests including direct markers of extracellular matrix remodelling and indirect markers (liver function tests, transaminases, platelet count) and combined panels/scores have been elaborated on to respond to different clinical questions such as fibrosis staging, diagnosis of cirrhosis, presence of portal hypertension and prognosis of patients with cACLD. Table 3 summarizes the most validated serum tests for diagnosis of cirrhosis and clinically significant portal hypertension validated in comparison with HVPG measurement [47–64].
Table 3.
Serum tests used for diagnosis of cirrhosis and portal hypertension
| Indirect serum test | ||
|---|---|---|
| Platelet count | Very well validated [47–49] | Very well validated [50,51] |
| ALT/AST index | Few evidences [49,52] | No evidence |
| AST/platelet ratio index | Very well validated [49,53,54] | No evidence |
| Lok | Well validated [49,55] | Little evidence [56] |
| FIB-4 | Very well validated [57–59] | Little evidence [56] |
| Forns | Little evidence [60] | No evidence |
| Direct fibrosis markers included | ||
| Fibrotest | Very well validated [61–63] | Little evidence [50] |
| Fibrometer | Very well validated [62,63] | No evidence |
| Hepascore | Very well validated [62,63] | No evidence |
| Hyaluronic acid | Very well validated [57,64] | No evidence |
| Enhanced liver fibrosis | Very well validated [63,64] | No evidence |
As may be seen, the majority of serum scores are very well validated for the diagnosis of cirrhosis and could be easily used according to the local availability. However, as previously shown, the main cause of complications in patients with cACLD is clinically significant portal hypertension and this should be the key diagnostic feature for risk stratification of these patients. Unfortunately, serum tests are not sufficiently validated for this purpose and, therefore, none can be used alone for selecting patients who would eventually avoid endoscopy. Although never used alone, platelet count is probably the most routinely used test to look for portal hypertension in cACLD. Low platelet count is very common in patients with cirrhosis, with 78% of patients developing thrombocytopenia [65], and, most of the time, it represents a sign of portal hypertension [51]. In the absence of thrombocytopenia, portal hypertension can be haemodynamically present, but the likelihood of large oesophageal varices is low; a normal platelet count added to < 20 kPa at liver-stiffness measurement by transient elastography, screening endoscopy constitutes a simple rule to exclude the presence of gastroesophageal varices requiring treatment [7].
A valuable non-invasive surrogate should also have prognostic relevance. Simple serum variables as albumin, bilirubin, prothrombin time and creatinine have been extensively validated within different prognostic models used to classify cirrhosis stage, such as Child-Pugh or MELD (Model for End-stage Liver Disease) score [66,67]. Only a few of the non-invasive scores designed to diagnose fibrosis have been tested for prognostic aims. Among them, the enhanced liver fibrosis (ELF) test and Fibrotest are the most validated, the latter having similar performance in predicting 5-year survival to liver-stiffness measurement by transient elastography in a large cohort of HCV patients [68]; nonetheless, only a minority of patients included in this study had cirrhosis and, therefore, the results are difficult to interpret in the context of cACLD.
Elastography
From a physics point of view, elasticity (reciprocal of stiffness) is defined as the hability of a tissue of maintaining its shape after being challenged by a mechanical stress. This is an intrinsic characteristic of each tissue, and is expressed by the Young’s elastic module [69,70]. The application of a mechanical stimulus such as a vibration or an ultrasound impulse to a tissue induces the formation of shear waves in the tissue itself. These propagate into the tissue with a velocity depending on the tissue elasticity according to the following formula: Elasticity = 3×ρ (density) × V (propagation velocity) [69,70].
If the amplitude and frequency of the initial stimulus are known, by using ultrasound or magnetic resonance, it is possible to measure the velocity of the propagation of the shear waves and, consequently, it is possible to estimate the elasticity of a given tissue.
The healthy liver is an elastic (low-stiffness) organ, while fibrosis induces an increase in its stiffness [71,72]. This is the rationale for the use of elastography methods to estimate fibrosis; however, it should be underlined that any process occupying space in the liver tissue (e.g. inflammation, venous congestion, cholestasis and infiltrative neoplastic processes) and meal ingestion increase liver stiffness independently of fibrosis, and this should be taken into account in the interpretation of elastography results.
Among ultrasound-elastography methods, transient elastography (TE; Fibroscan®, Echosens, Paris, France) has been the first developed to assess liver stiffness. A detailed explanation of the technical aspects can be found elsewhere [69,70]. TE has proved to be very accurate in identifying and ruling out cirrhosis in patients with chronic liver disease of many different aetiologies (most data have been provided in patients with chronic viral hepatitis), with an area under the ROC curve (AUC) of 0.94 on meta-analysis [73,74]. Values above 10 kPa are suggestive of ACLD and values above 12.5 kPa have an accuracy of over 90% in detecting cirrhosis [75], provided the above-mentioned confounders are excluded. Given that liver fibrosis is the major component of hepatic resistance, and given that hepatic resistance is the major factor leading to portal hypertension in patients with compensated cirrhosis (Ohm’s law applied to hydrodynamics: Pressure = Resistance × Flow), liver stiffness has been tested as a surrogate of portal pressure in cirrhosis. Interestingly, it was observed that liver stiffness is able to identify clinically significant portal hypertension with a high accuracy (AUC of 0.93 on meta-analysis [76]). Values ≥21 kPa are highly specific of clinically significant portal hypertension [26,51,77] and are associated with increased risk of clinical decompensation of cirrhosis and with increased risk of HCC [78].
Despite the fact that liver-stiffness measurement (LSM) is not an optimal method to identify gastroesophageal varices [79], it is now accepted that the combination of values of LSM by TE < 20 kPa and a platelet count >150 × 109/L can rule out large oesophageal varices in compensated patients, so leading to a reduction in the number of unnecessary endoscopies to screen for varices [7]. It is important to notice that LSM cannot be used as a perfect surrogate of HVPG, since, above the threshold of 10–12 kPa, LSM and HVPG are no longer strictly correlated [80]. Furthermore, LSM changes in patients on non-selective beta-blockers do not mirror changes in HVPG and TE cannot be used to monitor the haemodynamic response to beta-blockers.
Other newer ultrasound-elastography methods that are incorporated in standard ultrasound equipments include point shear-wave elastography (pSWE; taking advantage of acoustic radiation force impulse imaging) and two-dimensional shear-wave elastography (2D-SWE) [69,70]. These techniques allow visualizing in real time the area where the measurement of elastic-wave velocity is performed; the measurement needs to follow reliability criteria based on the quality of the ultrasound image and the result is given either as metres/second or as kPa. The first pSWE available in the market (VirtualTouch, Siemens, Germany) is now considered validated and provides a higher applicability for the measurement of liver stiffness as compared to TE, with similar accuracy for detecting liver cirrhosis [81]; the suggested cut-off is 1.80–1.85 m/sec. The first 2D-SWE available in the market (Aixplorer, Supersonic Imagine, France) is close to validation; its applicability and diagnostic performance seem comparable to those of TE [82]. The suggested cut-off for cirrhosis is 11.5 kPa.
Limited data are available regarding the accuracy of pSWE and 2D-SWE for portal hypertension, but pilot experiences suggest that these methods yield similar results as compared to TE [83].
Since the spleen in patients with cirrhosis undergoes enlargement and changes mostly related to portal hypertension [84], spleen-stiffness measurement (SSM) has been proposed as a novel parameter, not yet routinely used, to better mirror portal hypertension as compared to LSM [83]. In the first studies using TE, SSM showed a close correlation to HVPG and, in a meta-analytic review of 16 studies performed by different ultrasound-elastography methods, SSM was superior to LSM to predict the presence of oesophageal varices [85]. SSM by using TE has substantial limitations, including low applicability in normal-sized spleens and a ceiling effect at 75 kPa, limiting risk stratification above this threshold.
Magnetic resonance elastography (MRE) has been proposed as a method to evaluate both liver and spleen stiffness, overcoming some of the limitations of ultrasound-elastography methods (no need for an acoustical window, freely oriented field of view, lack of sensitivity to body habitus) [86,87]. MRE has proved to be accurate in stage of liver fibrosis (being marginally better than TE and pSWE in two studies [88,89]) and is a highly promising method for diagnosing cirrhosis in patients unsuitable to ultrasound elastography. It holds high reproducibility and, interestingly, changes in MRE correlate with changes in fibrosis.
Recently, Ronot et al used multiparametric MRE in a small series of 36 patients on the waiting list for orthotopic liver transplantation undergoing HVPG measurement and endoscopy [90]. Three different liver and spleen parameters were assessed, namely storage, loss and shear moduli. The spleen loss modulus was the best parameter for identifying patients with severe portal hypertension (AUC = 0.81, p = 0.019) or high-risk varices (AUC = 0.93, p = 0.042), confirming previous data regarding the potential of spleen stiffness on the evaluation of portal hypertension in cirrhosis.
Limitations of MRE include its high cost and limited availability, which currently prevent its use as a routine diagnostic method.
Imaging methods
Ultrasound
Ultrasound is the first-line imaging examination to be performed in patients with suspected cirrhosis and/or portal hypertension. Ultrasound is safe, can be repeated easily, is not expensive and is highly sensitive in detecting thrombosis in the portal vein and hepatic veins, so allowing a correct differential diagnosis of new cases of portal hypertension [91]. As for the limitations, inter-observer variability is considered a major drawback, but appropriate training and knowledge markedly reduce it. Intestinal gas and obesity limit the exploration.
Ultrasound signs of cirrhosis on grey-scale (B mode) include changes in liver morphology and signs of portal hypertension (Table 4). Most signs have a high specificity and can be considered sufficient to confirm the diagnosis of cirrhosis. On the other hand, the sensitivity of most individual signs is low, indicating that a negative result cannot fully rule out cirrhosis in patients with compensated chronic liver disease.
Table 4.
Most commonly observed signs of cirrhosis on imaging
| Liver morphology changes | Nodular liver surface (all imaging methods, but better visualized by high-frequency probe on ultrasound) |
| Coarse echopattern (ultrasound); heterogeneous density with nodular pattern in some cases (CT) | |
| Hypertrophy of the left lobe and atrophy of the segment IV (better visualized on CT and MRI); expanded gallbladder fossa (CT and MRI) | |
| Hypertrophy of caudate lobe | |
| Reduction of the medial segment of left hepatic lobe | |
| Hepatic veins | Narrowing and loss of normal plasticity of flow by Doppler |
| Altered straightness | |
| Non-uniformity of hepatic vein-wall echogenicity | |
| Hepatic artery | Increased diameter (all techniques) and tortuosity (CT) |
| Portal venous system | Dilatation of portal vein (≥13 mm), splenic vein and superior mesenteric vein (≥11 mm) |
| Reduction of portal vein blood-flow velocity | |
| Reversal of portal vein blood flow | |
| Spleen | Increased size (splenomegaly: diameter >12 cm and/or area ≥45 cm2 by ultrasound) |
| Presence of porto-systemic collateral circulation | |
| Minimal perihepatic ascites | |
The most accurate single sign for the diagnosis of cirrhosis, which can be found even in early phases and should be always specifically investigated, is nodularity of the liver surface [92,93]. The use of high-frequency transducers increases the diagnostic performance of conventional abdominal ultrasound probes and should be preferred [92,93]. False-positive findings are rare but have been described (e.g. fulminant hepatitis leading to the collapse of large parenchyma areas). The combination of nodular liver surface and portal vein mean velocity below 12 cm/sec holds an accuracy of 80% for discriminating between patients with chronic hepatitis with severe fibrosis and those with cirrhosis [94]. In patients with clinical suspicion of cirrhosis and confounding conditions, the detection of nodular liver surface is an excellent non-invasive method to rule in cirrhosis, while the combination of ultrasound and TE allows the best diagnostic performance [93].
Similarly to what has previously been discussed regarding cirrhosis, most ultrasound signs of portal hypertensions are specific, but their sensitivity is moderate, especially in compensated cirrhosis; therefore, while the presence of a sign or a combination of signs permits confirming portal hypertension, the absence of ultrasound signs cannot exclude this diagnosis [95,96]. Only two signs are 100% specific (pathognomonic) signs of portal hypertension, namely porto-systemic collaterals (e.g. paraumbilical vein, spleno-renal collaterals, etc.) and reversal of flow in the portal vein system.
Splenomegaly is commonly associated with portal hypertension; this sign is more sensitive than other signs, but less specific. However, increasing spleen size is an independent predictor of gastroesophageal varices in compensated cirrhosis [51].
Other signs include dilatation of the portal venous system vessels, lack of or reduced respiratory variations of splenic and superior mesenteric vein diameter, reduced portal vein velocity, increased congestion index of the portal vein and an altered Doppler pattern in the liver veins. Less commonly explored signs include changes in the arterial flow pattern of the hepatic, splenic, mesenteric and renal arteries.
Despite the fact that most of these signs show some degree of correlation with the HVPG, none of them can be used as a reliable surrogate for haemodynamic measurement either at first examination or after starting non-selective beta-blocker therapy. Nonetheless, ultrasound parameters hold prognostic value and can suggest a worsening of portal hypertension on follow-up [95,96]. For instance, the development of or increase in the number of visible porto-systemic collaterals and progressive spleen size increase are associated with variceal formation and growth. Finally, Doppler ultrasound can be used to follow up the patency of transjugular intrahepatic porto-systemic shunt (TIPS) and proved useful to save unnecessary invasive haemodynamic procedures [97].
Computed tomography (CT) and magnetic resonance imaging (MRI)
Most of the signs mentioned in the ultrasound section suggest cirrhosis on other cross-sectional imaging (Table 4) [98]; following an approach first suggested by our group on ultrasound images [93], it has been recently described that the quantitative measurement of liver surface nodularity from routine CT images in the portal venous phase is accurate in differentiating cirrhosis from non-cirrhotic livers [99]. If validated, this measurement could be implemented routinely in patients with chronic liver disease undergoing CT.
Contrast-enhanced cross-sectional imaging allows very accurate visualization of the portal venous system and of the porto-systemic collaterals, so potentially providing useful data to detect oesophageal varices. However, while large oesophageal varices can be reliably diagnosed by single- or multidetector CT (sensitivity 84–100%; specificity 90–100%), inter-observer reproducibility is moderate and the sensitivity of the method for the detection of small varices is low. Due to these limitations, endoscopic screening is more cost-effective than contrast-enhanced CT followed by endoscopic screening in positive cases [100].
Dynamic contrast-enhanced techniques on CT and MRI (compartmental analysis of intensity versus time curves for magnetic resonance images of the liver after injection of a gadolinium chelate; phase contrast MR angiography) allow quantitative measurement of perfusion [101] and portal and azygos blood flow [102,103]. In one paper, HVPG correlated with a portal fraction of liver perfusion [104] and, in another, it correlated with azygos blood flow [105]; the latter parameter weakly correlates with the presence of oesophageal varices. In recent work, the combination of longitudinal relaxation time (T1) of the liver and splenic artery velocity remained independently associated with HVPG [106]. Future studies should address the practical use and the cost-effectiveness of these possible predictors in clinical practice.
Conclusions and future directions
As shown in the present review, it is currently possible to diagnose liver cirrhosis and portal hypertension accurately by non-invasive methods in a fair proportion of patients with chronic liver disease. However, all methods have pros and cons (Table 5). New, more sophisticated non-invasive diagnostic methods such as MRE, new software analysis of images obtained with the existing technology such as the analysis of the nodularity of liver surface on ultrasound [93] and CT images [99] and dynamic techniques on MRI are emerging tools further improving this possibility. Nonetheless, it should be remembered that an accurate diagnosis requires not only practical skills and specific knowledge of the methods to be used, however, but also clinical experience and critical judgment to identify potential false negatives and false positives of the tests used. In this context, liver biopsy is and will continue to be a crucial tool for patients in whom the clinical features are not typical, in whom the results of non-invasive tests are discordant and in acquiring better knowledge of the natural history/regression of cACLD after effective cure of the cause leading to cirrhosis.
Table 5.
Pros and cons of the available non-invasive diagnostic methods used for cirrhosis and portal hypertension
| Test | Pros | Cons | |
|---|---|---|---|
| Serum tests | Standard laboratory tests (Child score; platelet count) |
|
|
| Serum markers of fibrosis | Easily obtained | False positives can occur (inflammation) | |
| Validated for cirrhosis (AUC 85–90% for cirrhosis) | Not validated for PH | ||
| Ultrasound elastography | Transient elastography |
|
|
| Point shear-wave elastography |
|
|
|
| 2D shear-wave elastography |
|
||
| Ultrasound | Ultrasound and Doppler ultrasound complemented by contrast-enhanced ultrasound |
|
|
| Computed tomography (CT) | Abdominal contrast-enhanced CT scan |
|
|
| Magnetic resonance imaging (MRI) | Abdominal contrast-enhanced MRI |
|
|
| Magnetic resonance elastography | Allows measurement of liver and spleen stiffness in large areas of the organs | Expensive; not available in many centres | |
| Lack of data regarding PH |
PH, portal hypertension; CSPH, clinically significant portal hypertension; AUC, area under the ROC curve.
Funding
Interdisciplinary Grant 2015 of the University of Bern (UniBe-ID 2015).
Conflict of interest statement: none declared.
References
- 1. Ripoll C, Groszmann R, Garcia-Tsao G. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133:481–8. [DOI] [PubMed] [Google Scholar]
- 2. Mallet V, Gilgenkrantz H, Serpaggi J. et al. Brief communication : the relationship of regression of cirrhosis to outcome in chronic hepatatis C. Ann Intern Med 2008;149: 399–403. [DOI] [PubMed] [Google Scholar]
- 3. Conti F, Buonfiglioli F, Scuteri A. et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727–33. [DOI] [PubMed] [Google Scholar]
- 4. Anrs T, Co A, Cirvir CO. et al. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: data from three ANRS cohorts. J Hepatol 2016;65:734–40. [DOI] [PubMed] [Google Scholar]
- 5. Kumar M, Kumar A, Hissar S. et al. Hepatic venous pressure gradient as a predictor of ¢ brosis in chronic liver disease because of hepatitis B virus. Liver Int 2008;28:690–8. [DOI] [PubMed] [Google Scholar]
- 6. Regev A, Berho M, Jeffers LJ. et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002;97:2614–18. [DOI] [PubMed] [Google Scholar]
- 7. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:543–5. [DOI] [PubMed] [Google Scholar]
- 8. EASL. Management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- 9. Rockey DC, Caldwell SH, Goodman ZD. et al. Liver biopsy. Hepatology 2009;49:1017–44. [DOI] [PubMed] [Google Scholar]
- 10. Ble M, Procopet B, Miquel R. et al. Transjugular liver biopsy. Clin Liver Dis 2014;18:767–78. [DOI] [PubMed] [Google Scholar]
- 11. Kalambokis G, Manousou P, Vibhakorn S. et al. Transjugular liver biopsy: indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007;47:284–94. [DOI] [PubMed] [Google Scholar]
- 12. Saffioti F, Pinzani M.. Development and regression of cirrhosis. Dig Dis 2016;34:374–81. [DOI] [PubMed] [Google Scholar]
- 13. Kumar M, Sakhuja P, Kumar A. et al. Histological subclassification of cirrhosis based on histological–haemodynamic correlation. Aliment Pharmacol Ther 2008;27:771–9. [DOI] [PubMed] [Google Scholar]
- 14. Tsochatzis E, Bruno S, Isgro G. et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol 2014;60:948–54. [DOI] [PubMed] [Google Scholar]
- 15. Kim MY, Cho MY, Baik SK. et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 2011;55:1004–9. [DOI] [PubMed] [Google Scholar]
- 16. Kutami R, Girgrah N, Wanless I. et al. The Laennec grading system for assessment of hepatic fibrosis: validation by correlation with wedged hepatic vein pressure and clinical features. Hepatology 2000;32:407A. [Google Scholar]
- 17. Kim SU, Jung KS, Lee S. et al. Histological subclassification of cirrhosis can predict recurrence after curative resection of hepatocellular carcinoma. Liver Int 2014;34:1008–17. [DOI] [PubMed] [Google Scholar]
- 18. Kim SU, Oh HJ, Wanless IR. et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012;57:556–63. [DOI] [PubMed] [Google Scholar]
- 19. Wanless IR, Nakashima E, Sherman M.. Regression of human cirrhosis: morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med 2000;124:1599–1607. [DOI] [PubMed] [Google Scholar]
- 20. Hytiroglou P, Snover DC, Alves V. et al. Beyond ‘cirrhosis’ A proposal from the International Liver Pathology Study Group. Am J Clin Pathol 2012;137:5–9. [DOI] [PubMed] [Google Scholar]
- 21. Bosch J, Abraldes JG, Berzigotti A. et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009;6:573–82. [DOI] [PubMed] [Google Scholar]
- 22. Llop E, Berzigotti A, Reig M. et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol 2012;56:103–8. [DOI] [PubMed] [Google Scholar]
- 23. Rossle M, Blanke P, Fritz B. et al. Free hepatic vein pressure is not useful to calculate the portal pressure gradient in cirrhosis: a morphologic and hemodynamic study. J Vasc Interv Radiol 2016;27:1130–7. [DOI] [PubMed] [Google Scholar]
- 24. Silva-Junior G, Baiges A, Turon F. et al. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology 2015;62:1584–92. [DOI] [PubMed] [Google Scholar]
- 25. La Mura V, Abraldes JG, Berzigotti A. et al. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: a clinical-hemodynamic correlation study. Hepatology 2010;51:2108–16. [DOI] [PubMed] [Google Scholar]
- 26. Berzigotti A, Seijo S, Reverter E, Bosch J.. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 2013;7:141–55. [DOI] [PubMed] [Google Scholar]
- 27. Blasco A, Forns X, Carrion JA. et al. Hepatic venous pressure gradient identifies patients at risk of severe hepatitis C recurrence after liver transplantation. Hepatology 2006;43: 492–9. [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Tsao G, Groszmann RJ, Fisher RL. et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5:419–24. [DOI] [PubMed] [Google Scholar]
- 29. Groszmann RJ, Garcia-Tsao G, Bosch J. et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005;353:2254–61. [DOI] [PubMed] [Google Scholar]
- 30. Ripoll C, Groszmann RJ, Garcia-Tsao G. et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009;50:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berzigotti A, Reig M, Abraldes JG. et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526–36. [DOI] [PubMed] [Google Scholar]
- 32. Abraldes JG, Villanueva C, Bañares R. et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229–36. [DOI] [PubMed] [Google Scholar]
- 33. Rincon D, Lo Iacono O, Ripoll C. et al. Prognostic value of hepatic venous pressure gradient for in-hospital mortality of patients with severe acute alcoholic hepatitis. Aliment Pharmacol Ther 2007;25:841–8. [DOI] [PubMed] [Google Scholar]
- 34. Groszmann RJ, Bosch J, Grace ND. et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990;99:1401–7. [DOI] [PubMed] [Google Scholar]
- 35. Villanueva C, Balanzó J, Novella M. et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for prevention of variceal rebleeding. N Engl J Med 1996;334:1624–9. [DOI] [PubMed] [Google Scholar]
- 36. Villanueva C, Minana J, Ortiz J. et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med 2001;345:647–55. [DOI] [PubMed] [Google Scholar]
- 37. D’Amico G, Garcia-Pagan JC, Luca A. et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 2006;131: 1611–24. [DOI] [PubMed] [Google Scholar]
- 38. Hernández-Gea V, Aracil C, Colomo A. et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol 2012;107:418–27. [DOI] [PubMed] [Google Scholar]
- 39. Bureau C, Alric JL, Morales J. et al. ‘ A la carte’ treatment of portal hypertension: adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology 2002;1053:1361–6. [DOI] [PubMed] [Google Scholar]
- 40. Reiberger T, Ulbrich G, Ferlitsch A. et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut 2013;62:1634–41. [DOI] [PubMed] [Google Scholar]
- 41. Merkel C, Bolognesi M, Berzigotti A. et al. Clinical significance of worsening portal hypertension during long-term medical treatment in patients with cirrhosis who had been classified as early good-responders on haemodynamic criteria. J Hepatol 2010;52:45–53. [DOI] [PubMed] [Google Scholar]
- 42. Mandorfer M, Kozbial K, Schwabl P. et al. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol 2016;65:692–9. [DOI] [PubMed] [Google Scholar]
- 43. Lens S, Rincón D, Albillos A. et al. Association between severe portal hypertension and risk of liver decompensation in patients with hepatitis C, regardless of response to antiviral therapy. Clin Gastroenterol Hepatol 2015;13:1846–53 [DOI] [PubMed] [Google Scholar]
- 44. de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762–8. [DOI] [PubMed] [Google Scholar]
- 45. Rudler M,, Benosman H,, Lebray P. et al. Screening for esophageal varices in patients newly diagnosed in 2011: 84% of upper gastrointestinal endoscopies are futile. Hepatology 2011;54(Suppl):935A. [Google Scholar]
- 46. Child C, Turcotte J.. Surgery and portal hypertension In: Child C. (ed.). The Liver and Portal Hypertension. Philadelphia: W. B. Saunders Co., 1964, 50. [Google Scholar]
- 47. Lackner C, Struber G, Liegl B. et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005;41:1376–82. [DOI] [PubMed] [Google Scholar]
- 48. Macías J, Girón-González JA, González-Serrano M. et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut 2006;55:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Castera L, Le Bail B, Roudot-Thoraval F. et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009;50:59–68. [DOI] [PubMed] [Google Scholar]
- 50. Thabut D, Imbert-Bismut F, Cazals-Hatem D. et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther 2007;26:359–68. [DOI] [PubMed] [Google Scholar]
- 51. Berzigotti A, Seijo S, Arena U. et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013;144: 102–11. [DOI] [PubMed] [Google Scholar]
- 52. Sheth SG, Flamm SL, Gordon FD CS.. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44–8. [DOI] [PubMed] [Google Scholar]
- 53. Wai CT, Greenson JK, Fontana RJ. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 54. Corpechot C, Carrat F, Poujol-Robert A. et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198–208. [DOI] [PubMed] [Google Scholar]
- 55. Lok ASF, Ghany MG, Goodman ZD. et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 2005;42:282–92. [DOI] [PubMed] [Google Scholar]
- 56. Procopet B, Cristea VM, Robic MA. et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis 2015;47:411–16. [DOI] [PubMed] [Google Scholar]
- 57. Corpechot C, Carrat F, Poujol-Robert A. et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56:198–208. [DOI] [PubMed] [Google Scholar]
- 58. Sterling RK, Lissen E, Clumeck N. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43: 1317–25. [DOI] [PubMed] [Google Scholar]
- 59. Kim BK, Kim DY, Park JY. et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
- 60. Floreani A, Cazzagon N, Martines D. et al. Performance and utility of transient elastography and noninvasive markers of liver fibrosis in primary biliary cirrhosis. Dig Liver Dis 2011;43:887–92. [DOI] [PubMed] [Google Scholar]
- 61. Lucidarme D, Foucher J, Le Bail B. et al. Factors of accuracy of transient elastography (fibroscan) for the diagnosis of liver fibrosis in chronic hepatitis C. Hepatology 2009;49:1083–9. [DOI] [PubMed] [Google Scholar]
- 62. Naveau S, Gaudé G, Asnacios A. et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology 2009;49:97–105. [DOI] [PubMed] [Google Scholar]
- 63. Zarski J-P, Sturm N, Guechot J. et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol 2012;56:55–62. [DOI] [PubMed] [Google Scholar]
- 64. Lichtinghagen R, Pietsch D, Bantel H. et al. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 2013;59:236–42. [DOI] [PubMed] [Google Scholar]
- 65. Qamar A a, Grace ND, Groszmann RJ. et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol 2009;7:689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kamath PS, Wiesner RH, Malinchoc M. et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–70. [DOI] [PubMed] [Google Scholar]
- 67. Nunes D, Fleming C, Offner G. et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol 2010;105:1346–53. [DOI] [PubMed] [Google Scholar]
- 68. Vergniol J, Foucher J, Terrebonne E. et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:1970–9. [DOI] [PubMed] [Google Scholar]
- 69. Bamber J, Cosgrove D, Dietrich CF. et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med 2013;34:169–84. [DOI] [PubMed] [Google Scholar]
- 70. Shiina T, Nightingale KR, Palmeri ML. et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic principles and terminology. Ultrasound Med Biol 2015;41:1126–47. [DOI] [PubMed] [Google Scholar]
- 71. Cosgrove D, Piscaglia F, Bamber J. et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med 2013;34:238–53. [DOI] [PubMed] [Google Scholar]
- 72. Ferraioli G, Filice C, Castera L. et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound Med Biol 2015;41: 1161–79. [DOI] [PubMed] [Google Scholar]
- 73. Friedrich-Rust M, Ong MF, Martens S. et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960–74. [DOI] [PubMed] [Google Scholar]
- 74. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237–64. [DOI] [PubMed] [Google Scholar]
- 75. Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142: 1293–1302. [DOI] [PubMed] [Google Scholar]
- 76. Shi KQ, Fan YC, Pan ZZ. et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 2013;33:62–71. [DOI] [PubMed] [Google Scholar]
- 77. You MW, Kim KW, Pyo J. et al. A meta-analysis for the diagnostic performance of transient elastography for clinically significant portal hypertension. Ultrasound Med Biol 2016;43:59–68. [DOI] [PubMed] [Google Scholar]
- 78. Singh S, Fujii LL, Murad MH. et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Castera L, Pinzani M, Bosch J.. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol 2012;56:696–703. [DOI] [PubMed] [Google Scholar]
- 80. Vizzutti F, Arena U, Romanelli RG. et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007;45:1290–7. [DOI] [PubMed] [Google Scholar]
- 81. Friedrich-Rust M, Nierhoff J, Lupsor M. et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat 2012;19:e212–19. [DOI] [PubMed] [Google Scholar]
- 82. Feng JC, Li J, Wu XW. et al. Diagnostic accuracy of supersonic shear imaging for staging of liver fibrosis: a meta-analysis. J Ultrasound Med 2016;35:329–39. [DOI] [PubMed] [Google Scholar]
- 83. Berzigotti A. ( 2017) Non invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol, Published online: February 13, 2017. DOI: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 84. Mejias M, Garcia-Pras E, Gallego J. et al. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol 2010;52:529–39. [DOI] [PubMed] [Google Scholar]
- 85. Ma X, Wang L, Wu H. et al. Spleen stiffness is superior to liver stiffness for predicting esophageal varices in chronic liver disease: a meta-analysis. PLoS One 2016;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Venkatesh SK, Yin M, Ehman RL.. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 2013;37:544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Godfrey EM, Mannelli L, Griffin N. et al. Magnetic resonance elastography in the diagnosis of hepatic fibrosis. Semin Ultrasound CT MR 2013;34:81–8. [DOI] [PubMed] [Google Scholar]
- 88. Cui J, Heba E, Hernandez C. et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Imajo K, Kessoku T, Honda Y. et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016;150:626–37.e7. [DOI] [PubMed] [Google Scholar]
- 90. Ronot M, Lambert S, Elkrief L. et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol 2014;24:1394–1402. [DOI] [PubMed] [Google Scholar]
- 91. Margini C, Berzigotti A.. Portal vein thrombosis: The role of imaging in the clinical setting. Dig Liver Dis 2016;49:113–20. [DOI] [PubMed] [Google Scholar]
- 92. Simonovsky V. The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. Br J Radiol 1999;72:29–34. [DOI] [PubMed] [Google Scholar]
- 93. Berzigotti A, Abraldes JG, Tandon P. et al. Ultrasonographic evaluation of liver surface and transient elastography in clinically doubtful cirrhosis. J Hepatol 2010;52:846–53. [DOI] [PubMed] [Google Scholar]
- 94. Gaiani S, Gramantieri L, Venturoli N. et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997;27:979–85. [DOI] [PubMed] [Google Scholar]
- 95. Berzigotti A, Piscaglia F.. Ultrasound in portal hypertension—Part 1. Ultraschall Med 2011;32:548–71. [DOI] [PubMed] [Google Scholar]
- 96. Berzigotti A, Piscaglia F.. Ultrasound in portal hypertension—Part 2—and EFSUMB recommendations for the performance and reporting of ultrasound examinations in portal hypertension. Ultraschall Med 2012;33:8–32. [DOI] [PubMed] [Google Scholar]
- 97. Abraldes JG, Gilabert R, Turnes J. et al. Utility of color Doppler ultrasonography predicting TIPS dysfunction. Am J Gastroenterol 2005;100:2696–2701. [DOI] [PubMed] [Google Scholar]
- 98. Brancatelli G, Federle MP, Ambrosini R. et al. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 2007;61:57–69. [DOI] [PubMed] [Google Scholar]
- 99. Smith AD, Branch CR, Zand K. et al. Liver surface nodularity quantification from routine ct images as a biomarker for detection and evaluation of cirrhosis. Radiology 2016;280:151542. [DOI] [PubMed] [Google Scholar]
- 100. de Franchis R. Non-invasive (and minimally invasive) diagnosis of oesophageal varices. J Hepatol 2008;49:520–7. [DOI] [PubMed] [Google Scholar]
- 101. McAvoy NC, Semple S, Richards JMJ. et al. Differential visceral blood flow in the hyperdynamic circulation of patients with liver cirrhosis. Aliment Pharmacol Ther 2016;43:947–54. [DOI] [PubMed] [Google Scholar]
- 102. Materne R, van Beers BE, Smith AM. et al. Non-invasive quantification of liver perfusion with dynamic computed tomography and a dual-input one-compartmental model. Clin Sci (Lond) 2000;99:517–25. [PubMed] [Google Scholar]
- 103. Van Beers BE, Materne R, Annet L. et al. Capillarization of the sinusoids in liver fibrosis: noninvasive assessment with contrast-enhanced MRI in the rabbit. Magn Reson Med 2003;49:692–9. [DOI] [PubMed] [Google Scholar]
- 104. Annet L, Materne R, Danse E. et al. Measured with MR Imaging and Doppler US : correlations with degree of cirrhosis and portal hypertension. Radiology 2003;2:409–14. [DOI] [PubMed] [Google Scholar]
- 105. Gouya H, Grabar S, Vignaux O. et al. Portal hypertension in patients with cirrhosis: indirect assessment of hepatic venous pressure gradient by measuring azygos flow with 2D-cine phase-contrast magnetic resonance imaging. Eur Radiol 2016;26:1981–90. [DOI] [PubMed] [Google Scholar]
- 106. Palaniyappan N, Cox E, Bradley C. et al. Non-invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol 2016;65:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


