Abstract
Background
The SAMe-TT2R2 score was developed to predict which patients on oral anticoagulation with vitamin K antagonists (VKAs) will reach an adequate time in therapeutic range (TTR) (> 65%-70%). Studies have reported a relationship between this score and the occurrence of adverse events.
Objective
To describe the TTR according to the score, in addition to relating the score obtained with the occurrence of adverse events in patients with nonvalvular atrial fibrillation (AF) on oral anticoagulation with VKAs.
Methods
Retrospective cohort study including patients with nonvalvular AF attending an outpatient anticoagulation clinic of a tertiary hospital. Visits to the outpatient clinic and emergency, as well as hospital admissions to the institution, during 2014 were evaluated. The TTR was calculated through the Rosendaal´s method.
Results
We analyzed 263 patients (median TTR, 62.5%). The low-risk group (score 0-1) had a better median TTR as compared with the high-risk group (score ≥ 2): 69.2% vs. 56.3%, p = 0.002. Similarly, the percentage of patients with TTR ≥ 60%, 65% or 70% was higher in the low-risk group (p < 0.001, p = 0.001 and p = 0.003, respectively). The high-risk group had a higher percentage of adverse events (11.2% vs. 7.2%), although not significant (p = 0.369).
Conclusions
The SAMe-TT2R2 score proved to be effective to predict patients with a better TTR, but was not associated with adverse events.
Keywords: Atrial Fibrillation, Anticoagulants / adverse effects, Decision Support Techniques, Warfarin, Phenprocoumon, Vitamin K
Introduction
Vitamin K antagonists (VKAs) reduce the risk for ischemic stroke in patients with atrial fibrillation (AF) by approximately 60%.1 The efficacy of the treatment with VKAs is directly related to the time in therapeutic range (TTR), that is, percent time with prothrombin time/international normalized ratio (PT/INR) between 2.0 and 3.0.2 A previous study3 has suggested that the target TTR would be 58%-65%, below which there appears to be little benefit of oral anticoagulation with VKAs over dual antiplatelet therapy. Additional evidence has emphasized that stroke prevention with the use of VKAs is effective when individual mean TTR is high (> 70%).4
Predicting which patients are good candidates for anticoagulation therapy would be very useful. Scores are currently used to assess the risk for thromboembolic events (CHADS2 and CHA2DS2-VASc),5,6 as well as the risk for the major adverse effect from that therapy, bleeding (HAS-BLED).7 Those scores allow us to assess the indication for that therapy and its risk; however, they provide no information on how the patient will respond to treatment, that is, whether the patient will maintain the target TTR. An easy prediction of which AF patients are likely to reach the target TTR by using VKAs could guide decision making in the strategy of anticoagulation with VKAs or new oral anticoagulants (NOACs).8 Recently, Apostolakis et al.9 have proposed and validated the SAMe-TT2R2 score. Those authors have reported the possibility of identifying AF patients on VKAs who reached the target TTR (score 0-1), as well as those who required additional interventions to reach the target TTR, achieving a low TTR with the use of VKAs (score ≥ 2), being thus likely candidates for the use of NOACs. Later studies have validated that score for the prediction of both TTR8,10-17 and adverse events.8,10-12,16,17 Others, however, have shown that the score cannot do that.18-20
In a previous study,21 we have described our experience in an outpatient anticoagulation clinic of a Brazilian tertiary hospital, with a mean TTR of 64.8%. This study aimed at describing the TTR according to the SAMe-TT2R2 score, in addition to relating the score obtained with the occurrence of adverse events in patients with nonvalvular AF on anticoagulation with VKAs.
Methods
This is a retrospective cohort including patients on oral anticoagulation with VKAs being followed up at the Outpatient Anticoagulation Clinic of the Internal Medicine Service of the Hospital de Clínicas de Porto Alegre (HCPA), a university-affiliated hospital for tertiary care in the Southern region of Brazil. Decisions regarding anticoagulation management were based on the protocol by Kim et al.22 All patients attending consultations from January to March 2014 were screened, and those with nonvalvular AF were included in this study. Valvular AF was considered when moderate to severe mitral stenosis or prosthetic heart valve coexisted.4
The risk for ischemic stroke was estimated based on the CHADS2 and CHA2DS2-VASc scores, while the risk for bleeding was estimated based on the HAS-BLED score.5-7 To analyze the SAMe-TT2R2 score (0-8 points), the following variables were assessed: female sex (1 point), age < 60 years (1 point), presence of > 2 comorbidities (1 point), use of amiodarone to control heart rhythm (1 point), smoking within 2 years (2 points), and non-Caucasian race (2 points). The following were considered comorbidities: previous stroke, diabetes, peripheral artery disease, coronary artery disease, liver disease, lung disease, kidney disease, hypertension, and heart failure. Patients were categorized based on the SAMe-TT2R2 score into two groups: low risk (0-1 point) and high risk (≥ 2 points).9
Demographic and clinical data and results from complementary tests were obtained via retrospective assessment to electronic medical records, outpatient clinic consultations, visits to the emergency unit and admissions to the HCPA from January to December 2014. Patients lost to follow-up, those who died or whose anticoagulation with VKAs was suspended were also included in the analysis, and the TTR was analyzed up to the last available PT/INR test. Patients were assessed regarding anticoagulation control (via PT/INR tests) and occurrence of adverse events [major bleeding, stroke, transient ischemic attack (TIA), systemic embolism or death]. The TTR was estimated by use of the Rosendaal´s linear interpolation method.23
The laboratory tests, left ventricular ejection fraction (preferably assessed on echocardiogram) and number of drugs used were recorded based on the information available on the date closest to the beginning of follow-up. Anemia was considered when hemoglobin (Hb) < 13.0 g/dL for men or < 12 g/dL for women.24 Uncontrolled hypertension was defined as systolic blood pressure > 160 mm Hg at the outpatient clinic visit closest to the beginning of follow-up.7 Major bleeding was characterized as an event requiring hospitalization or transfusion of red blood cell concentrate, or Hb drop ≥ 2 g/dL.7 Kidney disease was considered in the presence of kidney transplantation, chronic dialysis, or serum creatinine ≥ 2.26 mg/dL.7 Liver disease was considered in the presence of chronic liver disease (ex.: cirrhosis) or biochemical evidence of significant liver damage (ex.: bilirubin > 2x the upper limit of normality, associated with aspartate aminotranferase, alanine aminotranferase or alkaline phosphatase levels > 3x the normal limit).7
Statistical analysis
Data were analyzed with the Statistical Package for Social Sciences (SPSS) software, version 21.0. Descriptive analysis was performed based on the distribution of absolute and relative frequency for qualitative variables, and based on mean ± standard deviation and median for quantitative variables with symmetrical and asymmetrical distribution, respectively. The median 25-75% percentiles were presented when deemed suitable. The groups were compared by using non-paired Student t test for symmetrical quantitative variables, Mann-Whitney U test for asymmetrical quantitative variables, and chi-square test for categorical variables. In low-frequency situations, Fisher exact test was used. The normality of the distribution of each variable was assessed by using Shapiro-Wilk test. Area under the Receiver Operating Characteristic (ROC) curve was calculated to assess the ability of the SAMe-TT2R2 score to predict the outcome 'TTR ≥ 65%' and the occurrence of adverse events, the best cutoff point of the score being considered that with the highest sensitivity x specificity product. Event-free survival was assessed by using Kaplan-Meier curves with the Log-Rank test. The significance level adopted for all tests was 5%. This study was submitted to the Committee on Ethics and Research from the HCPA, and approved.
Results
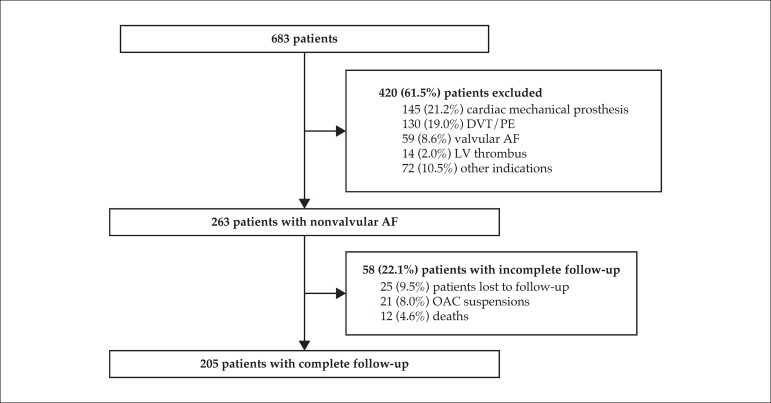
This study assessed 263 patients on oral anticoagulation with VKAs due to nonvalvular AF, corresponding to 38.5% of those being followed up at the Outpatient Anticoagulation Clinic of the HCPA. Of those, 205 patients (77.9%) completed the follow-up (Figure 1). Table 1 shows the demographic characteristics of the sample.
Figure 1.
Study diagram. DVT: deep venous thrombosis; PTE: pulmonary embolism; AF: atrial fibrillation; LV: left ventricular; OAC: oral anticoagulation.
Table 1.
Demographic characteristics of the sample
| Variable | n = 263 |
|---|---|
| Female sex | 113 (43.0) |
| Age (years) | 71.2 (64.1-78.5) |
| Use of warfarin | 256 (97.3) |
| Labile PT/INR (TTR < 60%) | 124 (47.1) |
| Hypertension | 231 (87.8) |
| Uncontrolled hypertension | 22 (8.4) |
| HF/LVEF < 40% | 149 (56.7) |
| Diabetes | 108 (41.1) |
| Previous stroke/TIA | 96 (36.5) |
| Coronary artery disease | 76 (28.9) |
| Use of antiplatelet drugs/NSAIDs | 64 (24.3) |
| Anemia | 67 (25.5) |
| Pulmonary disease | 36 (13.7) |
| Previous major bleeding | 24 (9.1) |
| Peripheral artery disease | 25 (9.5) |
| Kidney disease | 7 (2.7) |
| Liver disease | 2 (0.8) |
| Number of medications | 7 (6-9) |
| CHADS2 | 3 (2-4) |
| CHA2DS2-VASc | 4 (3-5) |
| HAS-BLED | 2 (1-3) |
PT/INR: prothrombin time / international normalized ratio; TTR: time in therapeutic range; HF: heart failure; LVEF: left ventricular ejection fraction; TIA: transient ischemic attack; NSAIDs: non-steroidal anti-inflammatory drugs. Categorical variables are shown as n (%), and continuous variables, as median (25%-75%).
During follow-up, 2,754 PT/INR tests (median: 10 tests/patient) were performed, and 1,270 (46.1%) resulted between 2.0 and 3.0. Median TTR was 62.5% (P25-75 44.2%-79.5%). The median of subtherapeutic PT/INR time (< 2.0) was 18.9%, and that of supratherapeutic PT/INR time (> 3.0), 9.6%.
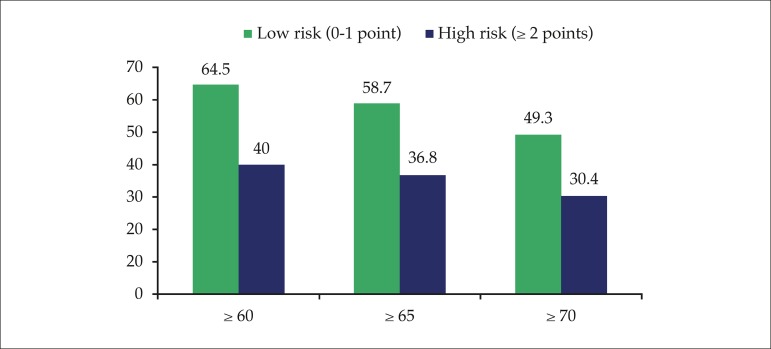
Regarding the SAMe-TT2R2 score, 138 patients (52.5%) had it 0-1 (low risk), while 125 (47.5%) had it ≥ 2 (high risk), the median being 1 (1-2). When assessing the SAMe-TT2R2 score criteria individually (Table 2), the criterion "medical history" (presence of > 2 comorbidities) was the most prevalent (57.0%). Low-risk (score 0-1) patients had a significantly higher median TTR as compared to high-risk (score ≥ 2) ones: 69.2% vs. 56.3% (p = 0.002). Likewise, the percentage of patients with TTR ≥ 60%, 65% or 70% was higher among low-risk patients for all cutoff points analyzed (Figure 2).
Table 2.
Prevalence of the SAMe-TT2R2 score components
| Score Component | n (%) | |
|---|---|---|
| S | Sex (female) | 113 (43.0) |
| A | Age (< 60 years) | 41 (15.6) |
| Me | Medical history (> 2 comorbidities*) | 150 (57.0) |
| T | Treatment (amiodarone) | 26 (9.9) |
| T2 | Tobacco use (within 2 years) | 37 (14.1) |
| R2 | Race (non-Caucasian) | 22 (8.4) |
Previous stroke, diabetes, peripheral artery disease, coronary artery disease, liver disease, lung disease, kidney disease, hypertension, and heart failure.
Figure 2.
Percentage of patients with TTR ≥ 60%, 65% and 70% according to the points obtained in the SAMe-TT2R2 score (p < 0.001, 0.001 and 0.003, respectively).
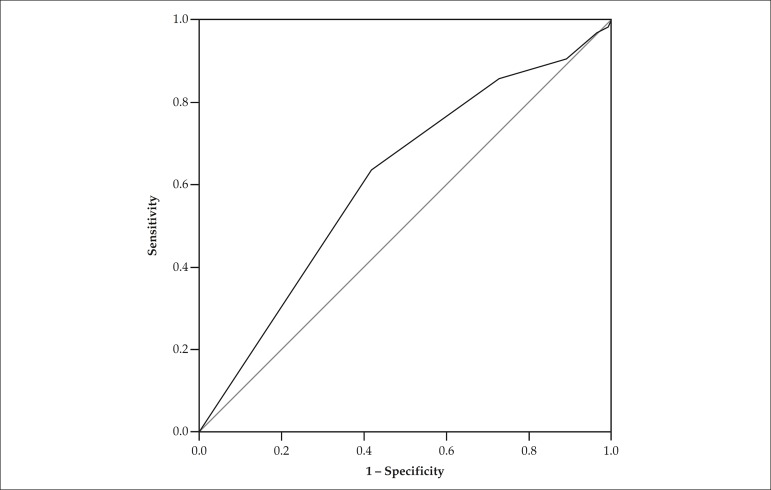
When assessing the ability of the SAMe-TT2R2 score to predict the outcome 'TTR ≥ 65%' by using the ROC curve (Figure 3), the cutoff point ≥ 2 showed the best combination of sensitivity and specificity (63.8% and 58.1%, respectively). The area under the curve was 0.612 (95%CI: 0.544 - 0.681; p = 0.002).
Figure 3.
ROC curve for the outcome 'TTR ≥ 65%'.
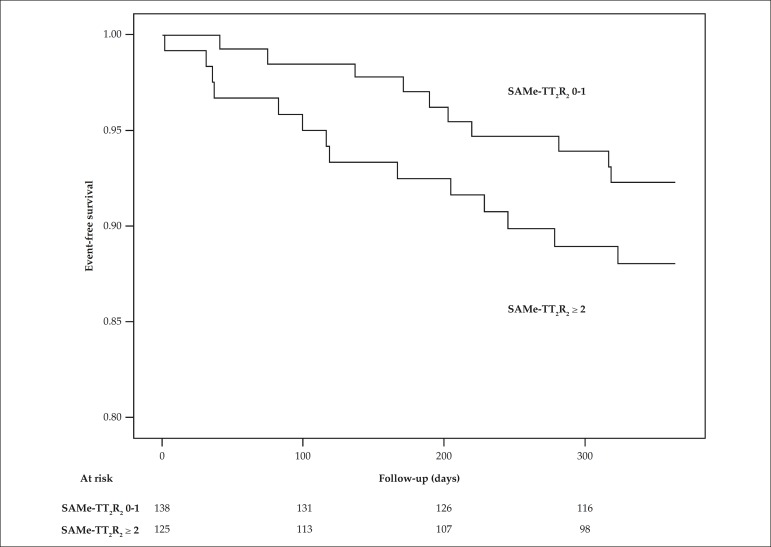
During follow-up, there were 24 (9.1%) adverse events, whose complete description is shown in Table 3. Neither TIA nor systemic embolism occurred during the period studied. High-risk patients (score ≥ 2) had more events, but with no statistically significant difference (11.2% vs. 7.2%; p = 0.369). The area under the ROC curve of the score for the occurrence of adverse events was 0.566 (95%CI: 0.449 - 0.682; p = 0.289), ≥ 2 being again the best cutoff point, with sensitivity and specificity of 58.3% and 53.6%, respectively. Figure 4 shows the event-free survival curves.
Table 3.
Adverse events in total follow-up and according to the points obtained in the SAMe TT2R2 score.
| Adverse Events | n = 263 | SAMe-TT2R2 | p | |
|---|---|---|---|---|
| 0-1 point | ≥ 2 points | |||
| Major bleeding | 15 (5.7) | 6 (4.3) | 9 (7.2) | 0.465 |
| Stroke | 4 (1.5) | 1 (0.7) | 3 (2.4) | 0.349 |
| Death | 12 (4.6) | 5 (3.6) | 7 (5.6) | 0.637 |
| TOTAL | 24 (9.1) | 10 (7.2) | 14 (11.2) | 0.369 |
Data shown as n (%).
Figure 4.
Event-free survival curve according to the points obtained in the SAMe-TT2R2 score (p = 0.224).
Discussion
The use of anticoagulation in patients with AF to prevent thromboembolic events is known to be effective and TTR-dependent. Predicting which patients on VKAs are more likely to reach the target TTR is important, especially currently when new drugs that do not require PT/INR monitoring are available. In this study with a Brazilian sample, the SAMe-TT2R2 score proved to be a good predictor of TTR for nonvalvular AF patients on oral anticoagulation with VKAs. That score can be useful in the initial assessment of patients with indication for anticoagulation. Median TTR, as well as the percentage of patients with TTR ≥ 60%, 65% and 70%, were higher among patients with a low SAMe-TT2R2 score (0-1 point) as compared to the group whose score was ≥ 2.
The usefulness of that score in other populations and clinical settings has been reported. Ruiz-Ortiz et al.,15 in a prospective analysis of Spanish cardiology outpatients, have reported a progressive decrease in mean TTR according to the score obtained. In their study, patients who scored 0 had a mean TTR of 67.5% ± 24.6%, while those who scored ≥ 4 had a mean TTR of 52.7% ± 28.7% (p < 0.01), with an area under the ROC curve for the outcome 'TTR ≥ 65%' of 0.57 (95%CI: 0.53 - 0.60; p < 0.0005). Roldán et al.,14 assessing 459 patients of an outpatient anticoagulation clinic, have reported that those with a score of 0-1 had a mean TTR of 67% ± 18%, while those with a score ≥ 2 had a mean TTR of 61% ± 16% (p < 0.001). In their study, the odds ratio for reaching a TTR < 65% was 2.10 (95%CI: 1.44 - 3.06; p < 0.001) in patients with a score ≥ 2. In a retrospective study including 4,468 patients selected from a registry of primary care units in the United Kingdom, Martinez et al.17 have reported that the proportion of patients with TTR ≥ 60% was 44.1% among those with a score of 0-1, and 37.1% among those with a score ≥ 2 (p < 0.01).
The association of the points obtained in the score with the occurrence of anticoagulation adverse events (major bleeding, stroke, systemic embolism and/or death) has been described in other studies8,10-12,16,17 after the original study,9 always relating the quality of anticoagulation, assessed via TTR, with the occurrence of those outcomes. Only the study by Poli et al.13 has not observed that relationship. In a retrospective study including 4,468 AF patients on VKAs with a 3-year follow-up, Martinez et al.17 have reported a higher risk for stroke in patients with score ≥ 2 as compared to those with score of 0-1 (log rank p < 0.01). Lip et al.,12 in a retrospective study with 8,120 patients (mean follow-up, 1,016 ± 1,108 days), have reported that the SAMe-TT2R2 score predicted stroke/thromboembolism, severe bleeding and death, reflecting a suboptimum TTR in patients with score ≥ 2. In the present study, the lack of association between the score and the occurrence of adverse events, specifically stroke, can be attributed to the low incidence of that complication.
Several studies have proposed the inclusion of the SAMe-TT2R2 score in the flowchart for strategic decision-making about which anticoagulant should be used for patients recently diagnosed with AF.14,25-28 Based on the score obtained, for patients with ≥ 2 points, the use of NOACs should begin immediately, while those with a score of 0-1 should begin their treatment with VKAs, which should be changed to NOACs if target TTR (> 70%) was not achieved during follow-up. Current guidelines for AF management, however, have not included that strategy.4,29,30
Our study has some limitations. Its retrospective design has inherent limitations, which can affect the quality of the data analyzed. Nevertheless, we believe that there was no great loss of data necessary for this study, because at our institution patients undergo systematic care, by use of protocols and structured outpatient clinic visits. Thus, most data necessary for the study was systematically collected during outpatient visits. Another limitation is that the medical record review identified only in-hospital adverse events or events reported by patients during their visits to the outpatient clinic, and some events, especially the adverse ones, might have been missed. Finally, the single-center characteristic of this study ensures the uniform follow-up of the patients described in this cohort, but might have decreased its external validity.
Conclusion
Based on our findings, the SAMe-TT2R2 score proved to be effective to predict TTR for AF patients on anticoagulation with VKAs. Thus, the association of that score with the scores to assess the indication of anticoagulation (CHADS2 and/or CHA2DS2-VASc), as well as the risk for bleeding (HAS-BLED), will provide a high-quality assessment of the treatment. For patients with a high SAMe-TT2R2 score (≥ 2), anticoagulation with VKAs is more likely to be less effective, and, thus, the use of NOACs should be considered. Low-risk patients (score 0-1), however, respond better to VKAs. Therefore, an intervention based on patients' risk allows the use of new technologies (in our case, NOACs), usually more expensive and less available, to be directed to a group of patients with a more specific indication.
Footnotes
Author contributions
Conception and design of the research, Analysis and interpretation of the data and Critical revision of the manuscript for intellectual content: Pivatto Júnior F, Scheffel RS, Amon LC, Biolo A; Acquisition of data: Ries L, Wolkind RR, Marobin R, Barkan SS; Statistical analysis: Pivatto Júnior F, Scheffel RS; Writing of the manuscript: Pivatto Júnior F, Scheffel RS, Ries L, Wolkind RR, Marobin R, Barkan SS, Amon LC, Biolo A.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Fernando Pivatto Júnior, from Universidade Federal do Rio Grande do Sul.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.White HD, Gruber M, Feyzi J, Kaatz S, Tse HF, Husted S, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007;167(3):239–245. doi: 10.1001/archinte.167.3.239. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–2037. doi: 10.1161/CIRCULATIONAHA.107.750000. [DOI] [PubMed] [Google Scholar]
- 4.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. ESC Committee for Practice Guidelines 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. Erratum in: Eur Heart J. 2013;34(10):790. Eur Heart J. 2013;34(36):2850-1. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 6.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 7.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 8.Gallego P, Roldán V, Marin F, Gálvez J, Valdés M, Vicente V, et al. SAMe-TT2R2 score, time in therapeutic range, and outcomes in anticoagulated patients with atrial fibrillation. Am J Med. 2014;127(11):1083–1088. doi: 10.1016/j.amjmed.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: The SAMe-TT2R2 score. Chest. 2013;144(5):1555–1563. doi: 10.1378/chest.13-0054. [DOI] [PubMed] [Google Scholar]
- 10.Chan PH, Hai JJ, Chan EW, Li WH, Tse HF, Wong IC, et al. Use of the SAMe-TT2R2 score to predict good anticoagulation control with warfarin in Chinese patients with atrial fibrillation: relationship to ischemic stroke incidence. PLoS One. 2016;11(3):e0150674. doi: 10.1371/journal.pone.0150674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abumuaileq RR, Abu-Assi E, Raposeiras-Roubin S, López-López A, Redondo-Diéguez A, Álvarez-Iglesias D, et al. Evaluation of SAMe-TT2R2 risk score for predicting the quality of anticoagulation control in a real-world cohort of patients with non-valvular atrial fibrillation on vitamin-K antagonists. Europace. 2015;17(5):711–717. doi: 10.1093/europace/euu353. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Haguenoer K, Saint-Etienne C, Fauchier L. Relationship of the SAMe-TT2R2 score to poor-quality anticoagulation, stroke, clinically relevant bleeding, and mortality in patients with atrial fibrillation. Chest. 2014;146(3):719–726. doi: 10.1378/chest.13-2976. [DOI] [PubMed] [Google Scholar]
- 13.Poli D, Antonucci E, Testa S, Lip GY. A prospective validation of the SAME-TT2R2 score: how to identify atrial fibrillation patients who will have good anticoagulation control on warfarin. Intern Emerg Med. 2014;9(4):443–447. doi: 10.1007/s11739-014-1065-8. [DOI] [PubMed] [Google Scholar]
- 14.Roldán V, Cancio S, Gálvez J, Valdés M, Vicente V, Marín F, et al. The SAMe-TT2R2 score predicts poor anticoagulation control in AF patients: a prospective 'real-world' inception cohort study. Am J Med. 2015;128(11):1237–1243. doi: 10.1016/j.amjmed.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Ortiz M, Bertomeu V, Cequier Á, Marín F, Anguita M. Validation of the SAMe-TT2R2 score in a nationwide population of nonvalvular atrial fibrillation patients on vitamin K antagonists. Thromb Haemost. 2015;114(4):695–701. doi: 10.1160/TH15-02-0169. [DOI] [PubMed] [Google Scholar]
- 16.Proietti M, Lane DA, Lip GY. Relation of the SAMe-TT2R2 score to quality of anticoagulation control and thromboembolic events in atrial fibrillation patients: observations from the SPORTIF trials. Int J Cardiol. 2016;216:168–172. doi: 10.1016/j.ijcard.2016.04.131. [DOI] [PubMed] [Google Scholar]
- 17.Martinez C, Katholing A, Reitbrock S, Lip GY, Freedman B. SAMeTT2R2 scores predict stroke risk after initiation of vitamin K antagonist therapy for atrial fibrillation: a real-world practice study. Circulation. 2014;130:A19565–A19565. Abstract. [Google Scholar]
- 18.Al Janubi H, Mohamad S, Mahfouz A, Muabby NE, Tawengi K, Alismaaial M, et al. PM 184 The Same-TT2R2 score does not predict the quality of anticoagulation or outcomes of atrial fibrillation in middle eastern patients. Global Heart. 2016;11(2):e101 [Google Scholar]
- 19.Andreu-Cayuelas JM, Puche CM, Caro-Martínez C, Flores-Blanco PJ, Valdés M, Manzano-Fernández S. SAMe-TT2R2 score does not predict time in therapeutic range in atrial fibrillation patients after hospitalization for acute decompensated heart failure. Rev Esp Cardiol. 2016;69(4):453–454. doi: 10.1016/j.rec.2016.01.009. Letter. [DOI] [PubMed] [Google Scholar]
- 20.Skov J, Bladbjerg E, Bor MV, Gram J. SAMeTT2R2 does not predict time in therapeutic range of the international normalized ratio in patients attending a high-quality anticoagulation clinic. Chest. 2014;145(1):187–188. doi: 10.1378/chest.13-1897. [DOI] [PubMed] [Google Scholar]
- 21.Pivatto Jr F, da Silva AL, Simionato BM, Fuzinatto F, Oliveira JC, Pires LM, et al. Management of anticoagulation with vitamin K antagonists in a tertiary hospital outpatient clinic. Clin Biomed Res. 2014;34(2):139–144. [Google Scholar]
- 22.Kim YK, Nieuwlaat R, Connolly SJ, Schulman S, Meijer K, Raju N, et al. Effect of a simple two-step warfarin dosing algorithm on anticoagulant control as measured by time in therapeutic range: a pilot study. J Thromb Haemost. 2010;8(1):101–106. doi: 10.1111/j.1538-7836.2009.03652.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. [PubMed] [Google Scholar]
- 24.World Health Organization. Department of Nutrition for Health and Development . Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva: 2001. [Google Scholar]
- 25.Fauchier L, Angoulvant D, Lip GY. The SAMe-TT2R2 score and quality of anticoagulation in atrial fibrillation: a simple aid to decision-making on who is suitable (or not) for vitamin K antagonists. Europace. 2015;17(5):671–673. doi: 10.1093/europace/euv088. [DOI] [PubMed] [Google Scholar]
- 26.Fauchier L, Poli D, Olshansky B. The SAMe-TT2R2 score and quality of anticoagulation in AF: Can we predict which patient benefits from anticoagulation? Thromb Haemost. 2015;114(4):657–659. doi: 10.1160/TH15-06-0518. [DOI] [PubMed] [Google Scholar]
- 27.Voukalis C, Lip GY, Shantsila E. Emerging tools for stroke prevention in atrial fibrillation. EBioMedicine. 2016;4:26–39. doi: 10.1016/j.ebiom.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esteve-Pastor MA, Roldán V, Valdés M, Lip GY, Marín F. The SAMe-TT2R2 score and decision-making between a vitamin K antagonist or a non-vitamin K antagonist oral anticoagulant in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2016;14(2):177–187. doi: 10.1586/14779072.2016.1116941. [DOI] [PubMed] [Google Scholar]
- 29.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Magalhães LP, Figueiredo MJ, Cintra FD, Saad EB, Kuniyishi RR, Teixeira RA, et al. Sociedade Brasileira de Cardiologia II Diretrizes brasileiras de fibrilação atrial. Arq Bras Cardiol. 2016;106(4) Supl. 2:1–22. [Google Scholar]