Abstract
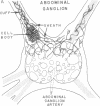
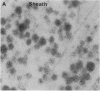
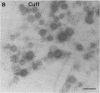
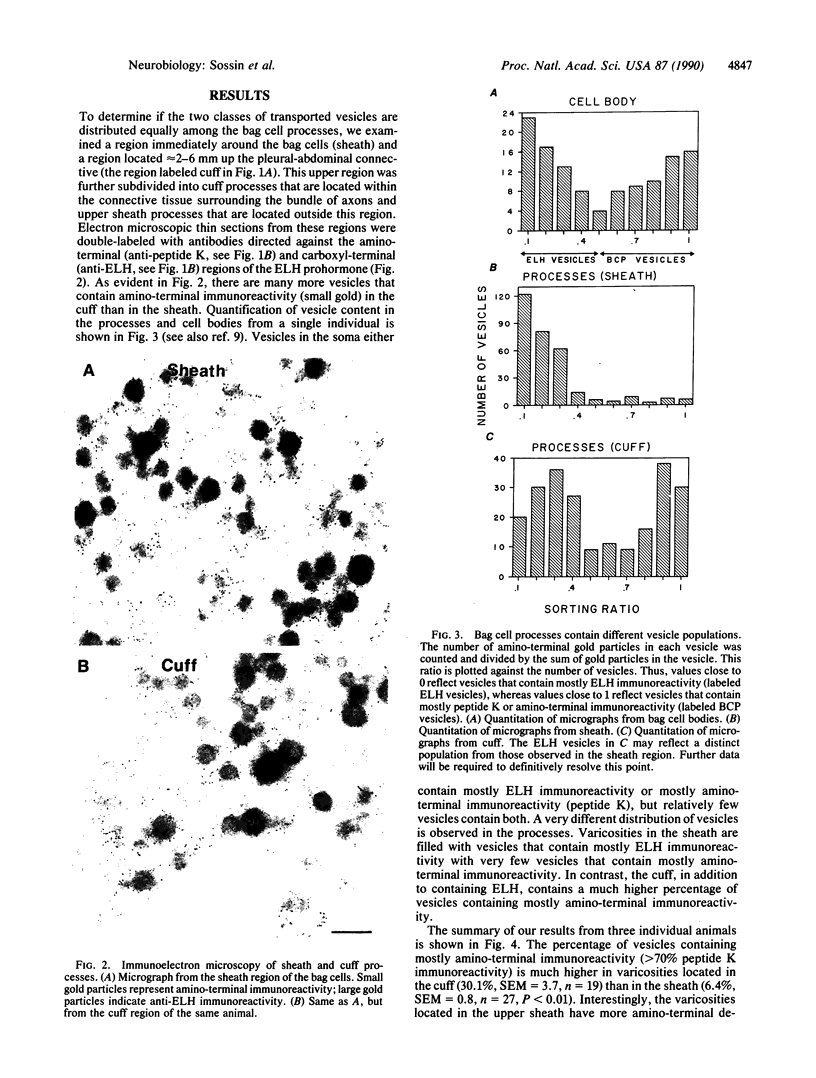
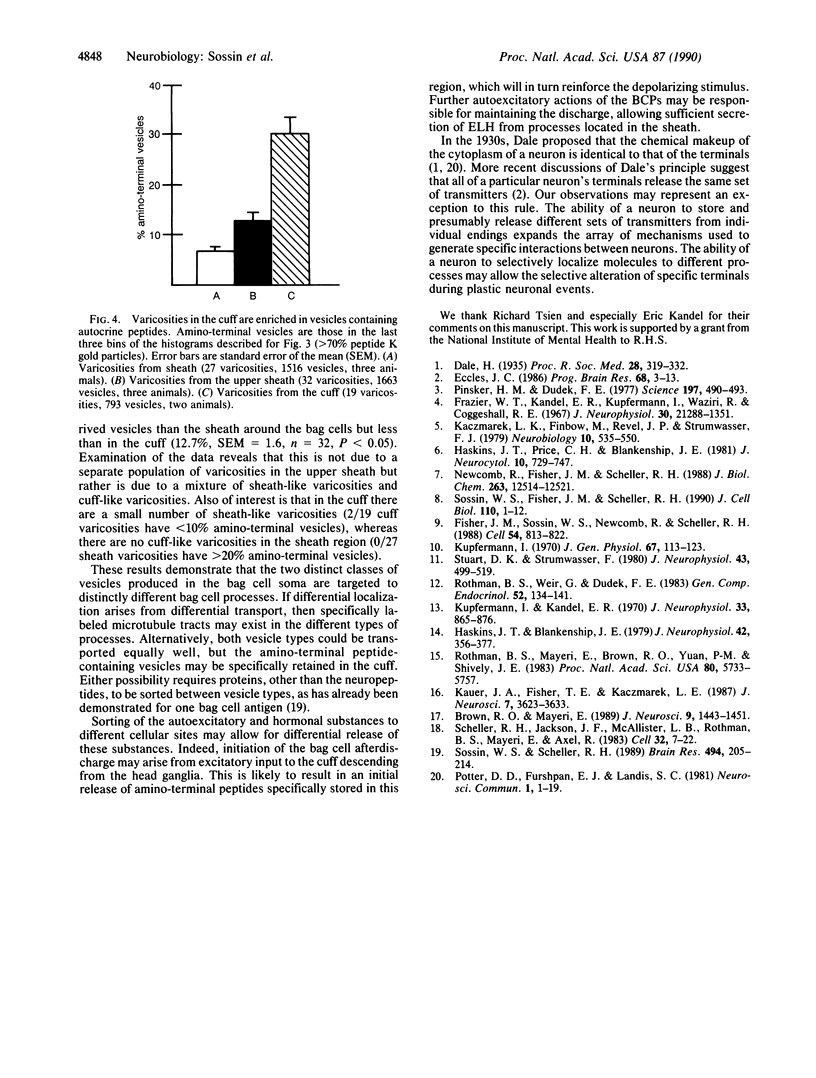
In the bag cells of Aplysia californica, the prohormone of egg-laying hormone is processed by means of endoproteolytic cleavage into two sets of peptides. The amino-terminal region of the prohormone gives rise to the bag cell peptides (alpha, beta, and gamma). These serve an autocrine function; they are autoexcitatory on the bag cells and also act locally to alter the firing patterns of neurons in the abdominal ganglion. The carboxyl-terminal portion of the prohormone gives rise to the egg-laying hormone. This peptide acts as a hormone on nearby neurons and by means of the circulation on peripheral tissues to bring about egg-laying. We have previously reported that the first cleavage of the prohormone, which occurs in the trans-Golgi network, results in two intermediates that are sorted into distinct vesicle classes prior to further processing. Here we show that these distinct vesicles are localized to separate processes, thus spatially segregating autocrine and hormonal release sites. The findings of segregation indicate that neurons need not always release the same set of chemical messengers from all of their endings.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. O., Mayeri E. Positive feedback by autoexcitatory neuropeptides in neuroendocrine bag cells of Aplysia. J Neurosci. 1989 Apr;9(4):1443–1451. doi: 10.1523/JNEUROSCI.09-04-01443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. Chemical transmission and Dale's principle. Prog Brain Res. 1986;68:3–13. doi: 10.1016/s0079-6123(08)60227-7. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Sossin W., Newcomb R., Scheller R. H. Multiple neuropeptides derived from a common precursor are differentially packaged and transported. Cell. 1988 Sep 9;54(6):813–822. doi: 10.1016/s0092-8674(88)91131-2. [DOI] [PubMed] [Google Scholar]
- Haskins J. T., Blankenship J. E. Interactions between bilateral clusters of neuroendocrine cells in Aplysia. J Neurophysiol. 1979 Mar;42(2):356–367. doi: 10.1152/jn.1979.42.2.356. [DOI] [PubMed] [Google Scholar]
- Haskins J. T., Price C. H., Blankenship J. E. A light and electron microscopic investigation of the neurosecretory bag cells of Aplysia. J Neurocytol. 1981 Oct;10(5):729–747. doi: 10.1007/BF01262650. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Finbow M., Revel J. P., Strumwasser F. The morphology and coupling of Aplysia bag cells within the abdominal ganglion and in cell culture. J Neurobiol. 1979 Nov;10(6):535–550. doi: 10.1002/neu.480100604. [DOI] [PubMed] [Google Scholar]
- Kauer J. A., Fisher T. E., Kaczmarek L. K. Alpha bag cell peptide directly modulates the excitability of the neurons that release it. J Neurosci. 1987 Nov;7(11):3623–3632. doi: 10.1523/JNEUROSCI.07-11-03623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Kandel E. R. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol. 1970 Nov;33(6):865–876. doi: 10.1152/jn.1970.33.6.865. [DOI] [PubMed] [Google Scholar]
- Kupfermann I., Weiss K. Water regulation by a presumptive hormone contained in identified neurosecretory cell R15 of Aplysia. J Gen Physiol. 1976 Jan;67(1):113–123. doi: 10.1085/jgp.67.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R., Fisher J. M., Scheller R. H. Processing of the egg-laying hormone (ELH) precursor in the bag cell neurons of Aplysia. J Biol Chem. 1988 Sep 5;263(25):12514–12521. [PubMed] [Google Scholar]
- Pinsker H. M., Dudek F. E. Bag cell control of egg laying in freely behaving aplysia. Science. 1977 Jul 29;197(4302):490–493. doi: 10.1126/science.197.4302.490. [DOI] [PubMed] [Google Scholar]
- Rothman B. S., Mayeri E., Brown R. O., Yuan P. M., Shively J. E. Primary structure and neuronal effects of alpha-bag cell peptide, a second candidate neurotransmitter encoded by a single gene in bag cell neurons of Aplysia. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5753–5757. doi: 10.1073/pnas.80.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman B. S., Weir G., Dudek F. E. Egg-laying hormone: direct action on the ovotestis of Aplysia. Gen Comp Endocrinol. 1983 Oct;52(1):134–141. doi: 10.1016/0016-6480(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Rothman B. S., Mayeri E., Axel R. A single gene encodes multiple neuropeptides mediating a stereotyped behavior. Cell. 1983 Jan;32(1):7–22. doi: 10.1016/0092-8674(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Sossin W. S., Fisher J. M., Scheller R. H. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol. 1990 Jan;110(1):1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin W. S., Scheller R. H. A bag cell neuron-specific antigen localizes to a subset of dense core vesicles in Aplysia californica. Brain Res. 1989 Aug 14;494(2):205–214. doi: 10.1016/0006-8993(89)90588-x. [DOI] [PubMed] [Google Scholar]
- Stuart D. K., Strumwasser F. Neuronal sites of action of a neurosecretory peptide, egg-laying hormone, in Aplysia californica. J Neurophysiol. 1980 Feb;43(2):499–519. doi: 10.1152/jn.1980.43.2.499. [DOI] [PubMed] [Google Scholar]