Abstract
Acute haemorrhage from ruptured gastroesophageal varices is perhaps the most serious consequence of uncontrolled portal hypertension in cirrhotic patients. It represents a medical emergency and is associated with a high morbidity and mortality. In those who survive the initial bleeding event, the risks of further bleeding and other decompensated events remain high. The past 30 years have seen a slow evolution of management strategies that have greatly improved the chances of surviving a variceal haemorrhage. Liver cirrhosis is a multi-staged pathological process and we are moving away from a one-size-fits-all therapeutic approach. Instead there is an increasing recognition that a more nuanced approach will yield optimal survival for patients. This approach seeks to risk stratify patients according to their disease stage. The exact type and timing of treatment offered can then be varied to suit individual patients. At the same time, the toolbox of available therapy is expanding and there is a continual stream of emerging evidence to support the use of endoscopic and pharmacological therapies. In this review, we present a summary of the treatment options for a variety of different clinical scenarios and for when there is failure to control bleeding. We have conducted a detailed literature review and presented up-to-date evidence from either primary randomized–controlled trials or meta-analyses that support current treatment algorithms.
Keywords: varices, acute varices haemorrhage, cirrhosis, prophylaxis, non-selective beta-blockers, variceal band ligation
Introduction
Portal hypertension in cirrhosis develops when there is increased hepatic vascular resistance to portal blood flow. The origin of this increased hepatic vascular resistance is multifactorial, with mechanical distortion of normal sinusoid architecture that results from cirrhosis being an important factor. It is now well understood that other factors are also important, such as increased portal inflow from progressive splanchnic vasodilation due to increased extrahepatic overproduction of nitric oxide (NO), with sGC-PKG signalling and smooth muscle relaxation. Intrahepatically, there is an imbalance between vasoconstrictors (endothelin-1) and vasodilators (NO), and there is reduced activity of intrahepatic endothelial nitric oxide synthase (eNOS). Hyperdynamic circulation and high cardiac output also play a role [1,2]. About 90% of cases of portal hypertension in the West are due to liver cirrhosis, with the remainder due to non-cirrhotic portal hypertension, where the aetiological factors are usually vascular in nature, affecting the portal or hepatic venous systems, for example thrombotic occlusion.
Portal hypertension is defined as a hepatic venous pressure gradient (HVPG) of > 5 mmHg. Portal pressure is measured by passing a catheter under radiological guidance into the hepatic vein and wedging it into a small venule or by using an inflatable balloon to occlude a larger branch of the hepatic vein—this gives a measure of the hepatic venous wedge pressure, which correlates very closely to the portal pressure. The wedge pressure is corrected for increased abdominal pressure form ascites by subtracting the free hepatic venous pressure or intra-abdominal inferior vena cava (IVC) pressure. The resulting pressure difference gives the HVPG. HVPG is a measure of sinusoidal pressure and will therefore be elevated in cirrhosis but will be normal in pre-hepatic causes of portal hypertension such as portal vein thrombus. HVPG measurements are less accurate where there is a pre-sinusoidal component, for example in primary biliary cholangitis (PBC).
Clinically significant portal hypertension occurs when the HVPG is >10 mmHg and it can herald the development of the following complications: upper gastrointestinal (GI) bleeding form gastroesophageal varices, portal hypertensive gastropathy, ascites and spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, splenomegaly, hepatocellular carcinoma and hepatic encephalopathy [3–6].
Actual variceal haemorrhage is unlikely to occur when the HVPG is less than 12 mmHg and a HVPG above 20 mmHg predicts failure to control bleeding and increased mortality [7–10]. The corollary is that, to reduce the risk of variceal bleeding, the goal should be to reduce the HVPG to below 12 mmHg or by 20% or more from baseline. The available approaches to reducing HVPG include non-selective beta-blockers (NSBBs), transjugular intrahepatic portosystemic stent-shunt (TIPSS) and vasopressors.
The past 20–30 years have seen significant advances in the management of portal hypertension and acute variceal haemorrhage, and this has led to marked improvement in survival. In the early 1980s, it was reported that the 6-week mortality following an acute variceal bleed was 40–50%, with re-bleeding rates of over 33% over the same period [11]. In 1995, a multi-centre prospective audit of all-cause acute upper GI bleeding was conducted [12]. The study analysed data from 4000 cases of acute upper GI bleeding across 73 centres—4% of cases were due to acute variceal haemorrhage and these were associated with an in-hospital mortality of 23%. More recently, in 2014, a post hoc analysis of data from the 2007 UK national audit of acute upper GI bleeding was presented [13]. During an 8-week period, 526 cases of acute variceal haemorrhage were identified from 212 hospitals. Overall 30-day mortality was 15%. The re-bleeding rate was 26% and the 30-day mortality in those patients who did not re-bleed was 7%.
The improved survival from acute variceal bleeding observed over the past 30 years is due to several factors, including: improvement is access to and quality of critical and high-dependency care; improvements in general supportive management and use of early-warning scores; early use of vasopressors; the routine use of prophylactic antibiotics; access to out-of-hours emergency endoscopy; early involvement of anaesthetics and intensive-care medicine; and improved access to interventional radiology services for TIPSS. The utility of these interventions in improving outcomes from acute variceal bleeding have been demonstrated in numerous randomized–controlled trials (RCTs) and have therefore been incorporated into current clinical guidelines.
Another important factor that has advanced our knowledge, understanding and clinical practice in this area are expert-led consensus conferences. The most recent of these conferences, Baveno VI, took place in 2015 and the major focus was on risk stratification and individualized care [14]. The conference acknowledged that the severity of cirrhosis varies and patients at different points on the continuum have different risks of developing complications and death. Table 1 illustrates the different features associated with each stage of liver cirrhosis as well as the attendant 1-year mortality. The general approach to the management of varices in any given patient, including screening interval, should now be stratified to take into account the exact stage of portal hypertensive disease.
Table 1.
Different stages of the natural history of portal hypertension—patients at each stage should have a tailored treatment and screening protocol
| Stage | 1a | 1b | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Features | Compensated cirrhosis; no varices | Compensated cirrhosis; no varices | Cirrhosis; gastroesophageal varices but never bled | Cirrhosis presenting with acute variceal bleeding; no other complication | First non-bleeding decompensated event* | Any second decompensated event* |
| HVPG >10 mmHg? | No | Yes | Yes | Yes | Yes | Yes |
| 1-year mortality | 1.5% | – | 2% | 10% | 21% | 87%—5-year mortality |
Decompensated events include ascites, hepatic encephalopathy, spontaneous bacterial peritonitis and jaundice, etc. HVPG, hepatic venous pressure gradient. Adapted from Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int 2017;37(Suppl):104–15.
In this review article, we present an overview of the current evidence-based management strategies for portal hypertension and variceal haemorrhage. Wherever possible, we have assessed RCTs, prospective studies and meta-analyses. The focus of this review is on screening, primary prophylaxis, management of acute variceal haemorrhage and secondary prophylaxis. We conducting a detailed PubMed literature search using search terms: portal hypertension, oesophageal and gastric varices, gastroesophageal bleeding/haemorrhage, management of gastroesophageal bleeding/haemorrhage. From the many thousands of results, we selected about 400 papers of relevance. In addition, we took into account current BSG (British Society of Gastroenterology) guidelines for managing variceal bleeding in cirrhotic patients [15], the 6th Baveno Consensus Workshop [14] and AASLD (American Association for the Study of Liver Disease) Single Topic Conferences and resulting guidance [16,17].
The incidence, prevalence and outcome of varices
Gastroesophageal varices are present in about 50% of all patients with cirrhosis and a good predictor of their presence is the severity of liver disease. Cumulative incidence of varices over 10 years is 44% as calculated using a competing risk model [18]. For example, 85% of Child C cirrhotic patients have varices compared to 40% of Child A patients [19]. In cirrhotic patients without varices, the rate of developing them is 8% per year and the main risk factor and predictor of developing varices is a HVPG is >10 mmHg [20]. Patients with small varices go on to develop large varices at an annual rate of 8% as well. The main risk factors for developing large varices are: decompensated disease, alcoholic cirrhosis and the presence of red whale marks at initial endoscopy [21]. The rate of variceal haemorrhage is 5–15% per year and the risk of bleeding increases in patients with larger varices, the presence of red-signs and more advanced liver disease in particular Child’s B and C cirrhosis [22]. The wall tension of the varix is one of the main factors that predicts rupture and wall tension is directly related to vessel diameter. For any given equal pressure, a large-diameter vessel is more likely to rupture than a small-diameter vessel [23]. The other major factor that influences wall tension is the pressure within the varix and this is related to the HVPG. Hence, decreasing the HVPG can reduce the risk of variceal rupture and haemorrhage. In the context of secondary prophylaxis, the risk of variceal haemorrhage is very low when the HVPG is < 12 mmHg [24,25]. Reductions in HVPG of more than 20% from baseline or to an absolute value below 12 mmHg is associated with reduced rates of recurrent bleeding, ascites, SBP and death [26–28].
The mortality from an index variceal bleed is 20% at 6 weeks [29–31]. An important cut-off for HVPG is 20 mmHg; in patients where the HVPG is > 20 mmHg within 24 hours of a variceal bleed, there is a higher risk of recurrent bleeding within 1 week and higher risk of failure to control bleeding (83% vs 29%) as well as a higher 12-month mortality (64% vs 20%) [32,33].
Gastric varices
The management of bleeding gastric varices continues to present a significant clinical challenge. In general, gastric varices are less prevalent than oesophageal varices. In addition, gastric varices are about 50% less likely to bleed than oesophageal varices. However, once gastric varices rupture, transfusion requirements and mortality are higher than bleeding oesophageal varices [34,35]. The overall incidence of gastric varices in cirrhotic patients who have not previously bled is 4%. At screening endoscopy, 25% of patients had gastric varices and 18% had both gastric and oesophageal varices [36]. The reported incidence of bleeding from gastric varices is about 25% in 2 years, with a higher bleeding rate for fundal varices (IGV1—see below). The main risk factors identified for gastric variceal haemorrhage are:
size of varix, with the greatest risk of bleeding occurring in large (>10 mm) > medium (5–10 mm) > small (<5 mm);
cirrhosis severity—Child C > B > A;
Gastric varices are classified based on their relationship with oesophageal varices as well as their location in the stomach [34]. Gastro-oesophageal varices (GOV) are where the gastric varices are associated with oesophageal varices. This association can be along the lesser curve (GOV1) or along the fundus (GOV2). Isolated gastric varices (IGV) are isolated varices that form in the fundus (IGV1) or ectopically in the stomach or duodenum (IGV2). The commonest type of gastric varices are GOV 1 (about 70% of all gastric varices) followed by fundal varices GOV2 (21%) and IGV1 (7%); only about 2% of gastric varices are IGV2. IGV1 has the highest incidence of bleeding at 78%, with GOV2 the second most likely to bleed with an incidence of 55%. The incidence of bleeding from GOV1 and IGV2 is 10% [37].
Diagnosis and screening of gastroesophageal varices
All patients should be screened for gastroesophageal varices at the point where liver cirrhosis is first diagnosed. However, this may not be necessary in compensated cirrhotics as per Baveno VI if the transient elastography (TE) is less than 20 kPa and platelet count is above 150 x 109 /l (Table 2). If there is evidence of decompensated disease, then the patient should have annual screening regardless of whether varices are present or not. It is also important to screen more frequently if there is disease progression, especially in primary sclerosing cholangitis (PSC). A patient with compensated cirrhosis and known varices can be screened every 1–2 years and compensated cirrhosis without varices can be screened every 2–3 years [17,38].
Table 2.
Different treatment recommendations for the primary prophylaxis of oesophageal varices in cirrhosis
| British Society of Gastroenterology (BSG) [15] | Baveno VI [14] | American Association for the Study of Liver Disease (AASLD) [16] | |
|---|---|---|---|
| Surveillance |
|
|
|
| Prophylactic Rx |
|
|
|
| When to stop NSBBs | Stop NSBBs if SBP |
|
|
| Not recommended | PPI, isosorbide mononitrate, shunt surgery or TIPSS, sclerotherapy | NSBBs in combination with VBL, TIPSS |
For example, ongoing alcohol use, obesity or lack of sustained virological response in hepatitis C. OGD, oesophago-gastro-duodenosopy; TE, transient elastography; HVPG, hepatic venous pressure gradient; NSBBs, non-selective beta-blockers; VBL, variceal band ligation; SBP, spontaneous bacterial peritonitis; PPI, proton pump inhibitor; TIPSS, transjugular intrahepatic portosystemic stent-shunt; BP, blood pressure.
Oesophago-gastro-duodenosopy (OGD)
The gold-standard for screening is OGD. However, the main disadvantages are that OGD is an invasive technique that invariably causes patient discomfort; the diagnosis of varices and classification of varices, especially small oesophageal and gastric varices, are subject to inter-observer variability; and the cost is relatively high.
If oesophageal varices are found at endoscopy, they should be photographed after washing and described as follows: Grade I—collapse on inflation of the oesophagus with air; Grade II—these are varices that cannot be categorized as either Grade I or Grade III; Grade III—these are varices that are large enough to occlude more than 50% of the lumen.
Transnasal endoscopy
A multi-centre prospective blinded study evaluated transnasal endoscopy with flexible imaging colour enhancement to assess 50 patients. The analysis showed that the technique was at least as good as conventional OGD at detecting varices and patients preferred it to standard OGD [39]. In another study of 100 patients with liver cirrhosis, transnasal small-calibre endoscopy performed without sedation showed that the accuracy of lesion detection was comparable to conventional OGD (96% vs 99%) but the technique was much better tolerated [40]. Transnasal endoscopy cannot be used to undertake variceal band ligation (VBL).
Capsule endoscopy
A recent systematic review and structured meta-analysis evaluated the efficacy of capsule endoscopy for screening oesophageal varices [41]. Seventeen eligible studies (n = 1328) were analysed and the diagnostic accuracy of capsule endoscopy in diagnosis of oesophageal varices was 90% (95% confidence interval [CI] 0.88–0.93). The pooled sensitivity and specificity were 83% and 85%, respectively. The conclusion from this work is that capsule endoscopy is not currently sensitive enough to replace OGD. Grading of oesophageal varices is not possible and the capsule is incapable of reliably detecting fundal varices. Therefore, capsule endoscopy is not recommended for variceal screening and staging.
Transient elastography (TE)
TE is a non-invasive technique that can derive a value for tissue stiffness based on the speed of propagation of low-frequency ultrasound. It has been shown to have a high sensitivity for predicting severe portal hypertension but is associated with a large variation in specificity (50–93%) [42]. In one study of 61 patients with hepatitis C, the sensitivity of TE in predicting oesophageal varices was 90% with a cut-off of 17.6 kPa, but specificity was only 43% [43]. More recently, a study of 298 patients found that the optimal cut-off for predicating oesophageal varices was 21.5 kPa, as this gave a sensitivity of 76% and sensitivity of 78% [44].
TE does have some use as a non-invasive tool for risk prediction in people with compensated advanced chronic liver disease. In a recent cross-sectional study, data from 542 patients across four centres were retrospectively analysed (Anticipate Study) [45]. Non-invasive tests (TE, platelet count, spleen size, platelet/spleen ratio, liver stiffness to spleen/platelet score [LSPS]) and invasive tests (endoscopy and HVPG) were paired. The LSPS had the highest discrimination factor such that a ratio of 2.65 was associated with a > 80% risk of clinically significant portal hypertension. Conversely, the LSPS ratio of < 1.33 was also able to identify patients with <5% risk of having varices that required treatment.
Primary prophylaxis
Patients with cirrhosis but no varices
In 2005, a large RCT established that NSBBs are ineffective in preventing varices in unselected patients with cirrhosis and portal hypertension [3]. In this study, 213 patients with cirrhosis and portal hypertension (minimum HVPG 6 mmHg) were randomized to receive timolol (n = 108) or placebo (n = 105) and the primary end-point was the development of varices or variceal haemorrhage. The mean follow-up was 54.9 months, with annual HVPG and OGD. The rate of primary end-point was essentially the same for the timolol group 39% and placebo 40% (p = 0.89). Neither was there a significant difference in the rates of ascites, encephalopathy, transplant or death. However, the rate of serious adverse events was significantly greater in the timolol group (18%) vs placebo (6%) (p = 0.006). The strongest predictor for the development of varices was a HVPG of >10 mmHg.
Patients with cirrhosis and grade I varices that have never bled
The incidence of variceal haemorrhage in patients with portal hypertension is 30–50% and, in those that do bleed, mortality is 20% at 6 weeks. The mainstay of primary prophylaxis for moderate to large varices in order to prevent an index bleed is NSBBs and repeat sessions of elective VBL [38]. In cirrhotic patients with grade I varices at initial endoscopy, the rate of progression to large varices is 5–30% per year [16]. The main risk factors for small varices becoming larger are: severity of liver disease (Child-Pugh B and C), red-sign on initial endoscopy and an alcoholic aetiology. However, the benefit of NSBBs as primary prophylaxis of small grade I varices is uncertain. At least three separate studies have been equivocal. In one RCT, 102 patients were randomized to propranolol (160 mg od) and 104 to placebo; at 2-year follow-up, 31% of the propranolol group had large varices compared to just 14% in the control group; however, about 33% did not attend for follow-up [46]. In a second study, patients were selected who had cirrhosis and small varices—these were randomized into two groups—nadolol (dose adjusted to reduce resting heart rate by 25%, n = 83) or placebo (n = 78). After mean follow-up of 36 months, the cumulative risk of developing large varices was 20% in the nadolol group and 51% in the placebo group (p < 0.001) [47]. However, there was no demonstrable difference in survival (p = 0.33). A more recent study failed to show any effect with propranolol, despite a significant effect on portal pressure [48].
A recent placebo-controlled RCT examined the effectiveness of carvedilol in preventing the progression of small varices [49]. Patients with cirrhosis predominantly due to non-alcoholic fatty liver disease (NAFLD) who had small oesophageal varices were prospectively randomized to either carvedilol or placebo (n = 70 in each group) and followed up for 24 months. OGD was performed at baseline and every 6 months, and HVPG was measured at baseline and at 12 months—the primary end-point was development of large varices. A larger proportion of the carvedilol group had non-progression to large oesophageal varices than the placebo group (79% vs 61.4%, p = 0.04). There was also a modest reduction in HVPG in the carvedilol group compared to placebo (–8.64% vs +0.33%, p = 0.22). Therefore, based on this one study, carvedilol may be safe and effective in delaying the progression of small oesophageal varices.
Baveno V and AASLD guidelines recommend that NSBBs should be used for primary prophylaxis in patients with small varices who are judged to be at increased risk of bleeding, i.e. those that have red-sign at initial endoscopy or who are Child-Pugh C [16,38]. UK guidelines recommend NSBBs as primary prophylaxis in grade I varices only if red-signs are present, but grade I varices should have annual OGD surveillance [15].
Patients with cirrhosis and grade II–III varices that have never bled
The treatment goal here is to prevent the first variceal bleed as well as other complications of portal hypertension such as ascites. Recommendations from Baveno VI and current BSG guidelines are similar (Table 2): in this group of patients, either a NSBB (propranolol, carvedilol or nadolol) or VBL can be used as primary prophylaxis. The choice will depend on patient preference and the availability of expertise and infrastructure and side-effects of NSBBs. It should be borne in mind that VBL involves local obliteration of oesophageal varices and therefore does not help to prevent portal hypertensive gastropathy, ascites and SBP [50–52].
Carvedilol is an attractive first-line agent due to promising results from heamodynamic studies. To date, there are no controlled trials comparing carvedilol with propranolol or nadolol in primary prophylaxis. In a RCT, the mean HVPG decrease with carvedilol was 22.2% vs 15.6% with propranolol/nadolol [53]. The same study showed that 56% of patients that did not respond to propranolol or nadolol did achieve good hemodynamic response with carvedilol. The likely mechanism for the observed superiority of carvedilol is that it has intrinsic anti-alpha-adrenergic activity as well as its blockade of beta1 and beta2 receptors. A recent meta-analysis showed that the mean relative HVPG reduction was 22% for carvedilol and 16% for propranolol, which is a weighted mean difference of 7% in favour of carvedilol [54]. Whilst carvedilol is more potent in reducing portal pressure, it does also lead to more pronounced decreases in systemic arterial pressure. The optimal dose of carvedilol is 12.5 mg once a day; higher doses are not necessary and have no additional benefit and lower doses are much less effective [55,56].
Two RCTs have compared carvedilol and VBL for primary prophylaxis. One study showed significantly reduced bleeding in the carvedilol group (10%) compared to the VBL patients (23%), but without any effect on overall survival [57]. The second trial did not show any significant difference, although, in this study, compliance with VBL was better and the underlying aetiology of liver disease was different (mainly viral hepatitis rather than alcohol) [58].
The efficacy of VBL has been compared with NSBB in a Cochrane meta-analysis of 19 RCTs involving 1504 patients. These data showed that VBL was associated with lower rates of variceal haemorrhage (relative risk [RR] = 0.67, 95%CI 0.46–0.98). However, there was no difference between VBL and NSBBs in overall mortality or bleeding-related mortality and no difference was seen when looking at high-quality trials or trials published as full papers [59]. VBL is associated with a higher risk of fatal adverse events compared to NSBBs, such as banding-induced bleeding. A meta-analysis has shown that there are reduced fatal adverse events with NSBBs (RR = 0.14, 95%CI 0.02–0.99) [60]. There is one RCT that compared the combination of NSBB plus VBL vs VBL alone—these data showed no difference in the incidence of the first variceal haemorrhage or death between the two groups; however, there was a higher incidence of side-effects and adverse events in the combination group [61]. Therefore, combination therapy is not recommended.
Current recommendations in the US and UK are similar in that either NSBBs or VBL can be used as primary prophylaxis. Table 2 summarizes the main recommendations for primary prophylaxis as described in the guidelines published by BSG, Baveno VI and AASLD. Whilst broadly similar, there are subtle differences. In general, an NSBB such as propranolol can be tried as first-line and then VBL can be offered if there are contraindications or adverse side-effects with beta-blockers. Once these patients have been commenced on NSBBs and tolerate them well, there is no need for repeat surveillance OGD.
The use of NSBBs has some advantages over VBL, as they are inexpensive and do not require any specific expertise or monitoring other than routine checks on blood pressure and heart rate. NSBBs act by causing splanchnic vasoconstriction that in turn leads to reduced portal pressure. Therefore, NSBBs may also reduce the rate of other decompensated events such as ascites and encephalopathy—not just variceal bleeding [62]. However, a major obstacle to NSBBs is that about 15% of patients have either a relative or absolute contraindication and a further 15% experience unpleasant side-effects such as shortness of breath, lethargy, fatigue and pre-syncope [63].
VBL can be performed at the same time as the screening endoscopy, but will likely require repeat sessions 2–4 weeks apart. The procedure can be poorly tolerated and there are risks associated with conscious sedation, as well as the usual risks with any type of OGD (bleeding and perforation). VBL can also lead to banding ulcers and subsequent bleeding from these sites in the distal oesophagus—bleeding can be severe enough to cause death. Whilst the number of side-effects is greater with NSBBs, the severity of side-effects is greater with VBL.
RCTs designed to examine the benefit of prophylactic shunt surgery show significantly higher rates of encephalopathy and mortality in the surgery group—hence, any shunt therapy such as TIPSS is not recommended as primary prophylaxis [64].
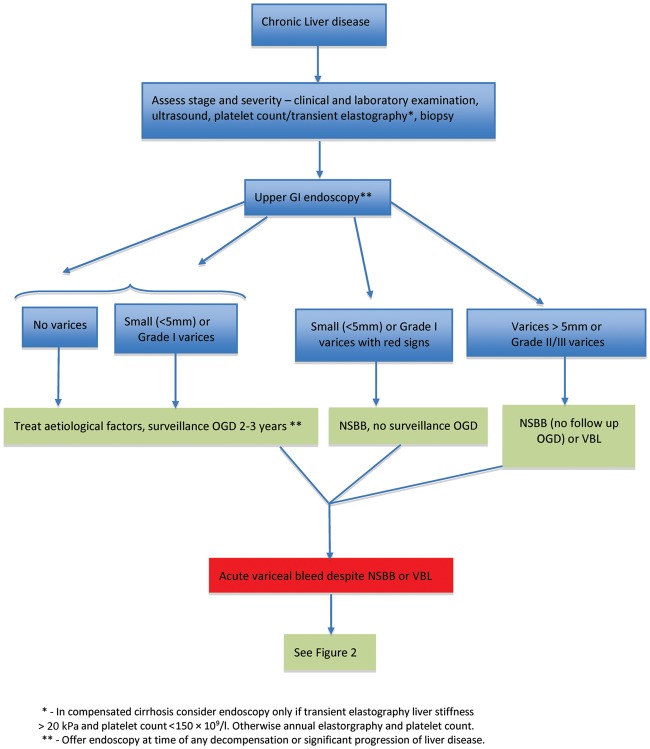
The overall treatment algorithm for the screening and management of gastroesophageal varices is illustrated in Figure 1.
Figure 1.
Treatment algorithm for the screening and management of gastroesophageal varices. GI, gastrointestinal; OGD, oesophago-gastro-duodenosopy; NSBB, non-selective beta-blockers; VBL, variceal band ligation. Adapted from Bosch J and Sauerbruch T. Esophageal varices: stage dependent treatment algorithm. J Hepatol 2016;64:746–8.
Isosorbide mononitrate (ISMN)
In the past, there has been interest in ISMN because it was shown to reduce portal pressure just as effectively as propranolol [65]. However, a subsequent trial comparing ISMN with propranolol showed no significant difference between the two [66]. Another trial compared ISMN with placebo and again there was no difference in the two arms [67]. Hence, ISMN is not recommended as monotherapy in primary prophylaxis.
The use of beta-blockers in end-stage liver disease or refractory ascites
It has been suggested that beta-blockers, whilst helpful in compensated or early decompensated disease, can cause worse outcomes if used in end-stage cirrhosis or in refractory ascites [68,69]. To a large extent, this perspective comes from a 2010 study that showed reduced survival in patients with refractory ascites who were treated with propranolol [70]. A consecutive crossover study also showed that there was a higher risk of paracentesis-induced circulatory dysfunction in patients with refractory ascites who were receiving propranolol. However, in both groups, a large proportion of patients were on high doses (160 mg) of propranolol. Nevertheless, Krag et al. based their so-called ‘window hypothesis’ on the results of this study [71]. This model states that there is a therapeutic window for safe NSBB use, which disappears in the later stages of cirrhosis because NSBBs reduce cardiac output in patients with refractory ascites, thus leading to increased mortality [72]. However, two large observational studies have shown that there is improved survival in patients with refractory ascites treated with beta-blockers, except in the presence of SBP [73,74]. A recent post-hoc analysis from three RCTs investigating the use of satavaptan for refractory ascites showed that treatment with propranolol and carvedilol had no impact on overall mortality (23% vs 25%) after 1 year [75]. It should be noted, however, that, in 29% of patients, NSBBs were discontinued.
In the context of SBP, NSBBs should be discontinued, especially if there is arterial hypotension and/or acute kidney injury/hepatorenal syndrome. NSBBs can be safely re-introduced after the episode of SBP has been treated, as long as the patient is hemodynamically stable [14].
Acute variceal haemorrhage
Acute variceal haemorrhage is a medical emergency requiring a coordinated multidisciplinary-team approach involving expertise from anaesthetics, emergency medicine, intensive-care medicine, hepatologist/gastroenterologist, haematologist, interventional radiologist as well as ancillary and support teams. At initial presentation, the management of acute variceal haemorrhage is the same as for any bleeding patient whatever the cause who will potentially be in hypovolaemic shock. The first priority is to assess and protect the airway, followed by an assessment of the respiratory and circulatory status. Resuscitation should be initiated immediately in order to maintain haemodynamic stability until more definitive steps can be taken.
A recent study showed that there is a strong correlation between Child-Pugh score and HVPG such that over 80% of Child C patients have a HVPG of >20 mmHg; hence the Child-Pugh score is a good, practical, albeit indirect, way of risk-stratifying these patients [76]. Several other studies have confirmed this association [77–80] and, more recently, the model for end-stage liver disease (MELD) score has been shown to be more accurate than the Child-Pugh score, with a MELD of > 19 associated with a 6-week mortality of 20% [81]. The benefit of undertaking this type of scoring and risk stratification is that it should allow a sub-group of patients to be identified as ‘high-risk’ and these could then be considered for early TIPSS once initially stabilized.
The immediate goals of managing an acute variceal haemorrhage are (i) to control the haemorrhage, (ii) reduce the risk of early re-bleeding (within 5 days) and (iii) prevent bleeding-related complications, such as infection, hepatic encephalopathy and acute kidney injury.
Transfusion strategy
In haemodynamically stable patients, transfusion of packed red blood cells (PRBC) should be restrictive with a target haemoglobin (Hb) of 7–8 g/dL and this should be assessed frequently either using a blood-gas analyser or from laboratory samples. A more liberal approach where the Hb is maintained at 9–11 g/dL is associated with a higher mortality 22% vs 11% (p = 0.05) [82]. In a sub-group analysis of patients from this RCT who had cirrhosis, it was found that patients randomized to the restrictive-transfusion arm had lower rates of re-bleeding and death. HVPG was found to increase in those given liberal transfusion but remained the same in those in the restrictive-transfusion group. It should be emphasized that a restrictive-transfusion strategy only applies to haemodynamically stable patients.
In chronic liver disease, there is often an equal and opposite balance of pro-coagulant and anticoagulant factors, and hence interpretation of the clotting profile can be difficult [83]. Thromboelastography (TEG) is a commercially available, quick, point-of-care assay that assesses clot formation in the whole blood and can therefore be a more accurate guide to the administration of pro-haemostatic factors [84]. However, currently, TEG is not routinely available outside the critical-care setting in most centres. Current BSG guidance recommends that the major haemorrhage protocol be activated, to give platelets if the count is below should be 50 x 109/L and to give Fresh Frozen Plasma if the international normalized ratio (INR) is above 1.5 and cryoprecipitate if the fibrinogen in below 1.5 [15]. Currently, there is no evidence to support the use of tranexamic acid or recombinant factor VIIa [85].
Pharmacological therapy
It is generally safe to commence pharmacological therapy, i.e. vasopressors and antibiotics, straightaway and certainly prior to organizing an endoscopy. NSBBs should not be started during an acute variceal bleed and, if the patient was taking these previously, they should be suspended during the acute crisis.
Vasopressors
The use of intravenous vasopressors such as terlipressin, somatostatin and octreotide for acute variceal haemorrhage have been shown, in a meta-analysis of 30 RCTs, to reduce 7-day all-cause mortality and give lower transfusion requirements [86]. Terlipressin is a synthetic analogue of vasopressin and it causes systemic vasoconstriction and reduces portal blood flow, portal-systemic collateral blood flow and hence variceal pressure. It has been shown in placebo-controlled trials to control bleeding, reduce the transfusion requirements and reduce 6-week mortality [52]. In a meta-analysis of seven RCTs, terlipressin was shown to improve survival (RR = 0.66, 95%CI 0.49–0.88) and reduce the failure to control bleeding (RR = 0.66, 95%CI 0.55–0.93) [87]. It is therefore current practice that, as soon as a variceal bleed is suspected, a vasoactive drug be commenced. In the UK, we use terlipressin 2 mg qds for 24–72 hours or until satisfactory haemostasis has been achieved. In fact, once haemostasis has been achieved with endoscopic band ligation, 24 hours of terlipressin is as effective as 72 hours [88].
In the US, octreotide is the only vasoactive drug available for acute variceal haemorrhage. Octreotide is a somatostatin analogue that works by causing selective splanchnic vasoconstriction and thus reduction in portal blood flow. Meta-analysis has shown that octreotide and somatostatin are as equally effective in controlling acute variceal haemorrhage as terlipressin [86]. A more recent study again demonstrated that there is an equivalence between the three agents, although, in this study, terlipressin was used at lower-than-recommended doses [89].
Prophylactic antibiotics
A meta-analysis of 12 RCTs shows clear survival benefit for the early use of prophylactic antibiotics during an acute variceal bleed (RR = 0.79, 95%CI 0.63–0.98) [90]. These trials also showed that antibiotics reduced the risk of bacterial infections and early re-bleeding. A retrospective analysis of 383 patients showed that the rates of infection and death are lower in Child-Pugh A patients presenting with an acute variceal bleed in the absence of prophylactic antibiotics compared to class B and C patients [91]. The use of routine antibiotics in this group requires further work. The current guidelines recommend routine antibiotics in all cases of acute variceal haemorrhage regardless of Child-Pugh class and regardless of whether there is a confirmed infection or suspected focus of infection. It is clearly good practice to undertake a full septic screen as part of the initial work up as a baseline and this should include an ascitic tap for diagnosis of SBP where appropriate.
Endoscopic VBL
An upper GI endoscopy should be performed as soon as possible after the patient has been stabilized and adequately resuscitated. Ideally, it should be performed in an operating theatre with a full anaesthetic team and with the patient intubated—as there is a high risk of aspiration during the OGD. It can be helpful to give a prokinetic about an hour before the endoscopy (e.g. metoclopramide or erythromycin) in order to help clear the stomach of blood and clots, as long as there are no contraindications such as QT prolongation [14].
There is some debate about the optimal timing of endoscopy, with many guidelines stating that it should be performed within 12 hours of presentation. However, there is no evidence for this and, in one study looking at urgent vs non-urgent endoscopy, there was no demonstrable advantage of performing an OGD within 12 hours [92]. The optimal timing is when the patient has been adequately resuscitated and terlipressin and antibiotics commenced, but it should be within 24 hours [93].
Acute variceal haemorrhage can be diagnosed endoscopically with confidence if there is an actively bleeding varix or a varix that shows signs of recent bleeding, e.g. fibrin plug, and if, after careful examination, no other cause of the haemorrhage can be found in the upper GI tract. During the initial examination without the banding device attached, the location and size of the varices should be carefully mapped in relation to the gastro-oesophageal junction. A detailed examination of the fundus after removal of blood and clots should be made to look for any GOV1 or GOV2 varices. Once satisfied with regard to the size and location of varices and where the optimal location would be to band, the endoscope is removed and a banding device can be attached before re-intubation. Band ligation can then be carried out with the emphasis on quality of bands rather than the quantity applied. The superiority of VBL over sclerotherapy is well established—a meta-analysis of seven RCTs showed that VBL reduced re-bleeding, mortality and resulted in fewer distal oesophageal strictures in comparison to sclerotherapy [94].
Endoscopic VBL should be combined with pharmacological therapy
A meta-analysis of eight trials showed that combination vasoactive drugs with VBL results in better initial control of bleeding (RR = 1.12, 95%CI 1.02–1.23) and no re-bleeding at 5 days (RR = 1.28, 95%CI 1.18–1.39), but with no difference in survival [95]. Adverse events are similar in both groups.
Failure to control an acute variceal haemorrhage
Balloon tamponade using, for example, a Sengstaken-Blakemore Tube (SBT) in patients with massive variceal haemorrhage or refractory bleeding can be a very effective holding measure or bridge to more definitive therapy. A SBT should only be left in place for a maximum of 24–48 hours. It can stem the acute bleed in about 90% of patients; however, 50% re-bleed when the gastric balloon is deflated [96,97]. In addition, it is associated with severe complications such as ulceration, and oesophageal and tracheal rupture. For this reason, the SBT should be placed under direct vision using an endoscope, the gastric balloon should only be inflated under direct vision to ensure it is in the stomach and the oesophageal balloon should not be used. In certain situations, for example if there is a delay in endoscopy, it may acceptable to place the SBT blindly, inflate the gastric balloon slightly and get an erect chest radiograph to confirm position before full inflation.
Self-expandable, oesophageal covered metal stents (SX-ELLA Danis) offer an alternative to balloon tamponade. These are placed endoscopically, without radiological guidance; they are removable and can be left in place for up to 2 weeks. In a recent multi-centre RCT involving 28 patients, removable metal stents were compared to balloon tamponade in patients with cirrhosis and variceal haemorrhage refractory to normal medical and endoscopic treatment [98]. The primary end-point was survival to day 15, control of bleeding and absence of serious adverse events. The primary end-point was reached more frequently in the stent group compared to the balloon tamponade group (66% vs 20%, p = 0.025), control of bleeding was better in the stent group (85% vs 47%, p = 0.037) and transfusion requirements and adverse serious events were fewer in the stent group. However, there was no significant difference in 6-week survival (54% vs 40%, p = 0.46).
TIPSS
Salvage TIPSS
If none of the above measures has managed to control an acute variceal haemorrhage, then the next step in the treatment algorithm is TIPSS; this may necessitate the urgent transfer of the patient to a specialist liver unit, as the appropriate interventional radiology expertise may not exist in every centre. Studies have shown that salvage TIPSS managed to achieve control of bleeding in 90–100% of cases, with re-bleeding rates of 6–16%. Mortality was 75% in hospital and 15% at 30 days [99].
Early TIPSS
A body of evidence is emerging to suggest that, amongst all patients that present with acute variceal haemorrhage, there is a sub-set of perhaps 20% that can be categorized as high-risk. RCTs have shown that, in this group, early TIPSS (before onset of treatment failure)—within 72 hours of admission—is associated with significantly lower mortality and treatment failure [100,101]. High-risk patients have been defined as those with a HVPG of >20 mmHg, Child-Pugh class C with a score of 10–13, Child-Pugh B with active bleeding seen endoscopically despite treatment with intravenous vasopressors. In one of these studies, there were multiple exclusion criteria and the patients entered into the study were highly selected [101]. Nevertheless, this group did demonstrate reduced risk of treatment failure with early TIPSS (3% vs 50%, p < 0.001) and improved survival at 1 year (86% vs 61%, p < 0.001), without increased risk of hepatic encephalopathy. Despite early TIPSS in these high-risk patients, two observational studies have failed to demonstrate long-term survival benefit [102,103].
Surgery
Shunt surgery—reserved mainly for Child-Pugh A patients—has been shown to be an effective option as salvage treatment for patients where there is failure to control bleeding with VBL and vasopressors [104,105]. A study spanning 30 years and 400 unselected patients who had portocaval shunt surgery within 8 hours of onset of bleeding reported almost universal success in controlling bleeding [106]. A RCT was conducted in 2012 that compared emergency portocaval surgery with bare-metal TIPSS within 24 hours of presenting with oesophageal variceal haemorrhage in unselected cirrhosis patients [107]. Surgery resulted in better outcomes in terms of bleeding control, encephalopathy and overall survival (p < 0.001). However, the difference may not be so clear cut when comparing to covered stents and more work is needed in this area. Portocaval shunt surgery is not routinely used in most centres, especially since the increased use of minimally invasive and simpler interventional radiology techniques such as TIPSS.
Liver transplantation is always an option but rarely used or appropriate in the setting of acute variceal haemorrhage. It is mainly considered for patients who bleed while active on a transplant waiting list. To date, there are no studies comparing VBL vs TIPSS vs liver transplantation and there are no trials on the use of liver transplantation in the context of active variceal bleeding. If the patient survives an acute episode, then of course they can be referred for elective assessment for liver transplantation assuming there are no excluding factors.
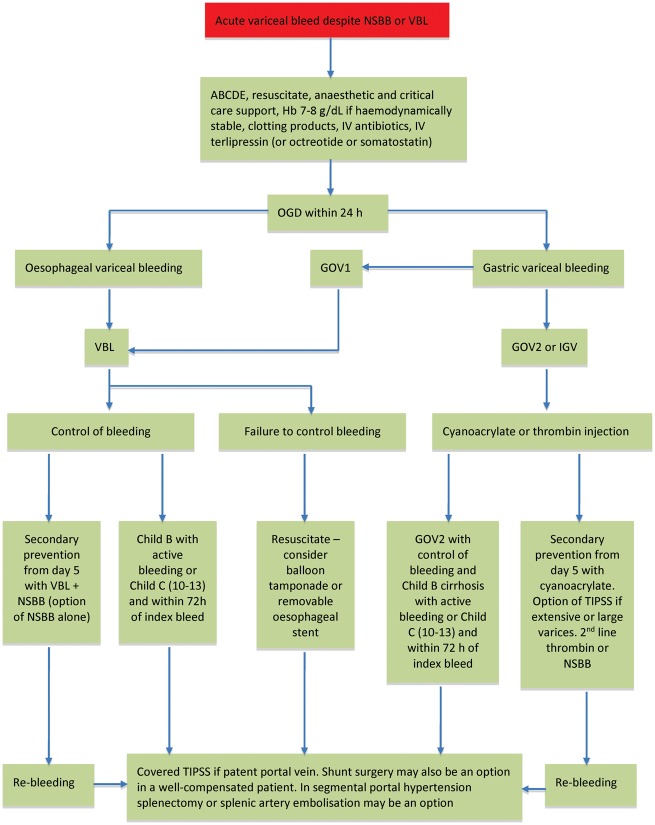
The management of an acute variceal bleed is summarized in Figure 2.
Figure 2.
Treatment algorithm for the management of acute variceal haemorrhage. NSBB, non-selective beta-blockers; VBL, variceal band ligation; ABCDE, Airway, Breathing, Circulation, Disability, Exposure; OGD, oesophago-gastro-duodenosopy; GOV, gastro-oesophageal varices; IGV, isolated gastric varices; TIPSS, transjugular intrahepatic portosystemic stent-shunt.
Patients who survive an index variceal bleed—secondary prophylaxis
The goal of secondary prophylaxis is to prevent further complications of liver cirrhosis, including further episodes of variceal haemorrhage and death. There may be subtle variations in approach, depending on how advanced the specific patient’s liver disease is. Once a patient has had one episode of variceal haemorrhage, they are at much greater risk of having a second episode (60% within the first year with a mortality of 33%) [50]—therefore, preventing further variceal bleeding remains a cornerstone of follow-up management.
If a patient had a salvage or early TIPSS during the acute-bleeding episode, they should be assessed and counselled with regard to possible liver transplantation. As long as there are no obvious precluding factors, e.g. non-abstinence from alcohol, then they should be referred to a liver-transplant centre for formal assessment. They should have surveillance ultrasound and Doppler’s for TIPSS patency every 6 months.
All other patients should have dual therapy with VBL (and this should continue until all varices are eradicated) and NSBBs. A recent meta-analysis of five studies involving 476 patients comparing VBL alone or in combination with NSBBs (+/– ISMN) showed a reduced risk of re-bleeding with combination therapy (RR = 0.44, 95%CI 0.28–0.69) and lower mortality (RR = 0.58, 95%CI 0.33–1.03) [108]. An analysis of a further four RCTs involving 409 patients where pharmacological therapy was used alone or in combination with VBL showed variceal bleeding rates decreased with combination therapy (p < 0.01) but re-bleeding from banding ulcers in the oesophagus increased (p = 0.01) [108]. This work shows that adding pharmacological treatment to VBL significantly reduces the risk of further variceal bleeding. However, adding VBL to pharmacological treatment alone gives a non-significant decrease in re-bleeding and no effect on mortality. The corollary is that, if patients are intolerant of beta-blockers, then they should be considered for TIPSS, as VBL alone may not be enough to prevent future bleeding events.
A recent multi-centre RCT of 158 patients examined the role of simvastatin in combination with standard therapy for reducing the risk of further variceal haemorrhage [109]. The main end-points were re-bleeding and death. This study showed that the addition of simvastatin does not reduce the risk of re-bleeding but did improve survival in Child-Pugh A and B patients (RR = 0.39, 95%CI 0.15–0.99), with no difference in serious adverse events. There was no survival benefit with simvastatin for Child-Pugh C patients. Survival was not a primary end-point of this trial and so these data need validation. The cirrhotic patients in both groups had either hepatitis C or hepatitis B viral infection. Many anti-virals with direct action on nucleoside or nucleotide analogues are known to improve liver function. Standard therapy with VBL and NSBBs were used in both groups but the simvastatin group had a high compliance at 83% vs 72% in the placebo groups. Nevertheless, it is known that simvastatin can lower portal pressure, improve hepatocellular function and even slow the rate of fibrosis. The mechanism for these effects is related to statin-induced improvement in endothelial function due to beneficial pleiotropic circulatory effects.
A 3-month prospective, randomized, triple-blind trial involving simvastatin vs placebo in patients with cirrhosis and portal hypertension was conducted in 2015 [110]. The primary end-point was a reduction in HVPG of at least 20% or to < 12 mmHg after 3 months of treatment. It was a small trial, with just 24 patients, but 55% of the simvastatin groups showed a clinically significant reduction in HVPG compared to 0% in the placebo group (p = 0.036). Large epidemiological studies have shown progression of chronic liver disease and mortality are reduced in patients receiving statins [111].
Management of gastric varices
The body of evidence to guide the management of gastric varices is much less robust than for oesophageal varices. A comprehensive review of the management of gastric varices has been published [112]. Based on one RCT of 89 patients designed to examine the role of glue (cyanoacrylate) injection vs NSBB vs observation alone in primary prophylaxis in selected patients with >10-mm gastric varices, the groups receiving glue injection had the lower bleeding rates and better survival [113]. Despite this, glue injection is not used routinely as primary prophylaxis for gastric varices. There may be a benefit to using NSBBs in this situation due to the potential for lowering HVPG and thus reducing risk of bleeding.
Acute haemorrhage from gastric varices
Both VBL and cyanoacrylate have been shown to be effective in achieving initial haemostasis. A meta-analysis of three RCTs showed that cyanoacrylate is associated with lower rates of bleeding [114]. The sample sizes in these studies are small and so the quality of evidence is relatively poor. On a practical level, VBL is not used for gastric varices either, as there is a high risk of the band falling off and leaving an ulcer on the gastric varix, substantially increasing the risk of rupture and death.
The use of thrombin injection for the management of gastric varices has a long history but has not been widely applied. Upon injection, thrombin catalyses the conversion of fibrinogen to fibrin, the monomers of which then aggregate to form a fibrin clot and eventually a cross-linked fibrin polymer. It also has effects on platelet aggregation. It has potential to be a technically simple and efficient alternative to cyanoacrylate and has fewer risks and complications. In one centre, 30 patients received this treatment over a 5-year period and haemostasis was achieved in 90% of patients treated acutely and 6-week survival was 83% [115]. However, the re-bleeding rate was 50% and so thrombin injections alone may have utility as a bridge to more definitive treatment options. Clearly, randomized–controlled studies are needed to evaluate this further.
Endoscopic ultrasound (EUS) is emerging as a useful diagnostic and therapeutic tool for the evaluation and management of gastroesophageal varices [116]. In some specialist centres, EUS is being used to guide the injection of coils or cyanoacrylate or thrombin. To date, there are no controlled studies that evaluate these techniques in comparison with existing practice.
As with oesophageal varices, TIPSS is the next step following failure to control bleeding using first-line methods. One study looked at the management of variceal haemorrhage—40 patients with gastric varices as well as 232 with oesophageal varices. This work showed that TIPSS is equally effective in terms of preventing re-bleeding form gastric and oesophageal varices and that a significant number of people with gastric varices bleed when the HVPG is less than or equal to 12 mmHg [116]. A RCT involving 72 patients where TIPSS was compared to cyanoacrylate injection for the prevention of re-bleeding showed a lower rate of re-bleeding with TIPSS (11% vs 38%, p = 0.014). The rate of encephalopathy was greater in the TIPSS group (26% vs 3%); overall complication rate and survival were similar in the two groups [117].
Surgical therapies such as spleno-renal shunts are not a good option at the time of acute haemorrhage from gastric varices because mortality can be as high as 70% (sepsis, renal and liver failure) [118]. In situations where there is segmental portal hypertension due to isolated splenic vein thrombus, then curative splenectomy or splenic artery embolization may be considered as options [119,120].
Conclusion
The last three decades have seen marked improvements in the management of variceal haemorrhage and this has led to improved survival from this devastating complication of portal hypertension. In part, these improvements have been due to better overall care in the acute setting, but also due to a better understanding of the underlying mechanisms of portal hypertension and the rational use of therapeutics that emanates from this. The cornerstone of preventing the first variceal bleed remains NSBBs, including carvedilol and VBL. For acute variceal haemorrhage, a multidisciplinary approach involving anaesthetics, critical care, endoscopist and hepatologist has markedly reduced mortality. The mainstay of treatment in this scenario remains antibiotics, vasoactive drugs and VBL, with early or rescue TIPSS for selected patients. Prevention of further bleeding episodes is based on the combined use of VBL and NSBBs.
Conflict of interest statement: none declared.
References
- 1. Tetangco EP, Silva RG, Lerma EV.. Portal hypertension: etiology, evaluation and management. Disease Mon 2016;62:411–26. [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Pagan JC, Gracia-Sncho J, Bosch J.. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol 2012;57:458–61. [DOI] [PubMed] [Google Scholar]
- 3. Groszmann RJ, Garcia-Tsao G, Bosch J. et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Eng J Med 2005;353:2254–61. [DOI] [PubMed] [Google Scholar]
- 4. Ripoll C, Groszmann RJ, Garcia-Tsao G. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007;133:481–8. [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Tsao G, Groszmann RJ, Fisher RL. et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5:419–24. [DOI] [PubMed] [Google Scholar]
- 6. Ripoll C, Groszmann RJ, Garcia-Tsao G. et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009;50:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Groszman RJ, Bosch J, Grace ND. et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal haemorrhage. Gastroenterology 1990;99:1401–7. [DOI] [PubMed] [Google Scholar]
- 8. Feu F, Garcia-Pagan JC, Bosch J. et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995;346:1056–9. [DOI] [PubMed] [Google Scholar]
- 9. D’Amico G, Garcia-Pagan JC, Luca A. et al. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review . Gastroenterology 2006;131:1611–24. [DOI] [PubMed] [Google Scholar]
- 10. Burroughs AK, Triantos CK.. Predicting failure to control bleeding and mortality in acute variceal bleeding . J Hepatol 2008;53:185–8. [DOI] [PubMed] [Google Scholar]
- 11. Graham DY, Smith JL.. The course of patients after variceal haemorrhage. Gastroenterology 1981;80:800–9. [PubMed] [Google Scholar]
- 12. Rockall TA, Logan RF, Devlin HB. et al. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingkom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ 1995;311:222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jairath V, Rehal S, Logan R. et al. Acute variceal haemorrhage in the United Kingdom: patient characteristics, management and outcomes in a nationwide audit. Dig Liver Dis 2014;46:419–26. [DOI] [PubMed] [Google Scholar]
- 14. de Francis R, Baveno VI faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: Stratifying risk and individualising care for portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 15. Tripathi D, Stanley AJ, Hayes PC. et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Tsao G, Abraldes JG, Berzigotti A. et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis and management: 2016 practice guidance by the American Association for the study of liver disease. Hepatology 2017;65:310–35. [DOI] [PubMed] [Google Scholar]
- 17. Grace ND, Groszmann RJ, Garcia-Tsao G. et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology 1998;28:868–80. [DOI] [PubMed] [Google Scholar]
- 18. D’Amico G, Pasta L, Morabito A. et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014;39:1180–93. [DOI] [PubMed] [Google Scholar]
- 19. Pagliaro L, D’Amico G, Pasta L. et al. Portal hypertension in cirrhosis: natural history In: Bosch J, Groszmann RJ (eds). Portal Hypertension: Pathophysiology and Treatment. Oxford: Blackwell Scientific Press, 1994, 72–92. [Google Scholar]
- 20. Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 2005;353:2254–61. [DOI] [PubMed] [Google Scholar]
- 21. Merli M, Nicolini G, Angeloni S. et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 2003;38:266–72. [DOI] [PubMed] [Google Scholar]
- 22. The North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices: a prospective multicenter study. N Engl J Med 1988;319:983–9. [DOI] [PubMed] [Google Scholar]
- 23. Polio J, Groszmann RJ, Reuben A. et al. Portal hypertension ameliorates arterial hypertension in spontaneously hypertensive rats. J Hepatol 1989;8:294–301. [DOI] [PubMed] [Google Scholar]
- 24. Groszmann RJ, Bosch J, Grace N. et al. Hemodynamic events in a prospective randomized trial of propranolol vs placebo in the prevention of the first variceal hemorrhage. Gastroenterology 1990;99:1401–7. [DOI] [PubMed] [Google Scholar]
- 25. Casado M, Bosch J, Garcia-Pagan JC. et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology 1998;114:1296–1303. [DOI] [PubMed] [Google Scholar]
- 26. Feu F, Garcia-Pagan JC, Bosch J. et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet 1995;346:1056–9. [DOI] [PubMed] [Google Scholar]
- 27. Abraldes JG, Tarantino I, Turnes J. et al. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology 2003;37:902–8. [DOI] [PubMed] [Google Scholar]
- 28. Bosch J, Garcia-Pagan JC.. Prevention of variceal rebleeding. Lancet 2003;361:952–4. [DOI] [PubMed] [Google Scholar]
- 29. El-Serag HB, Everhart JE.. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol 2000;95:3566–73. [DOI] [PubMed] [Google Scholar]
- 30. D’Amico G, de Franchis R.. Upper digestive bleeding in cirrhosis: post-therapeutic outcome and prognostic indicators. Hepatology 2003;38:599–612. [DOI] [PubMed] [Google Scholar]
- 31. Carbonell N, Pauwels A, Serfaty L. et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004;40:652–9. [DOI] [PubMed] [Google Scholar]
- 32. Moitinho E, Escorsell A, Bandi JC. et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999;117:626–31. [DOI] [PubMed] [Google Scholar]
- 33. Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L. et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology 2004;40:793–801. [DOI] [PubMed] [Google Scholar]
- 34. Sarin SK, Lahoti D, Saxena SP. et al. Prevalence, classification and natural history of gastric varices—a long term follow up study in 568 portal hypertension patients. Hepatology 1992;16:1323–49. [DOI] [PubMed] [Google Scholar]
- 35. De Francis R, Primignani M.. Natural history of portal hypertension in patients with cirrhosis. Clin Liv Dis 2001;5:645–63. [DOI] [PubMed] [Google Scholar]
- 36. Kim T, Shijo H, Kokawa H. et al. Risk factors for haemorrhage from gastric fundal varices. Hepatology 1997;25:307–12. [DOI] [PubMed] [Google Scholar]
- 37. Tripathi D, Ferguson JW, Therapondos G. et al. Recent advances in the management of bleeding gastric varices. Aliment Pharmacol Ther 2006;24:1–17. [DOI] [PubMed] [Google Scholar]
- 38. de Francis R, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosisand therapy in portal hypertension. J Hepatol 2010;53:762–8. [DOI] [PubMed] [Google Scholar]
- 39. Furuichi Y, Kawai T, Ichimura S. et al. Flexible imaging color enhancement improves visibility of transnasal endoscopic images in diagnosing esophageal varices: a multicenter prospective blinded study. J Dig Dis 2012;13:634–41. [DOI] [PubMed] [Google Scholar]
- 40. Choe WH, Kim JH, Ko SY. et al. Comparison of transnasal small-caliber vs. peroral conventional esophagogastroduodenoscopy for evaluating varices in unsedated cirrhotic patients. Endoscopy 2011;43:649–56. [DOI] [PubMed] [Google Scholar]
- 41. McCarty TR, Afinogenova Y, Njei B.. Use of wireless capsule endoscopy for the diagnosis and grading of esophageal varices in patients with portal hypertension—a systemic review and meta-analysis. J Clin Gastroenterol 2017;51:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castera L, Pinzani M, Bosch J.. Non-invasive evaluation of portal hypertension using transient elastography. J Hepatol 2012;56:696–703. [DOI] [PubMed] [Google Scholar]
- 43. Vizzutti F, Arena U, Romanelli RG. et al . Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology 2007;45:1290–7. [DOI] [PubMed] [Google Scholar]
- 44. Castera L, Le Bail B, Roudot-Thoraval F. et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009;50:59–68. [DOI] [PubMed] [Google Scholar]
- 45. Abraldes JG, Bureau C, Stefanescu H. et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the ‘Anticipate’ Study. Hepatology 2016;64:2173–84. [DOI] [PubMed] [Google Scholar]
- 46. Cales P, Oberti F, Payen JL. et al. Lack of effect of propranolol in the prevention of large oesophageal varices in patients with cirrhosis: a randomized trial: French-Speaking Club for the Study of Portal Hypertension. Eur J Gastroenterol Hepatol 1999;11:741–5. [DOI] [PubMed] [Google Scholar]
- 47. Merkel C, Marin R, Angeli P. et al. A placebo-controlled clinical trial of nadolol in the prophylaxis of growth of small esophageal varices in cirrhosis. Gastroenterology 2004;127:476–84. [DOI] [PubMed] [Google Scholar]
- 48. Sarin SK, Misra SR, Sharma P. et al . Early primary prophylaxis with beta-blockers does not prevent the growth of small esophageal varices in cirrhosis: a randomized controlled trial. Hepatol Int 2013;7:248–56. [DOI] [PubMed] [Google Scholar]
- 49. Bhardwaj A,, Kedarisetty CK,, Vashishtha C. et al. (13 June 2016) Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo controlled trial. Gut, 10.1136/gutjnl-2016–311735. [DOI] [PubMed] [Google Scholar]
- 50. Garcia-Tsao G, Bosch J.. Varices and variceal hemorrhage in cirrhosis: a new view of an old problem. Clin Gastroenterol Hepatol 2015;13:2109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de Franchis R. Expanding consensus in portal hypertension. J Hepatol 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 52. D’Amico G, Pagliaro L, Bosch J.. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis 1999;19:475–505. [DOI] [PubMed] [Google Scholar]
- 53. Reiberger T, Ulbrich G, Ferlitsch A. et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut 2013;62:1634–41. [DOI] [PubMed] [Google Scholar]
- 54. Sinagra E, Perricone G, D’Amico M. et al. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther 2014;39:557–68. [DOI] [PubMed] [Google Scholar]
- 55. Banares R, Moitinho E, Matilla A. et al. Randomized comparison of long-term varvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology 2002;36:1367–73. [DOI] [PubMed] [Google Scholar]
- 56. Banares R, Moitinho E, Piqueras B. et al. Carvedilol, a new nonselective beta-blocker with intrinsic anti-Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology 1999;30:79–83. [DOI] [PubMed] [Google Scholar]
- 57. Tripathi D, Ferguson JW, Kochar N. et al. Randomized controlled trial of carvedilol versus variceal band ligation for the prevention of the first variceal bleed. Hepatology 2009;50:825–33. [DOI] [PubMed] [Google Scholar]
- 58. Shah HA, Azam Z, Rauf J. et al. Carvedilol vs. esophageal variceal band ligation in the primary prophylaxis of variceal hemorrhage: a multicentre randomized controlled trial. J Hepatol 2014;60:757–64. [DOI] [PubMed] [Google Scholar]
- 59. Gluud LL, Krag A.. Banding ligation versus beta-blockers for primary prevention in oesophageal varices in adults. Cochrane Database Syst Rev 2012;8:CD004544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Funakoshi N, Duny Y, Valats JC. et al. Meta-analysis: beta-blockers versus banding ligation for primary prophylaxis of esophageal variceal bleeding. Ann Hepatol 2012;11:369–83. [PubMed] [Google Scholar]
- 61. Sarin SK, Wadhawan M, Agarwal SR. et al. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005;100:797–804. [DOI] [PubMed] [Google Scholar]
- 62. Hernandez-Gea V, Aracil C, Colomo A. et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with beta-blockers. Am J Gastroenterol 2012;107:418–27. [DOI] [PubMed] [Google Scholar]
- 63. Longacre AV, Imaeda A, Garcia-Tsao G. et al. A pilot project examining the predicted preferences of patients and physicians in the primary prophylaxis of variceal haemorrhage. Hepatology 2008;47:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. D’Amico G, Pagliaro L, Bosch J.. The treatment of portal hypertension: a meta-analytic review. Hepatology 1995;22:332–54. [DOI] [PubMed] [Google Scholar]
- 65. Navasa M, Chesta J, Bosch J. et al. Reduction of portal pressure by isosorbide-5- mononitrate in patients with cirrhosis: effects on splanchnic and systemic hemodynamics and liver function. Gastroenterology 1989;96:1110–18. [DOI] [PubMed] [Google Scholar]
- 66. Angelico M, Carli L, Piat C. et al. Isosorbide-5-mononitrate versus propranolol in the prevention of first bleeding in cirrhosis. Gastroenterology 1993;104:1460–5. [DOI] [PubMed] [Google Scholar]
- 67. Garcia-Pagan JC. Isosorbide-5-monitrate (ISMN) vs placebo (PLA) in the prevention of the first variceal bleeding (FVB) in patients with contraindications or intolerance to beta-blockers. J Hepatol 2000;32:28. [Google Scholar]
- 68. Mandorfer M, Reiberger T.. Beta-blockers and cirrhosis, 2016. Dig Liver Dis 2017;49:3–10. [DOI] [PubMed] [Google Scholar]
- 69. Quraishi MN, Khan F, Tripathi D.. How we manage variceal haemorrhage in cirrhotic patients. Pol Arch Med Wewn 2016;126:174–84. [DOI] [PubMed] [Google Scholar]
- 70. Serste T, Melot C, Francoz C. et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017–22. [DOI] [PubMed] [Google Scholar]
- 71. Krag A, Wiest R, Albillos A. et al. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61:967–9. [DOI] [PubMed] [Google Scholar]
- 72. Krag A, Bendtsen F, Henriksen JH. et al. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut 2010;59:105–10. [DOI] [PubMed] [Google Scholar]
- 73. Leithead JA, Rajoriya N, Tehami N. et al. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut 2015;64:1111–19. [DOI] [PubMed] [Google Scholar]
- 74. Mandorfer M, Bota S, Schwabl P. et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146:1680–90.e1. [DOI] [PubMed] [Google Scholar]
- 75. Bossen L, Krag A, Vilstrup H. et al. Nonselective β-blockers do not affect mortality in cirrhosis patients with ascites: post hoc analysis of three randomized controlled trials with 1198 patients. Hepatology 2016;63:1968–76. [DOI] [PubMed] [Google Scholar]
- 76. Abraldes JG, Villanueva C, Banares R. et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229–36. [DOI] [PubMed] [Google Scholar]
- 77. Bambha K, Kim WR, Pedersen R. et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008;57:814–20. [DOI] [PubMed] [Google Scholar]
- 78. Amitrano L, Guardascione MA, Manguso F. et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol 2012;107:1872–8. [DOI] [PubMed] [Google Scholar]
- 79. Hunter SS, Hamdy S.. Predictors of early re-bleeding and mortality after acute variceal haemorrhage. Arab J Gastroenterol 2013;14:63–7. [DOI] [PubMed] [Google Scholar]
- 80. Fortune B, Garcia-Tsao G, Ciarleglio M. et al. Child-Turcotte-Pugh Class is best at stratifying risk in variceal hemorrhage: analysis of a US multicenter prospective study. J Clin Gastroenterol 2017;51:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Reverter E, Tandon P, Augustin S. et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology 2014;146:412–19. [DOI] [PubMed] [Google Scholar]
- 82. Villanueva C, Colomo A, Bosch A. et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 83. Tripodi A, Mannucci PM.. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147–56. [DOI] [PubMed] [Google Scholar]
- 84. Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (N Y) 2012;8:513–20. [PMC free article] [PubMed] [Google Scholar]
- 85. Bendtsen F, D’Amico G, Rusch E. et al. Effect of recombinant Factor VIIa on outcome of acute variceal bleeding: an individual patient based meta-analysis of two controlled trials. J Hepatol 2014;61:252–9. [DOI] [PubMed] [Google Scholar]
- 86. Wells M, Chande N, Adams P. et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther 2012;35:1267–78. [DOI] [PubMed] [Google Scholar]
- 87. Ioannou G, Doust J, Rockey DC.. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003;1:CD002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Azam Z, Hamid S, Jafri W. et al. Short course adjuvant terlipressin in acute variceal bleeding: a randomized double blind dummy controlled trial. J Hepatol 2012;56:819–24. [DOI] [PubMed] [Google Scholar]
- 89. Seo YS, Park SY, Kim MY. et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology 2014;60:954–63. [DOI] [PubMed] [Google Scholar]
- 90. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F. et al. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding—an updated Cochrane review. Aliment Pharmacol Ther 2011;34:509–18. [DOI] [PubMed] [Google Scholar]
- 91. Tandon P, Abraldes JG, Keough A. et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on Child-Pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol 2015;13:1189–96. [DOI] [PubMed] [Google Scholar]
- 92. Cheung J, Soo I, Bastiampillai R. et al. Urgent vs. non-urgent endoscopy in stable acute variceal bleeding. Am J Gastroenterol 2009;104:1125–9. [DOI] [PubMed] [Google Scholar]
- 93. Chen PH, Chen WC, Hou MC. et al. Delayed endoscopy increases re-bleeding and mortality in patients with hematemesis and active esophageal variceal bleeding: a cohort study. J Hepatol 2012;57:1207–13. [DOI] [PubMed] [Google Scholar]
- 94. Laine L, Cook D.. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding: a meta-analysis. Ann Intern Med 1995;123:280–7. [DOI] [PubMed] [Google Scholar]
- 95. Banares R, Albillos A, Rincon D. et al. Endoscopic treatment versus endoscopic plus pharmacologic treatment for acute variceal bleeding: a meta-analysis. Hepatology 2002;35:609–15. [DOI] [PubMed] [Google Scholar]
- 96. Panes J, Teres J, Bosch J. et al. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices: results in 151 consecutive episodes. Dig Dis Sci 1988;33:454–9. [DOI] [PubMed] [Google Scholar]
- 97. Teres J, Cecilia A, Bordas JM. et al. Esophageal tamponade for bleeding varices: controlled trial between the Sengstaken-Blakemore tube and the Linton-Nachlas tube. Gastroenterology 1978;75:566–9. [PubMed] [Google Scholar]
- 98. Escorsell A, Pavel O, Cardenas A. et al. Esophageal balloon tamponade versus esophageal stent in controllong acute reftractory variceal bleeding: a multicentre randomised controlled trial. Hepatology 2016;63:1957–67. [DOI] [PubMed] [Google Scholar]
- 99. Vangeli M, Patch D, Burroughs AK.. Salvage tips for uncontrolled variceal bleeding. J Hepatol 2002;37:703–4. [DOI] [PubMed] [Google Scholar]
- 100. Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L. et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology 2004;40:793–801. [DOI] [PubMed] [Google Scholar]
- 101. Garcia-Pagan JC, Caca K, Bureau C. et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010;362:2370–9. [DOI] [PubMed] [Google Scholar]
- 102. Garcia-Pagan JC, Di PM, Caca K. et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol 2013;58:45–50. [DOI] [PubMed] [Google Scholar]
- 103. Rudler M, Cluzel P, Corvec TL. et al. Early-TIPSS placement prevents rebleeding in high-risk patients with variceal bleeding, without improving survival. Aliment Pharmacol Ther 2014;40:1074–80. [DOI] [PubMed] [Google Scholar]
- 104. Sanyal AJ, Freedman AM, Luketic VA. et al. Transjugular intrahepatic portosystemic shunts for patients with active variceal haemorrhage unresponsive to sclerotherapy. Gastroenterology 1996;111:138–46. [DOI] [PubMed] [Google Scholar]
- 105. McCormick PA, Dick R, Panagou EB. et al. Emergency transjugular intrahepatic portosystemic stent shunting as a salvage treatment for uncontrolled variceal hemorrhage. Br J Surg 1994;81:1324–7. [DOI] [PubMed] [Google Scholar]
- 106. Orloff MJ, Orloff MS, Orloff SL. et al. Three decades of experience with emergency portacaval shunt for acutely bleeding esophageal varices in 400 unselected patients with cirrhosis of the liver. J Am Coll Surg 1995;180:257–72. [PubMed] [Google Scholar]
- 107. Orloff MJ, Vaida F, Haynes KS. et al. Randomized controlled trial of emergency transjugular intrahepatic portosystemic shunt versus emergency portacaval shunt treatment of acute bleeding esophageal varices in cirrhosis. J Gastrointest Surg 2012;16:2094–2111. [DOI] [PubMed] [Google Scholar]
- 108. Puente A, Hernandez-Gea V, Graupera I. et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int 2014;34:823–33. [DOI] [PubMed] [Google Scholar]
- 109. Abraldes JG, Villanueva C, Aracil C. et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology 2016;150: 1160–70. [DOI] [PubMed] [Google Scholar]
- 110. Pollo-Flores P, Soldan M, Santos UC. et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis 2015;47:957–63. [DOI] [PubMed] [Google Scholar]
- 111. Mohanty A, Tate JP, Garcia-Tsao G.. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology 2016;150:430–440.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tripathi D, Ferguson JW, Therapondos JN. et al. Review article: recent advances in the management of bleeding gastric varices. Aliment Pharmacol Ther 2006;24:1–17. [DOI] [PubMed] [Google Scholar]
- 113. Mishra SR, Sharma BC, Kumar A. et al. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol 2011;54:1161–7. [DOI] [PubMed] [Google Scholar]
- 114. Rios CE,, Seron P,, Gisbert JP. et al. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev 2015;5:CD010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Smith MR, Tidswell R, Tripathi D.. Outcomes of endoscopic human thrombin injection in the management of gastric varices. Eur J Gastroenterol Hepatol 2014;26: 846–52. [DOI] [PubMed] [Google Scholar]
- 116. Tripathi D, Therapondos G, Jackson E. et al. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut 2002;51:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lo GH, Liang HL, Chen WC. et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy 2007;39:679–85. [DOI] [PubMed] [Google Scholar]
- 118. Krahenbuhl L, Seiler CA, Buchler MW.. Variceal hemorrhage in portal hypertension: role of surgery in the acute and elective situation. Schweiz Med Wochenschr 1999;129:631–8. [PubMed] [Google Scholar]
- 119. Tsuchida S, Ku Y, Fukumoto T. et al. Isolated gastric varices resulting from iatrogenic splenic vein occlusion: report of a case. Surg Today 2003;33:542–4. [DOI] [PubMed] [Google Scholar]
- 120. McDermott VG, England RE, Newman GE.. Case report: bleeding gastric varices secondary to splenic vein thrombosis successfully treated by splenic artery embolization. Br J Radiol 1995;68:928–30. [DOI] [PubMed] [Google Scholar]