Abstract
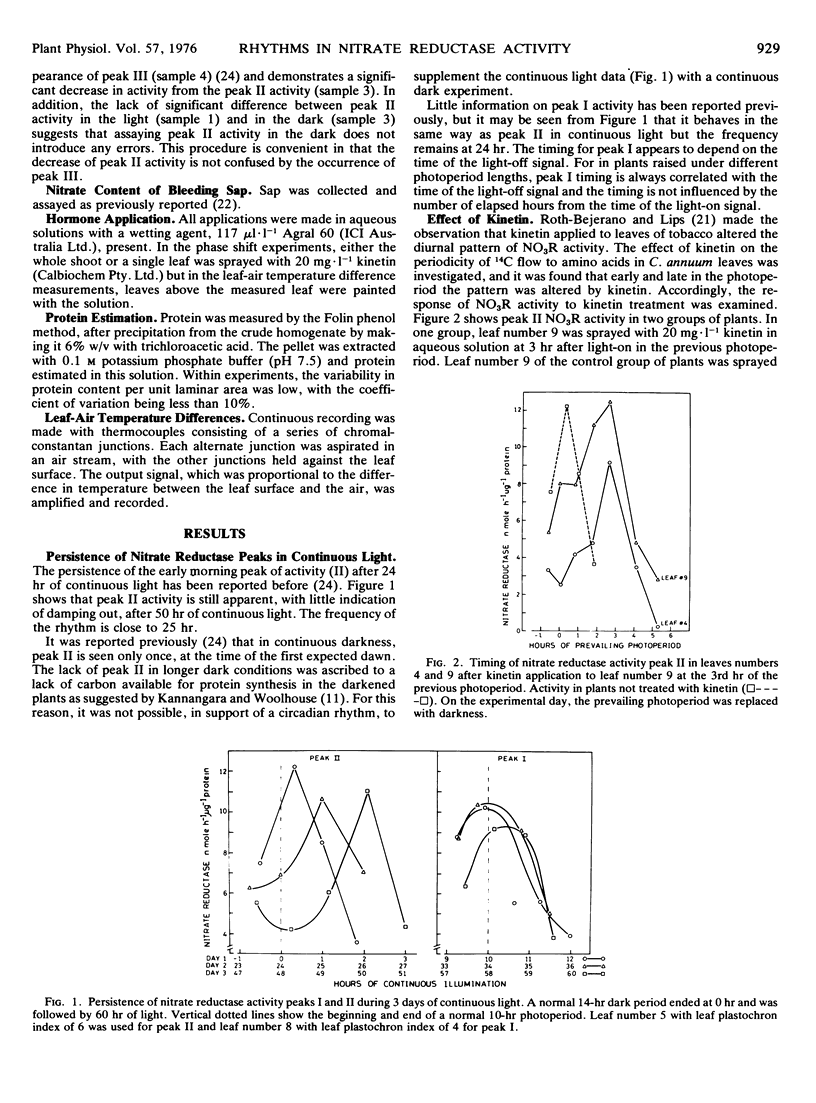
In the expanding leaves of Capsicum annuum L. cv. California Wonder, two of the three peaks of nitrate reductase activity associated with the light period exhibit a circadian rhythm that persists in continuous light.
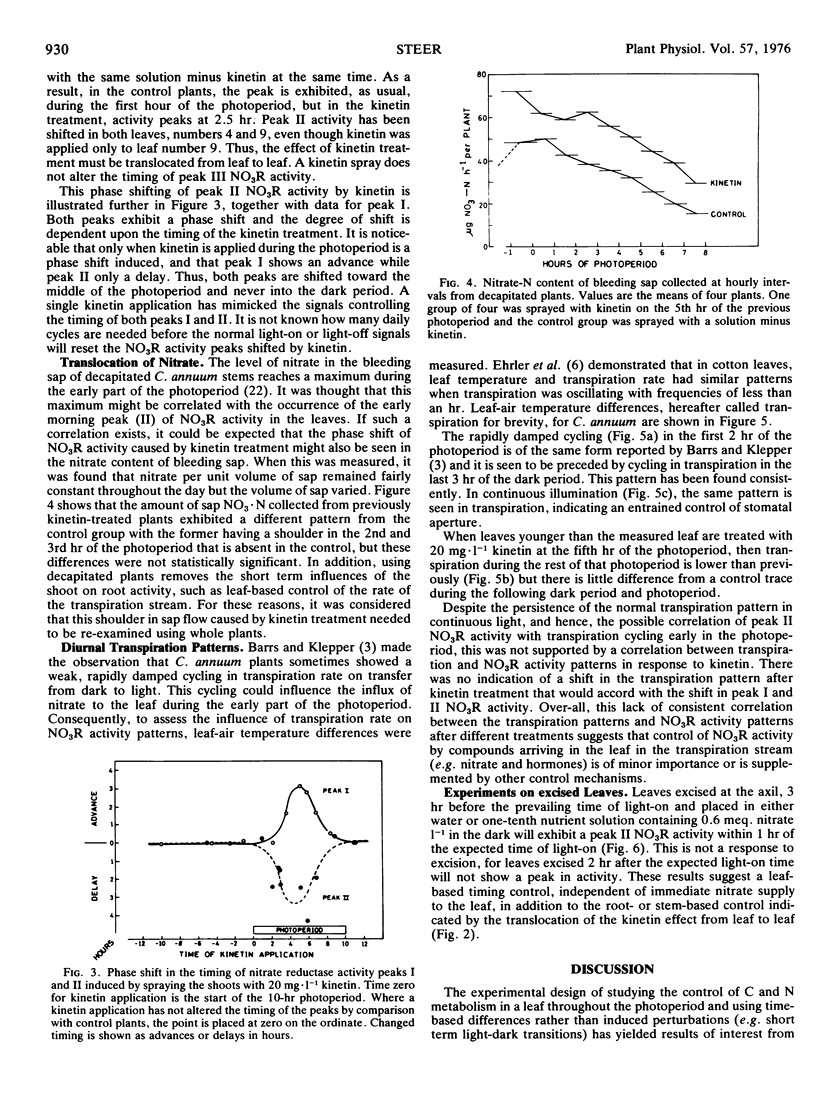
The spray application of kinetin to the whole shoot or to leaves other than the ones used for nitrate reductase assay causes a phase shift in the activity peaks and this has been used in preliminary investigations of the character of the mechanisms controlling the timing of the peaks.
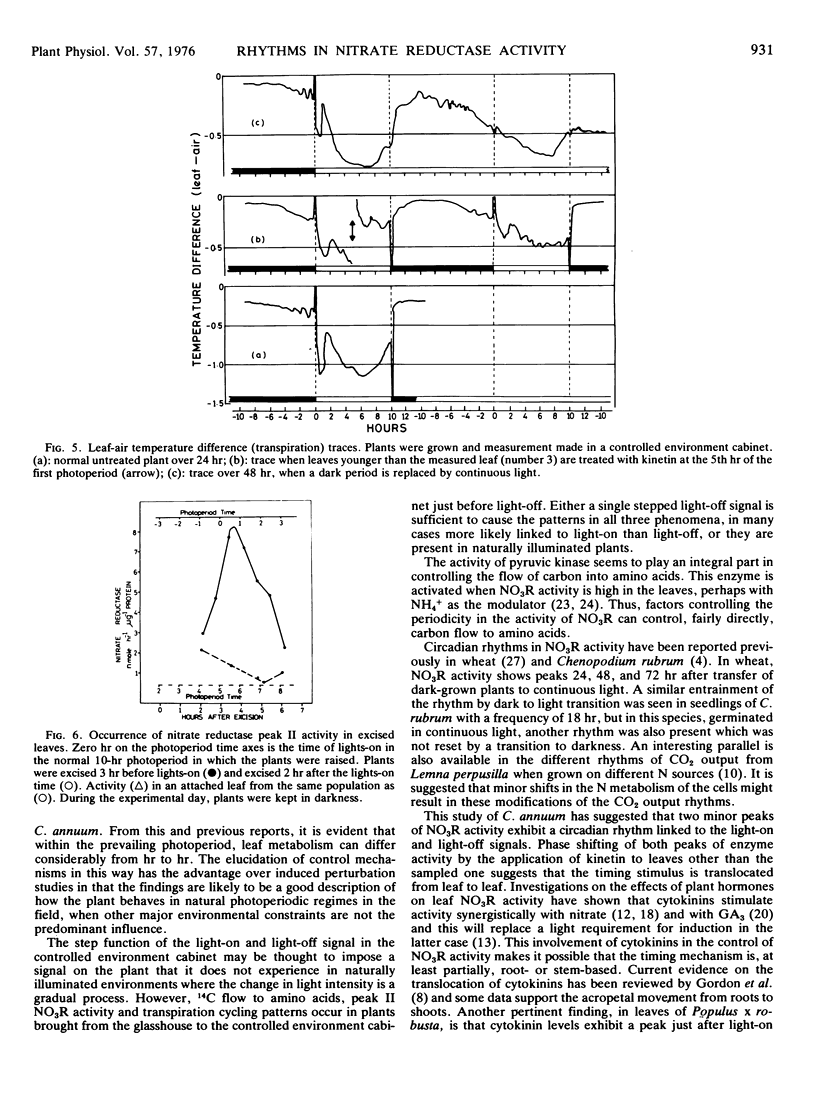
There was some indication that the rate of translocation of nitrate from the roots might be involved. The levels of nitrate moving up the stem after kinetin treatment were more dependent upon the rate of sap flow than on the concentration of nitrate in the sap. For this reason, transpiration rates in whole plants were measured after kinetin treatment but no change in pattern was seen that would correlate with the phase shift in nitrate reductase activity.
The occurrence of nitrate reductase peaks in excised leaves suggested a leaf-based in addition to a root-or stem-based mechanism in the timing of nitrate reductase activity in the leaves.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eaglesham A. R., Hewitt E. J. Kinetics and inhibition by adenosine phosphates and nitrite of nitrate reductase from Spinacea oleracea L. Biochem J. 1971 Mar;122(1):18P–19P. doi: 10.1042/bj1220018pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Yoder O. C., Filner P. Anaerobic nitrite production by plant cells and tissues: evidence for two nitrate pools. Plant Physiol. 1973 Mar;51(3):423–431. doi: 10.1104/pp.51.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman W. S. Effects of inorganic nitrogen on the response of Lemna carbon dioxide output to light quality and timing. Photochem Photobiol. 1975 Jan;21(1):30–47. doi: 10.1111/j.1751-1097.1975.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Lips S. H., Roth-Bejerano N. Light and hormones: interchangeability in the induction of nitrate reductase. Science. 1969 Oct 3;166(3901):109–110. doi: 10.1126/science.166.3901.109. [DOI] [PubMed] [Google Scholar]
- Njus D., Sulzman F. M., Hastings J. W. Membrane model for the circadian clock. Nature. 1974 Mar 8;248(5444):116–120. doi: 10.1038/248116a0. [DOI] [PubMed] [Google Scholar]
- Pavlidis T. Populations of interacting oscillators and circadian rhythms. J Theor Biol. 1969 Mar;22(3):418–436. doi: 10.1016/0022-5193(69)90014-9. [DOI] [PubMed] [Google Scholar]
- Steer B. T. Control of Diurnal Variations in Photosynthetic Products: II. Nitrate Reductase Activity. Plant Physiol. 1974 Nov;54(5):762–765. doi: 10.1104/pp.54.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T. Control of diurnal variations in photosynthetic products: I. Carbon metabolism. Plant Physiol. 1974 Nov;54(5):758–761. doi: 10.1104/pp.54.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T. Diurnal variations in photosynthetic products and nitrogen metabolism in expanding leaves. Plant Physiol. 1973 Apr;51(4):744–748. doi: 10.1104/pp.51.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney B. M. A physiological model for circadian rhythms derived from the acetabularia rhythm paradoxes. Int J Chronobiol. 1974;2(1):25–33. [PubMed] [Google Scholar]
- Upcroft J. A., Done J. Evidence for a complex control system for nitrate reductase in wheat leaves. FEBS Lett. 1972 Mar 15;21(2):142–144. doi: 10.1016/0014-5793(72)80123-6. [DOI] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]



