Abstract
Introduction
Seborrheic dermatitis is a chronic inflammatory skin disease. One of the components of metabolic syndrome is inflammation, and many inflammatory cytokines play a critical role in the disease. The aim of this study is to investigate metabolic syndrome and to evaluate the relationship between the parameters of the disease and disease severity in patients with seborrheic dermatitis.
Material and methods
Forty-seven patients with seborrheic dermatitis and 36 healthy controls were included in the study. The parameters of metabolic syndrome were recorded in both groups. In the patient group, disease severity was determined with the seborrheic dermatitis area and severity index (SDASI). All the venous blood samples were taken at 8 a.m. after 10 h of fasting.
Results
High-density lipoprotein (HDL) levels in the patient group were statistically significantly lower than in the controls. There was no significant difference between groups according to other parameters. In terms of history of metabolic disease in first degree relatives (diabetes mellitus, cardiovascular disease, and dyslipidaemia), 78.7% of those in the patient group (n = 37) and 55.6% of those in the control group (n = 20) had a history of metabolic disease in their families, and the difference between the patient and control groups was found to be statistically significant (p < 0.05). There was a significant correlation between disease severity and plasma HDL levels (p = 0.033, r = –0.312).
Conclusions
The presence of seborrheic dermatitis may be a predictive factor for metabolic syndrome.
Keywords: seborrheic dermatitis, metabolic syndrome, high-density lipoprotein
Introduction
Seborrheic dermatitis is a common chronic inflammatory disease which can last long years through recurrence and remissions. This disease seriously affects the quality of life of some patients due to its chronic course and the lack of a definitive treatment. Etiological factors commonly held accountable are seborrhea and Malassezia. Various studies have reported that the disease is frequently seen in cases of obesity and diabetes [1, 2]. Seborrheic dermatitis occurs in areas dense in sebaceous glands such as the face, ears, scalp, and upper body. The phrase ‘dermatitis of sebaceous areas’ is also used to define the disease [2, 3]. Although seborrheic dermatitis can be observed in all ages, it is most common amongst children 2 years of age. Seborrheic dermatitis occurs most commonly in infants within the first 3 months of life, in adolescents and young adults, with the incidence increasing again in patients older than 50 years of age [4].
The disease is an endocrinopathy characterized by obesity, hypertension, and dyslipidemia, and insulin resistance plays an important role in its metabolic syndrome pathophysiology. Metabolic syndrome is a multiple cardiovascular risk factor; both the syndrome itself and each of its components indicate an increased risk for cardiovascular complications. One of the components of metabolic syndrome is inflammation [5]. Numerous studies have been conducted on metabolic syndrome, as it is a significant cause of morbidity and mortality. The disease that is best known to be correlated with metabolic syndrome among dermatological diseases is psoriasis [6]. Seborrheic dermatitis and psoriasis are both chronic inflammatory diseases that share certain characteristics [7].
To our knowledge, no prospective study investigating the frequency of metabolic syndrome in seborrheic dermatitis has been conducted so far. The purpose of this study was to investigate metabolic syndrome, its components, and the correlation between these components and the severity of the disease among seborrheic dermatitis patients.
Material and methods
The study was approved by the local human ethics committee of Cumhuriyet University School of Medicine. The study included 47 seborrheic dermatitis patients who had applied to our dermatology outpatient clinic and were over the age of 18. The study also included 36 healthy volunteers who were similar in age and gender distribution, and did not have any known diseases. Pregnant women and those under the age of 18 were not included in the study. It was a case-control study.
Patients in the patient group were diagnosed with seborrheic dermatitis according to clinical findings. The onset of the disease and the disease duration were recorded. Disease severity, erythema, dandruff, and pruritus were evaluated and scored between 1 and 3 point(s). According to the scoring, 1 corresponded with mild, 2 corresponded with moderate, and 3 corresponded with severe. The disease’s severity was also measured using the seborrheic dermatitis area and severity index (SDASI) [8].
The heights and weights of all participants in the patient and control groups were measured and their body mass index (BMI) was calculated. Additionally, the waist circumference of the participants was measured.
After a minimum of a 20-minute relaxation period, the blood pressure (BP) of all the participants was measured using a pressurized sphygmomanometer.
Venous blood samples were taken from the patients and the volunteers at 8 a.m. following a 10-hour fasting period. Levels of fasting glucose, triglyceride (TG), total cholesterol, high density lipoprotein (HDL), low-density lipoprotein (LDL), and high-sensitivity C-reactive protein (hsCRP) were measured. The participants of the study were diagnosed with metabolic syndrome according to the NCEP ATPIII diagnostic criteria (Table I) [5, 9].
Table I.
ATP III Metabolic syndrome diagnostic criteria (7.93)
At least three of the following:
|
Statistical analysis
The results of the study were uploaded to Statistical Package for the Social Sciences (SPSS) data 20.0 program; Mann-Whitney U test, χ2 test, and Spearman correlation analysis were performed to assess the data, and the level of statistical significance was assumed to be 0.05.
Results
A total of 83 people over the age of 18, including 47 patients with seborrhoeic dermatitis and the control group of 36 who did not have any known diseases, were included in the study. While the average age of the patient group was 30.61 ±11.47 years, the average age in the control group was 34.50 ±12.71 years. No statistically significant difference was found between the groups in terms of gender or average age distribution (p > 0.05).
The difference between the groups in terms of hsCRP was statistically insignificant (p > 0.05). There was no significant correlation between SDASI and hsCRP in the patient group (p > 0.984, r = –0.003).
Table II shows the relationship between the groups according to metabolic syndrome parameters. High density lipoprotein levels in the patient group were statistically significantly lower than the controls. There was no significant difference between groups according to other parameters. In terms of history of the metabolic disease in first degree relatives (diabetes mellitus, cardiovascular disease, and dyslipidaemia), 78.7% of those in the patient group (n = 37) and 55.6% of those in the control group (n = 20) had a history of metabolic disease in their families, and the difference between the patient and control groups was found to be statistically significant (p < 0.05).
Table II.
Metabolic syndrome parameters and family history in both groups
| Groups | Abdominal obesity (x/n) | Hypertriglyceridemia (x/n) | Low HDL (x/n) | Hypertension (x/n) | Hyperglycemia (x/n) | Metabolic syndrome (x/n) | The presence of at least one metabolic disease in family history (x/n) |
|---|---|---|---|---|---|---|---|
| Patient group | 10/47 | 16/47 | 31/47 | 7/47 | 6/47 | 9/47 | 37/47 |
| Controls | 3/36 | 8/36 | 15/36 | 4/36 | 2/36 | 3/36 | 20/36 |
| P-value* | 0.135 | 0.329 | 0.044 | 0.749 | 0.456 | 0.216 | 0.032 |
x/n – the number of cases in the patient or control group
Fisher’s exact test.
No statistically significant correlation was found between the disease duration and metabolic syndrome in the patient group (p > 0.05). When parameters of the disease severity were compared with disease duration, no statistically significant correlation was found (p > 0.05).
The patient group was divided into two groups as mild and moderate-severe according to disease severity (adapted from SDASI). In both groups, there was no significant difference of metabolic syndrome parameters (p > 0.05).
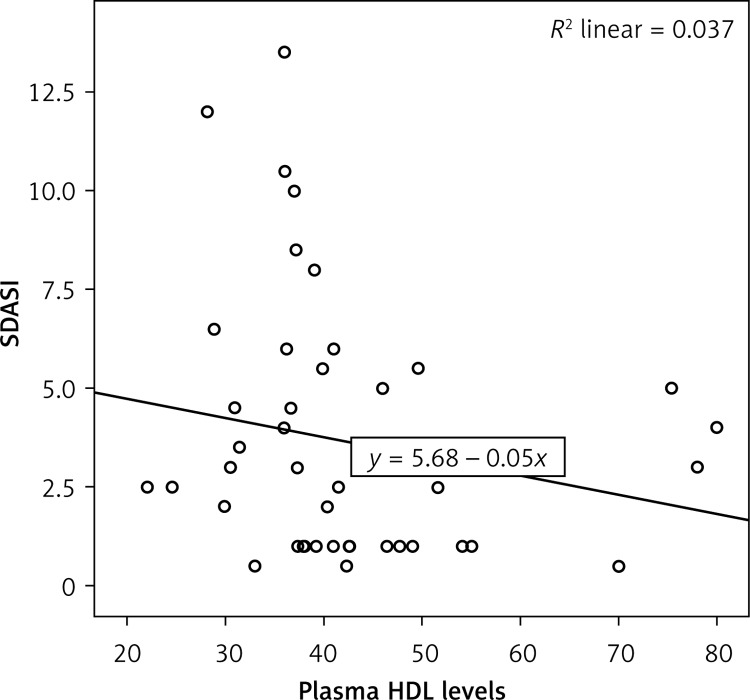
There was a significant correlation between disease severity and plasma HDL levels in the patient group (p = 0.033, r = –0.312) (Figure 1).
Figure 1.
Correlation between SDASI and plasma HDL levels in patient group
Discussion
Seborrheic dermatitis is a common chronic inflammatory disease which can last long years through recurrence and remission. Although various theories have been proposed regarding its etiology, no definitive results have been obtained yet. Etiological factors commonly held accountable are seborrhea, Plasmodium ovale, immune suppression, and hormonal factors [1, 2].
Metabolic syndrome is an endocrinopathy characterized by obesity, hypertension, and dyslipidemia, and insulin resistance plays an important role in metabolic syndrome pathophysiology. One of the components of metabolic syndrome is inflammation, and many inflammatory cytokines such as CRP, interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) play a critical role in the disease [5]. Numerous studies investigating the correlation between many inflammatory diseases and metabolic syndrome have been conducted so far. The disease that is best known to be correlated with metabolic syndrome among dermatological diseases is psoriasis. Seborrheic dermatitis and psoriasis are both chronic inflammatory diseases that share certain characteristics [7]. In various studies, the lesion-free skins of seborrheic dermatitis patients and healthy persons have been compared, and severe inflammatory reactions such as increased CD16+ expression on NK cells, the activation of complement systems, and increased inflammatory interleukins have been observed in skin of patients with seborrheic dermatitis [10].
In our study, it was found that 31 of 47 seborrheic dermatitis patients had low HDL levels, whereas 15 of the 36 individuals in the control group had low HDL levels. No statistically significant difference was found between the patient and control groups in terms of metabolic syndrome parameters, including triglycerides, hypertension, abdominal obesity and fasting blood glucose. In this study, the NCEP: ATPIII diagnosis criteria were used for the diagnosis of metabolic syndrome, and in accordance with the criteria, almost all patients diagnosed with metabolic syndrome had low HDL levels. Also in accordance with the criteria, the majority of the patients diagnosed with metabolic syndrome had high triglyceride values. The most crucial contributor for the diagnosis of dyslipidemia may be a low HDL value. The atherosclerosis protective effect of HDL takes the extra cholesterol from cells of the arterial wall and carries it back to the liver, and this is defined as reverse cholesterol transport [11].
Abdominal obesity and dyslipidemia are two of the most important components of metabolic syndrome. One of the abdominal obesity indicators is waist circumference. In addition to being an energy source, the adipose tissue also functions as an endocrine organ that releases many cytokines and peptides such as TNF-α, IL-6, leptin, resistin, adiponectin and visfatin. Increased TNF-α and decreased adiponectin also result in increased hepatic very-low-density lipoprotein (VLDL) and decreased peripheral clearing [12]. Abdominal obesity is accompanied by dyslipidemia and is associated with inflammation and some dermatological disorders, including psoriasis, lichen planus, pemphigus, granuloma annulare, discoid lupus erythematosus and histiocytosis, may cause dyslipidemias [13]. In our study, the abdominal obesity rate was not different from that in the control group.
Seborrheic dermatitis is thought to be initiated by the abnormal immune response developed against P. ovale and decomposition products [4]. Additionally, Malassezia species are known to produce lipase. Lipase leads to arachidonic acid release, which results in inflammation [14]. Various studies have reported that Malassezia type yeasts increase the release of inflammatory cytokines from keratinocytes such as IL-6, IL-8, and TNF-α [14, 15]. Although there is no antifungal activity specific to Malassezia, HDL is known to have antimicrobial activity and characteristics. Low HDL levels may decrease antimicrobial activity and indirectly result in inflammation and seborrheic dermatitis through the increase of Malassezia colonization.
In our study, no difference was found between the patient and control groups in terms of hsCRP levels, an indicator of inflammation. However, there was a correlation between plasma HDL levels and seborrheic dermatitis disease severity. The lower the HDL levels, the greater the disease severity in seborrheic dermatitis patients. This may be due to a decreased regulatory effect of lower HDL plasma levels on inflammation.
This study has some limitations. The small patient population of the study makes it difficult to draw a firm conclusion about the relationship between seborrheic dermatitis and metabolic syndrome. Furthermore, the follow-up was limited.
In conclusion, higher incidence of family history of metabolic syndrome accompanied by low HDL values in our patient group may be important in clinical practice. Therefore, the presence of seborrheic dermatitis should a warning for metabolic syndrome and dyslipidaemia. We are of the opinion that such patients must be clinically followed up in this aspect, and the planning of suitable treatments for patients diagnosed with metabolic syndrome or dyslipidaemia is important in the regulation of seborrheic dermatitis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Odom RB, James WB, Berger TG. Seborrheic dermatitis, psoriasis, recalcitrant palmoplantar eruptions, pustular dermatitis, and erythroderma. In: Fathman ME, Geisel EB, Salma A, editors. Andrew’s Diseases of the Skin. USA: WB Saunders Company; 2000. pp. 214–8. [Google Scholar]
- 2.Berth-Jones J. Eczema, lichenification, prurigo and erythrodermia. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s Textbook of Dermatology. Vol. 23. UK: Wiley-Blackwell Science; 2010. pp. 29–34. [Google Scholar]
- 3.Bieber T. Other types of dermatitis. In: Burgdorf WHC, Plewig G, Wolff HH, Landthaler M, editors. Braun-Falco’s Dermatology. Italy: Springer-Verlag; 2009. pp. 427–31. [Google Scholar]
- 4.Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, riskfactors and treatments: facts and controversies. Clin Dermatol. 2013;31:343–51. doi: 10.1016/j.clindermatol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Association of Endocrinology and Metabolism of Turkey Metabolic Syndrome Study Group. Guidelines of Metabolic Syndrome. 2009. http://www.turkendokrin.org/files/pdf/metabolik_sendrom.pdf.
- 6.Gelfand JM, Howa Y. Metabolic syndrome in patients with psoriatic disease. J Rheumatol Suppl. 2012;89:24–8. doi: 10.3899/jrheum.120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linder D, Drehier J, Zampatti A, Sampagna F, Cohen AD. Seborrheic dermatitis and hypertension in adults: a cross sectional study. J Eur Acad Dermatol Venerol. 2014;28:1450–5. doi: 10.1111/jdv.12310. [DOI] [PubMed] [Google Scholar]
- 8.Smith SA, Baker AE, Williams JH., Jr Effective treatment of seborrheic dermatitis using a low dose, oral homeopathic medication consisting of potassium bromide, sodium bromide, nickel sulfate, and sodium chloride in a double-blind, placebo-controlled study. Altern Med Rev. 2002;7:59–67. [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–241. [PubMed] [Google Scholar]
- 10.Faergmann J, Bergbrant IM, Dohse M, Scott A, Wesgate G. Seborrheic dermatitis and Pityriosporum follicülitis (Malassezia): characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144:549–56. doi: 10.1046/j.1365-2133.2001.04082.x. [DOI] [PubMed] [Google Scholar]
- 11.Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: it’s not about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15. doi: 10.1016/j.tem.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arslan M. Metabolic syndrome: diagnosis, pathogenesis, diagnostic criteria and components. Turkiye Klinikleri J Int Med Sci. 2006;2:1–7. [Google Scholar]
- 13.Shenoy C, Shenoy MM, RaO GK. Dislipidemia in dermatological disorders. North Am J Med Sci. 2015;7:421–8. doi: 10.4103/1947-2714.168657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riciputo RM, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses. 1996;39:233–5. doi: 10.1111/j.1439-0507.1996.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DS, Ingham E, Bojar RA, Holland KT. In vitro modulation of human keratinocyte pro-and anti-inflammatory cytokine production by the capsule of Malessezia species. FEMS Immunol Med Microbiol. 2008;54:203–14. doi: 10.1111/j.1574-695X.2008.00468.x. [DOI] [PubMed] [Google Scholar]