Abstract
Introduction
Body composition (BC) assessments in heart failure (HF) patients are mainly based on body weight, body mass index and waist-to-hip ratio. The present study compares BC assessments by basic anthropometry, dual energy X-ray absorptiometry (DXA), bioelectrical impedance spectroscopy (BIS), and air displacement plethysmography (ADP) for the estimation of fat (FM) and fat-free mass (FFM) in a HF population.
Material and methods
In this single-centre, observational pilot study we enrolled 52 patients with HF (33 HF with reduced ejection fraction (HFrEF), 19 HF with preserved ejection fraction (HFpEF); mean age was 67.7 ±9.9 years, 41 male) and 20 healthy controls. DXA was used as a reference standard for the measurement of FM and FFM.
Results
In the HF population, linear regression for DXA-FM and waist-to-hip ratio (r = –0.05, 95% CI: (–0.32)–0.23), body mass index (r = 0.47, 95% CI: 0.23–0.669), and body density (r = –0.87, 95% CI: (–0.93)–(–0.87)) was obtained. In HF, Lin’s concordance correlation coefficient of DXA-FM (%) with ADP-FM (%) was 0.76 (95% CI: 0.64–0.85) and DXA-FFM [kg] with DXA-ADP [kg] was 0.93 (95% CI: 0.88–0.96). DXA-FM (%) for BIS-FM (%) was 0.69 (95% CI: 0.54–0.80) and 0.73 (95% CI: 0.60–0.82) for DXA-FFM [kg] and BIS-FFM [kg].
Conclusions
Body density is a useful surrogate for FM. ADP was found suitable for estimating FM (%) and FFM [kg] in HF patients. BIS showed acceptable results for the estimation of FM (%) in HFrEF and for FFM [kg] in HFpEF patients. We encourage selecting a suitable method for BC assessment according to the compartment of interest in the HF population.
Keywords: heart failure, body composition, body mass index, cachexia, obesity
Introduction
The prognostic and clinical value of changes in body composition (BC) has been debated as a ‘signum mali ominis’ [1] in patients with heart failure (HF) since ancient times. The current prevalence of symptomatic heart failure (HF) is estimated to range from 0.4% to 2.0% in the general population [2], with approximately 14 million patients diagnosed with HF in the European Union alone [3]. Obesity has reached epidemic proportions, and clinical studies have shown that up to 49% of patients with HF are obese [4]. Cardiac cachexia is described in 10.5% of stable HF patients [5]. Obesity [6] and cachexia [7, 8] are relevant BC disorders in HF patients, and have recently been supplemented by the clinical picture of sarcopenia [9]. Sarcopenia affects 5–13% of healthy subjects of 60 years of age and up to 50% among octogenarians [10]. Among patients with HF with an average age of nearly 70 years, nearly 20% may meet the criteria for diagnosing sarcopenia [11]. In the form of loss of muscle mass and function, sarcopenia is of considerable functional interest in HF patients [10, 12]. Importantly, it does not necessarily involve weight loss, and may be compensated for by gain in fat tissue [13]. Given the vast number of HF patients at risk for multi-facetted BC disorders, a thorough assessment of BC, i.e. by estimating fat mass (FM), may prove worthwhile. The ‘obesity paradox’, a situation where obesity (as usually measured by body mass index (BMI)) is associated with incident HF, but paradoxically associated with better prognosis during chronic HF [14], highlights this need. The HF patients with higher FM have longer survival [15]. Skinfold measurements have allowed FM (%) to be reported as the strongest independent predictor of event-free survival in HF patients [16]. Additionally, adipose tissue represents a relevant physiological compartment with high endocrine and inflammatory activity [17]. FM assessment may hence bear relevant clinical consequences for our patients. Lavie et al. reported a 1% absolute increase in body fat to be associated with a more than 13% reduction in major clinical events in a HF population [18], highlighting the need to accurately assess FM in HF. In general, BC assessments are used as surrogate markers of morbidity and mortality not only in the clinical setting, but also in clinical trials involving HF.
To date, many clinicians have assessed body composition by body weight [19] and BMI. Increased BMI has been identified as a risk factor for HF [20]. When compared to BMI alone, the addition of waist-to-hip ratio (WHR) was not found to necessarily add prognostic potential in patients with HF [21]. Some question the accuracy of BMI in diagnosing obesity, particularly for individuals in the intermediate BMI ranges [22]. Oreopoulos et al. found BMI not to be a good indicator of adiposity and suggested that it may in fact be a better surrogate for lean body mass in patients with HF [23]. Aside from weight, BMI or WHR, HF clinics commonly monitor patients’ peripheral oedema. Such impaired body fluid distribution [24] may obscure changes in lean and adipose tissue in HF patients. Rarely have BC assessments been prospectively evaluated in a well-characterized target population, such as HF patients. With a large number of new therapeutic trials focusing on obesity, cachexia, sarcopenia and other BC imbalances, non-invasive and practically feasible approaches for endpoint assessment are becoming increasingly important. Hence, a thorough assessment of BC, i.e. by estimating FM as the compartment of interest, may prove pivotal. Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) [25] address this issue. The aim of the present study is to assess the association of simple anthropometric indices such as BMI and WHR with FM in a real-life sample of HF patients and to compare these established indices with some of the most commonly employed, non-invasive methods of BC assessment.
Material and methods
Study design, sample size
We performed an explorative, single-centre, observational, study assessing BC in HF patients using a multi-method, two-compartment approach. Specifically, BC was determined by dual energy X-ray absorptiometry (DXA) and used as a reference for the following indices and methods of BC assessment: BMI, WHR, bioelectrical impedance spectroscopy (BIS), and air displacement plethysmography (ADP). Hydration status was assessed by BIS. We aimed to enroll consecutive patients suffering from HF and 20 healthy reference subjects in this pilot cohort.
Recruitment, inclusion and exclusion criteria
We screened and recruited subjects presenting to our academic heart failure and general cardiology clinic (Department of Cardiology, Charité – Medical School, Campus Virchow Klinikum, Berlin, Germany) between April 2011 and March 2012. Before inclusion, all participants underwent focused transthoracic echocardiography to assess left ventricular ejection fraction (LVEF), left atrial diameter (LAD) and other cardiac parameters, and asked to give a medical history as part of the routine clinical care. Patients were included if they fulfilled the following inclusion criteria [25]:
Willing and able to give written informed consent, and
Established diagnosis with clinical signs and symptoms of HF with a New York Heart Association (NYHA) functional class ≥ II, previously documented NTproBNP > 400 pg/ml OR BNP > 150 pg/ml, and
- Echocardiographic findings as a surrogate of HF:
- with LVEF ≤ 40% (for HF with reduced ejection fraction, HFrEF), or
- with an LVEF > 40% and a LAD ≥ 40 mm (for HF with preserved ejection fraction, HFpEF).
Patients were excluded below the age of 18 years, with known pregnancy or with cardiac, or embolic events within 6 weeks prior to the baseline examination, previous heart transplantation, and on haemodialysis.
Twenty healthy subjects, age ≥ 18 years, non-pregnant with no known pre-existing medical conditions, chronic diseases, or on regular medication served as a reference sample.
Transthoracic echocardiography was performed by one of two experienced physicians using a GE Vivid-I ultrasound scanner and TTE transducer (by General Electric Company/GE Health Care, Buckinghamshire, UK). The LVEF was measured and calculated in per cent using Simpson’s method where possible, and LVEF was estimated visually in per cent according to LV impairment. The LAD was measured manually in mm.
Ethics
After the purpose, procedures and known risks had been explained, all participants provided written informed consent at enrolment. The local ethics committee approved the study. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Body composition assessments
All measurements were made on the same day of assessment within a time frame of a maximum of 4 h. Each system was calibrated in the morning of the day of assessment.
Anthropometry
Body weight was obtained by mass measurement with a high precision digital scale (BOD POD GS System, see below). Standing height was measured barefoot to the nearest 0.1 cm using a stadiometer. Waist circumference was measured at the midpoint between the lower margin of the last palpable rib and the top of the iliac crest. Hip circumference was measured around the widest portion of the buttocks. Body mass index was defined as the body mass, divided by the square of the body height, and expressed in units of kg/m2. The WHR was defined as waist measurement divided by hip measurement.
Dual-energy X-ray absorptiometry
DXA was performed when subjects were fasting (> 2 h), without shoes and jewellery and resting in a supine position using the LUNAR Prodigy DXA-Scanner (GE Medical Systems, Version 6.70.021; Prodigy en Core2002, 726 Heartland Trail, USA) at 38 keV and 70 keV [26] to determine total mass, fat-free mass (FFM) and FM by the EnCore2002 software according to the manufacturer’s instructions.
Air displacement plethysmography
Body density was determined by ADP in a seated position with subjects dressed in minimal clothes, and their hair covered with a swimming cap (BodPod GS, BOD POD Gold Standard, COSMED Italy, LMI Life Measurement Inc.; BodPod Version: 4.5.1.; DLL Version: 3.60; Controller Version: 13.40). The system allows the determination of body volume by measuring changes in air pressure induced by subjects seated in a closed measurement chamber. To account for lung volume, thoracic gas volume (TGV) was determined using a plethysmographic technique that is integrated into the measurement system. In cases where measurement of TGV was not possible or erroneous due to subject compliance, TGV was calculated from age, gender and height using McCrory’s approach [27]. Finally, body density was calculated from body mass and volume and used to determine FM, assuming constant densities for FM and FFM as suggested by Siri [28].
Body impedance spectroscopy
Impedance measurements were performed employing a bioelectrical impedance spectroscopy monitor (SFB7, Impedimed, Carlsbad, CA, USA). Measurements were taken from the right side of the body via a tetrapolar electrode arrangement following standard procedures [29]. Subjects, dressed in minimal clothes, were lying in a supine position with arms and legs 10° and 20° abducted from the body, respectively. Prior to electrode application, skin hair was removed, if necessary, and skin was rubbed with a 70% alcohol solution for 5 s and subsequently allowed to dry. Current introducing electrodes of the size of 4 cm2 (2.0 cm × 2.0 cm, Impedimed, Carlsbad, CA, USA) were placed in the middle on the dorsal surface of the right hand and foot just below the metacarpal-phalangeal and metatarsal-phalangeal joints, respectively. Whole body resistance was determined by positioning two voltage-sensing electrodes (the same type as current introducing electrodes) on the dorsal surfaces of the right wrist and ankle midline between the styloid processes of the ulna and radius and midline between the medial and lateral malleoli, respectively. After calibrating the measurement unit according to the guidelines of the manufacturer and lying 10 min supine to allow body fluids to stabilize, a sinusoidal current of 200 µA was applied, and impedance and phase angle were recorded at 256 exponentially-spaced frequencies between 4 kHz to 1000 kHz. Infinite resistance (R∞), extracellular resistance (R E = 0 kHz) and intracellular resistance (R I) were obtained by fitting impedance data to the Cole-Cole-equation (Cole and Cole 1941) and used to determine extracellular fluid (ECF) and intracellular fluid (ICF), respectively, using Hanai’s [30] second generation mixture theory [31]. Specific resistivities for intracellular and extracellular resistivity were 273.9 Ω·cm and 40.5 Ω·cm, respectively [32–34]. The FFM was computed assuming a fixed hydration constant of FFM of 73.2%. The FM was derived using the manufacturer’s software (SFB7 Version 1.55 and Bioimp Version 5.3.1.1, Impedimed, Carlsbad, CA, USA).
Data collection and documentation and storage
All participants underwent a focused medical interview on demographics, medical history, co-morbidities, risk factors, concomitant medication, and device therapy at baseline. A short physical examination focused on blood pressure, heart rate, heart rhythm, basic anthropometrics, and signs and symptoms of heart failure was performed. Transthoracic echocardiography and BC assessments were obtained as described above.
Statistical analysis
Data analysis was performed using the statistical software SPSS (version 20.0 IBM SPSS Statistics, Armonk, USA) and MedCalc (version 12.6. MedCalc Software, Ostend, Belgium). Figures were created using SigmaPlot (version 11.0 Systat Software, San Jose, USA). Descriptive data for primary outcome measures are presented as mean and 95% confidence intervals (CI). Categorical data are reported as frequency counts and percentages. Scatterplots and correlation analysis were performed for anthropometric indices and BC measurements. To scrutinize the association between different methods of BC DXA-determined FM was regressed on anthropometric indices as well as FM derived by BIA and ADP, respectively. Differences between DXA, BIS, and ADP-derived FM were tested for significance by repeated-measures ANOVA and t-tests, and Wilcoxon signed-rank and Friedman tests where appropriate. Agreement between methods was assessed by Lin’s concordance correlation coefficient (CCC) [35] and Bland-Altman plots [36]. The level of significance was set at α = 0.05 for all testing.
Results
Study population
This study enrolled 52 patients with an established diagnosis, clinical signs and symptoms, and echocardiographic evidence of HF. Twenty healthy controls were included. A detailed description of characteristics of the 72 subjects is given in Table I. Mean age was nearly 68 years in the HF population. Patients were predominantly male and considered overweight or obese. Most patients showed mild-to moderate HF with NYHA class II–III. Nearly half of patients showed traces of or mild clinical peripheral oedema. Two thirds of HF patients suffered from coronary heart disease, arterial hypertension was found in nearly 90%, and about 40% were diabetics. As for medical therapy, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were part of the daily medication of all patients. Four out of 5 patients were on β-blockers, and about 30% received mineralocorticoid receptor antagonists (Table II). Ten percent of the patients were bearing a conventional pacemaker. Internal cardioverter defibrillators (ICD) were found in a fifth of patients, and cardiac resynchronization therapy devices (with/without ICD) were present in a third of all patients (Table II).
Table I.
Baseline characteristics
| Variable | HF (n = 52) | HFrEF (n = 33) | HFpEF (n = 19) | REF (n = 20) | All (n = 72) |
|---|---|---|---|---|---|
| Demographics and basic anthropometry: | |||||
| Age [years], mean ± SD | 67.7 ±9.9 | 66.2 ±10.6 | 70.4 ±8.3 | 35.1 ±16.2 | 58.7 ±18.9 |
| Male sex, n (%) | 41 (78.8) | 27 (65.9) | 14 (73.7) | 12 (60) | 53 (73.6) |
| Weight [kg], mean ± SD | 85.4 ±18.8 | 82.6 ±18.6 | 90.2 ±18.7 | 71.4 ±15.0 | 81.5 ±18.8 |
| Height [cm], mean ± SD | 176.9 ±10.9 | 173.7 ±7.5 | 173.0 ±7.4 | 173.4 ±7.4 | 174.4 ±8.6 |
| Body mass index [kg/m2], mean ± SD | 28.4 ±5.8 | 27.4 ±5.7 | 30.0 ±5.5 | 22.6 ±2.6 | 26.8 ±5.7 |
| Waist circumference [cm], mean ± SD | 101.9 ±13.2 | 100.9 ±13.3 | 103.5 ±13.4 | 80.3 ±9.3 | 95.9 ±15.6 |
| Hip circumference [cm], mean ± SD | 106.9 ±12.8 | 105.5 ±13.1 | 109.3 ±12.3 | 99.1 ±8.1 | 104.7 ±12.2 |
| Wais-hip ratio, mean ± SD | 0.95 ±0.70 | 0.96 ±0.08 | 0.95 ±0.07 | 0.81 ±0.06 | 0.91 ±0.10 |
| Systolic blood pressure [mm Hg], mean ± SD | 123.8 ±23.4 | 116.6 ±21.7 | 137.9 ±20.2 | 123.0 ±10.7 | 123.6 ±20.5 |
| Diastolic blood pressure [mm Hg], mean ± SD | 72.5 ±11.0 | 69.4 ±9.7 | 78.5 ±11.0 | 76.5 ±6.6 | 73.6 ±10.0 |
| Heart rate [bpm], mean ± SD | 63.8 ±9.6 | 63.7 ±8.7 | 64.0 ±11.3 | 62.5 ±7.7 | 63.4 ±9.3 |
| Signs and symptoms of heart failure: | |||||
| NYHA II, n (%) | 27 (51.9) | 13 (39.4) | 14 (73.7) | – | – |
| NYHA III, n (%) | 24 (46.2) | 19 (57.6) | 5 (26.3) | – | – |
| Peripheral oedema, n (%) | 24 (46.2) | 16 (48.5) | 8 (42.1) | – | – |
| Jugular venous distension, n (%) | 9 (17.3) | 8 (24.2) | 1 (5.3) | – | – |
| Co-morbidities and risk factors: | |||||
| Hypertension, n (%) | 46 (88.5) | 29 (87.5) | 17 (89.5) | 0 (0) | 46 (63.9) |
| Hyperlipidaemia, n (%) | 40 (76.9) | 25 (75.8) | 15 (78.9) | 1 (5.0) | 41 (56.9) |
| Diabetes mellitus, n (%) | 21 (40.4) | 12 (36.4) | 9 (47.4) | 0 (0) | 21 (29.2) |
| Coronary heart disease, n (%) | 35 (67.3) | 21 (63.6) | 14 (73.7) | 0 (0) | 35 (48.6) |
| Chronic renal insufficiency, n (%) | 21 (40.4) | 16 (48.5) | 5 (26.3) | 0 (0) | 21 (29.2) |
| Chronic obstructive pulmonary disease, n (%) | 4 (7.7) | 4 (15.2) | 0 (0) | 0 (0) | 4 (5.6) |
NYHA – New York Heart Association functional class.
Table II.
Echocardiography parameters, medical and device therapy
| Variable | HF (n = 52) | HFrEF (n = 33) | HFpEF (n = 19) | REF (n = 20) | All (n = 72) |
|---|---|---|---|---|---|
| Echocardiographic parameters: | |||||
| LVEF [%], mean ± SD | 42.0 ±14.8 | 31.6 ±7.2 | 58.2 ±5.3 | 61.4 ±4.7 | 43.7 ±15.2 |
| LAD [mm], mean ± SD | 45.0 ±6.2 | 45.8 ±7.5 | 43.8 ±2.7 | 32.4 ±2.9 | 43.9 ±7.0 |
| LVEDD [mm], mean ± SD | 57.7 ±10.0 | 60.0 ±9.1 | 52.0 ±9.0 | – | – |
| IVSDd [mm], mean ± SD | 11.5 ±2.6 | 11.1 ±2.6 | 12.4 ±2.5 | – | – |
| Right-ventricular dilatation, n (%) | 11 (21.2) | 10 (30.3) | 1 (5.3) | – | – |
| E-wave [cm/s], mean ± SD | 62.4 ±32.5 | 55.4 ±47.6 | 69.3 ±14.8 | – | – |
| DT [ms], mean ± SD | 196.9 ±83.8 | 173.5 ±85.2 | 228.7 ±73.2 | – | – |
| IVRT [ms], mean ± SD | 125.7 ±34.1 | 130.7 ±40.1 | 119.2 ±22.1 | – | – |
| E/E’ [cm/s], mean ± SD | 13.9 ±8.8 | 15.5 ±10.5 | 11.4 ±4.9 | – | – |
| Diastolic dysfunction, n (%) | 48 (92.3) | 29 (87.9) | 19 (100) | – | – |
| Medical therapy: | |||||
| β-Blocker, n (%) | 43 (82.7) | 30 (90.9) | 13 (68.4) | – | – |
| ACE inhibitor, n (%) | 32 (61.5) | 20 (60.6) | 12 (63.2) | – | – |
| Angiotensin receptor blocker, n (%) | 20 (38.5) | 13 (39.4) | 7 (36.8) | – | – |
| Thiazide diuretics, n (%) | 17 (32.7) | 8 (24.2) | 9 (47.4) | – | – |
| Loop diuretic, n (%) | 28 (53.8) | 23 (69.7) | 5 (26.3) | – | – |
| Mineralocorticoid receptor antagonist, n (%) | 16 (30.8) | 12 (36.4) | 4 (21.1) | – | – |
| Statin, n (%) | 36 (69.2) | 23 (69.7) | 13 (68.4) | – | |
| ASA, n (%) | 39 (75.0) | 24 (72.7) | 15 (78.9) | ||
| Device therapy: | |||||
| Pacemaker, n (%) | 6 (11.5) | 4 (12.1) | 2 (10.5) | – | – |
| ICD, n (%) | 11 (21.2) | 10 (30.3) | 1 (5.3) | – | – |
| CRT-(D), n (%) | 15 (28.8) | 11 (33.3) | 4 (21.1) | – | – |
LVEF – left ventricular ejection fraction, LAD – left atrial diameter, LVEDD – left ventricular end-diastolic diameter, IVSDd – interventricular septal diameter in diastole, E-wave – early-wave, DT – deceleration time, IVRT – isovolumic relaxation time, ACE – angiotensin converting enzyme, ASA – acetylsalicylic acid, ICD – internal cardioverter-defibrillator, CRT-(D) – cardiac resynchronization therapy (-defibrillator).
Echocardiographic parameters are given in Table II. In the HF group mean LVEF was 42.0 ±14.8%. In the HFrEF subgroup (mean LVEF: 31.6 ±7.2%), still evidence of diastolic dysfunction was found in 87.9%. In the HFpEF subgroup (mean LVEF: 58.2 ±5.3%) mean LAD was 43.8 ±2.7 mm, where evidence of diastolic dysfunction was diagnosed in all patients.
The reference population consisted of 20 (60% male) healthy individuals. Mean LVEF was 61.4 ±4.7%, and mean LAD was 32.4 ±2.9 mm.
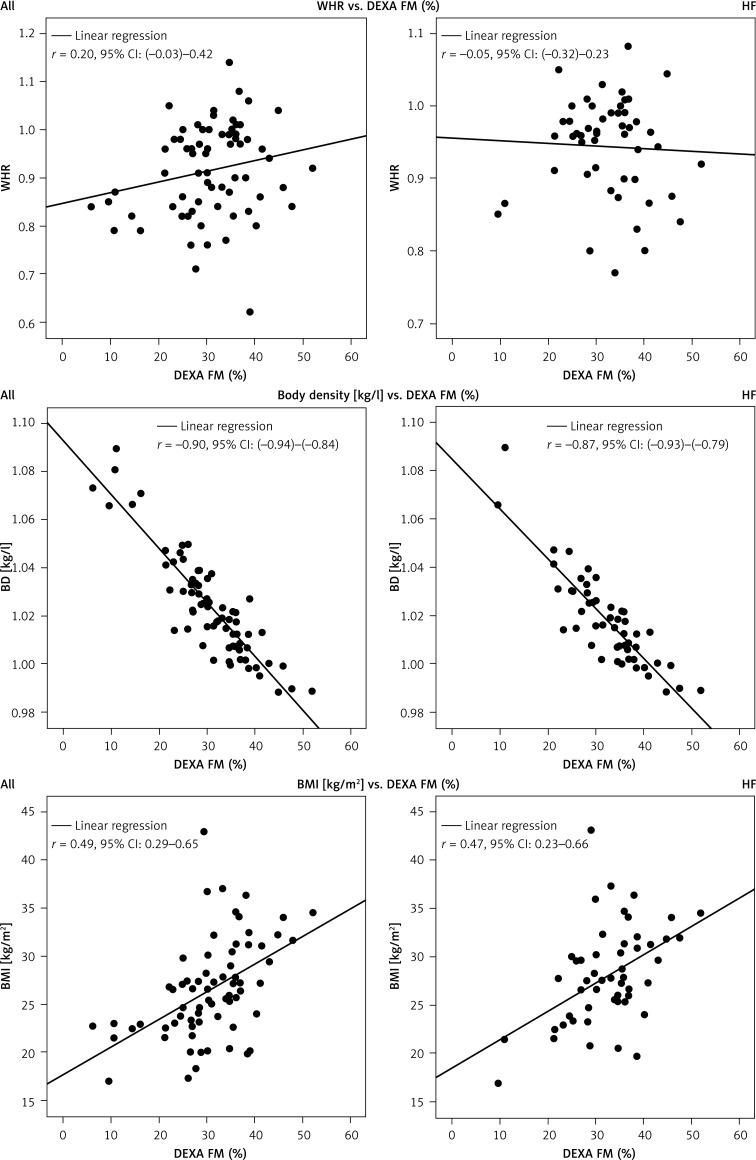
Estimating FM (%) by anthropometric indices
Linear regressions of anthropometric surrogates for obesity, such as BMI and WHR, and measured FM in DXA-Scan are presented in Figure 1. For all subjects, Pearson’s correlation for FM (%) from DXA-Scan and WHR was r = 0.20 (95% CI: (–0.03)–0.42), from DXA and BMI r = 0.49 (95% CI: 0.29–0.65). Body density was obtained by ADP; Pearson’s correlation was r = –0.90 (95% CI: (–0.94)–(–0.84)) for DXA-FM and BD. In the general HF population, Pearson’s correlation for FM (%) from DXA-Scan and WHR was r = –0.05 (95% CI: (–0.32)–0.23), for BMI r = 0.47 (95% CI: 0.23–0.669), and r = –0.87 (95% CI: (–0.93)–(–0.87)) for BD.
Figure 1.
Estimating FM by anthropometric indices. Linear regression of FM (%) by dual energy X-ray absorptiometry (DEXA) to BMI, WHR and BD (ADP). Left: All subjects. Right: HF population
BMI – body mass index, WHR – waist to hip ratio, BD – body density, r – Pearson’s correlation coefficient, CI – confidence interval.
An inverse relationship was found between FM (%) and body density [kg/l].
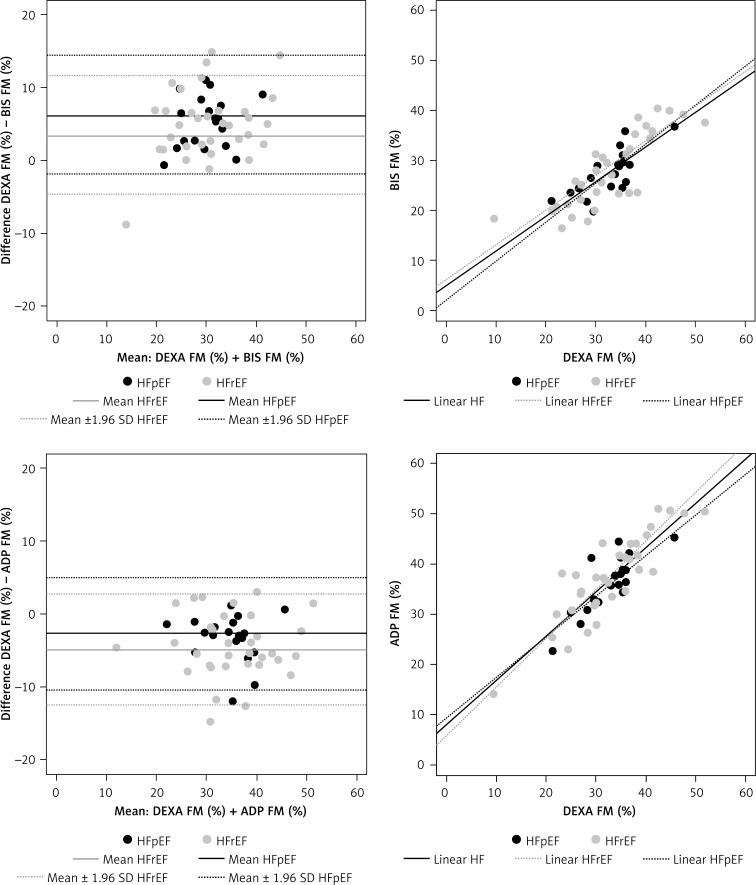
Measuring FM (%) by DXA, ADP, and BIS
Lin’s concordance correlation coefficients (CCC) and mean of differences between (xD) measured DXA FM (%) and ADP FM (%) results as well as measured DXA FM (%) and BIS FM (%) results, analogous to Bland-Altman (±1.96 SD), are given in Figure 2 and Table III. In HFrEF, Lin’s concordance correlation coefficient of DXA-FM with ADP-FM was 0.76 (95% CI: 0.59–0.86) and 0.79 (95% CI: 0.61–0.89) for BIS-FM. In healthy controls, Lin’s concordance correlation coefficient of DXA-FM with ADP-FM was 0.91 (95% CI: 0.81–0.96) and 0.71 (95% CI: 0.45–0.86) for BIS-FM.
Figure 2.
Bland-Altman analysis for FM (%) measurements ADP/DEXA and BIS/DEXA in HF
HFpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction, SD – standard deviation.
Table III.
Concordance analysis for FM (%) and FFM [kg] measurements ADP/DXA and BIS/DXA
| Variable | HF (n = 52) | HFrEF (n = 33) | HFpEF (n = 19) | REF (n = 20) | All (n = 72) |
|---|---|---|---|---|---|
| FM (%) from ADP vs. DXA: | |||||
| CCC | 0.76 | 0.76 | 0.74 | 0.91 | 0.86 |
| 95% CI | 0.64–0.85 | 0.59–0.86 | 0.47–0.88 | 0.81–0.96 | 0.79–0.90 |
| R | 0.87 | 0.89 | 0.80 | 0.92 | 0.89 |
| xD ±1.96 SD | –3.92 ±8.08 | –4.70 ±7.74 | –2.68 ±8.21 | 0.1 ±7.65 | –2.71 ±8.08 |
| Cb | 0.91 | 0.89 | 0.91 | 1.0 | 0.95 |
| FFM [kg] from ADP vs. DXA: | |||||
| CCC | 0.93 | 0.92 | 0.93 | 0.97 | 0.95 |
| 95% CI | 0.88–0.96 | 0.85–0.96 | 0.84–0.97 | 0.92–0.99 | 0.91–0.97 |
| R | 0.94 | 0.94 | 0.94 | 0.98 | 0.95 |
| xD ±1.96 SD | 1.30 ±7.30 | 1.60 ±6.20 | 0.70 ±9.10 | –1.8 ±5.80 | 0.40 ±7.50 |
| Cb | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 |
| FM (%) from BIS vs. DXA: | |||||
| CCC | 0.69 | 0.79 | 0.53 | 0.71 | 0.74 |
| 95% CI | 0.54–0.80 | 0.61–0.89 | 0.24–0.73 | 0.45–0.86 | 0.63–0.82 |
| R | 0.81 | 0.91 | 0.80 | 0.83 | 0.88 |
| xD ±1.96 SD | 4.82 ±8.26 | 3.59 ±7.64 | 6.51 ±8.15 | 4.47 ±9.30 | 4.71 ±8.54 |
| Cb | 0.79 | 0.86 | 0.72 | 0.85 | 0.82 |
| FFM [kg] from BIS vs. DXA: | |||||
| CCC | 0.73 | 0.59 | 0.82 | 0.91 | 0.79 |
| 95% CI | 0.60–0.82 | 0.38–0.75 | 0.66–0.91 | 0.80–0.96 | 0.71–0.86 |
| R | 0.89 | 0.79 | 0.97 | 0.97 | 0.92 |
| xD ±1.96 SD | –7.2 ±10.2 | –6.90 ±12.10 | –7.70 ±6.60 | –5.20 ±6.90 | –6.60 ±9.50 |
| Cb | 0.82 | 0.75 | 0.85 | 0.93 | 0.86 |
CCC – Lin’s concordance correlation coefficient, CI – confidence interval, R – Pearson’s correlation, xD – mean of differences between DXA FM (%)/FFM [kg] and ADP FM (%)/FFM [kg] and BIS FM (%)/FFM [kg] respectively (analogous to Bland-Altman), Cb – bias correction factor.
Measuring FFM [kg] by DXA, ADP, and BIS
Lin’s concordance correlation coefficients (CCC) and mean of differences between (xD) measured DXA FFM [kg] and ADP FFM [kg] results as well as measured DXA FFM [kg] and BIS FFM [%kg] results, analogous to Bland-Altman (±1.96 SD), are given in Table III.
Discussion
In this study we found the commonly employed anthropometric surrogates such as BMI and WHR to allow for an only vague estimation of FM in HF patients. We also demonstrated that body density as well as BIS show promise for assessing FM in these patients.
The present study enrolled a well-characterized, ambulatory, symptomatic cohort of HF patients with reduced or preserved ejection fraction on a guideline recommended heart failure therapy [3, 37].
In this work, we compared simple anthropometric measures such as BMI and WHR with an imaging-based approach by DXA for the estimation of FM. The anthropometric measures have been previously found to be independent predictors of the development [20, 21] and clinical outcome [38] of HF patients. They are straightforward to obtain and considered essential surrogates of adiposity by nearly all clinicians. However, we found the correlation of FM (%) with BMI and WHR to be far from satisfactory in our population (Figure 1). This suggests that the most commonly employed methods to characterize our patients’ risk and assess obesity in clinical routine may in fact fail as suitable surrogates for FM%. As previously suspected [22, 23], this may reflect the intrinsic limitations of BMI to differentiate between adipose tissue and lean mass. However, means to discriminate between these compartments are needed in all HF patients at risk for BC disorders such as cachexia, sarcopenia or obesity.
Especially under longitudinal follow-up in a HF clinic, when interpreting clinical trial data, or scrutinizing research questions, BMI may not necessarily be a suitable surrogate for FM estimation. Interestingly, physical density or body density, calculated from body mass and body volume, which can be measured by ADP, provided convincing results when evaluating percent FM in the present study. This obtained body volume index may be worthwhile to assess as a more accurate surrogate parameter in the future. Clinically available and feasible densitometry approaches, such as ADP, already allow for such assessments. ADP has previously shown to assess BD reliably in large, heterogeneous samples [39]. ADP does not require exposure to radiation, can be obtained within a few minutes and takes into account patients’ individual TGV. Infrastructural costs of the ADP system used are reasonably lower than costs of a DXA scan system, which consequently also reduces costs for the individual examination. While patient compliance regarding the size of the ADP test chamber or claustrophobia has been previously described, we did not observe any problem with this issue.
When BC was calculated from BD via Siri’s method [28], FM estimation results became less intriguing, yet still reasonable. On one hand this can partially be explained by the fairly young and relatively healthy control population Siri’s equation is based on, and on the other hand it is partly due to the impaired body fluid status and consequently altered tissue density in HF patients. Similarly, the currently used double-indirect pathway to derive FM (%) from total body water (TBW), extracellular and intracellular water (ECW/ICW) (which had been calculated from bio-impedance measurement using Cole modelling [40] and the Hanai mixture theory [30]) is prone to errors in HF patients. Impaired body fluid distribution and hence the validity of bioelectrical impedance models in clinical populations have already been seriously debated [32, 41, 42]. Yet, valuable tutorials for body composition assessment tools that try to account for fluid disturbances have recently become available [43].
In our population, BIS achieved a clinically acceptable accuracy of FM estimation in the subgroup of HFrEF (Table III), with less pronounced results regarding FM (%) estimation by BIS in the subgroup of HFpEF (HFrEF vs. HFpEF, p < 0.05). In the HFrEF subgroup, a comparable observation for FM (%) estimation by ADP/Siri’s was observed. Our results for BIS-FM in the general HF population and HFrEF are comparable not only to the enrolled reference group, but also to previously reported results for FM estimation by DXA and BIS in HF patients [44]. These findings are further supported by the respective 95% confidence interval reached. Poorer results from regression analysis, particularly FM estimation by BIS in the HFpEF subgroup, may in fact be linked to differences in body fluid imbalances in the respective subgroup, which could not be clinically objectified – these issues certainly call for further elucidation in the future. The low costs, portability and easy availability of BIS make the approach an attractive tool for BC assessments in a HF clinic. For this setting, it is especially noteworthy that we did not encounter any problems when using BIS in HF patients receiving device therapy.
As previously mentioned, impaired body fluid balance may be a striking problem for BC assessments in HF. Not only ECW/ICW water ratio, but also TBW (data not shown) are prone to clinical congestion, oedema status and diuretic therapy. The measurements of FFM or lean tissue mass in DXA (but also in ADP, BIS and anthropometry) can be complicated by oedema and impaired body fluid distribution [24]. Oedema can result in a false-high estimation of FFM that can indirectly lead to a false estimation of FM. In this analysis, we focused on hydrophobic FM, where water/fluid accumulation is generally thought to be negligible, but may still play a relevant role in obscuring body weight. Although to our knowledge currently not available for HF patients, population-specific BIS equations can incorporate and correct for the impaired body fluid distribution by distinguishing excess fluid from the hydration of major body tissues [45]. An approach of euvolaemia and over-hydration can allow for BC assessments that take into account fluid status and tissue mass in a two-compartment model. This is a clear advantage over whole-body absorptiometry and densitometry technologies, which are based on constant tissue densities or absorptions and intrinsically limit DXA scanning and Siri’s method to correct for oedema. Hence a DXA assessment, i.e. of appendicular muscle mass in the upper limbs, that is less prone to oedema, has been found a valuable modification of common DXA-Scan regimes in this population [11]. Alternatively, segmental BIS approaches might show promise for compensating errors and inconsistencies of whole-body measurements, when the fluid status and fluid distribution is altered [41]. Such approaches have been shown to represent valuable alternatives for determining segmental muscle volume compared to MRI imaging [46].
Due to its prognostic relevance, we focused on the assessment of FM percentage, but conversely its mirror image FFM was also studied in this two-compartment approach. FFM and lean-tissue mass show a strong correlation, which is of interest when assessing skeletal muscle mass [47]. Assessing absolute FFM by ADP proves convincing, with good CCC results and a narrow limit of agreement. BIS achieved clinically acceptable accuracy for FFM estimation in the subgroup of HFpEF (Table III). In the subgroup of HFrEF absolute FFM estimation by BIS showed poor results. We believe that estimating FM and FFM in HF patients and approaching the systemic disease by a two-compartment approach may (1) contribute to nutritional optimization [48], (2) be applicable to adjusted exercise training interventions, and (3) allow for significant clinical benefit in terms of body size and composition, laboratory parameters, and quality of life, as described previously [49].
In this cohort, a young and healthy control population was included as a reference sample. We did not intend to directly compare patients and healthy subjects, but rather confirm the methodological approach in this study’s setting. Accordingly, HF patients and healthy control subjects were not matched for age, weight, BMI, or FM and show variation in baseline demographic and clinical characteristics. Our findings in this healthy reference group, especially regarding the CCC and Pearson’s between FM (%) derived from ADP and DXA, are in line with the literature [50, 51], and indicate the validity of our study set up.
Considering the non-invasiveness and efficient and easy to use employment of the BC assessments performed in this study, future clinical trials and routine care could benefit from including these approaches and provide valuable insight into BC. Although the indirect measurements employed in this study warrant a cautious interpretation of our findings, we want to encourage future research to focus on the respective BC compartment of interest, rather than on mere anthropometric surrogates alone. After all, systematic FM estimation may prove indispensible for our patients.
It is argued by some clinicians that causality between fat mass, obesity and morbid conditions has not been established. However, since this is beyond the reach of a methodological pilot study, a closer examination of the role of FM and determining its mass, percentage and function is necessary in the future. Furthermore, it is noteworthy that indirect measurement approaches, especially the double-indirect approach, i.e. by BIS (as described above), may bear fundamental difficulties that contribute to impaired data reproducibility and validity. Estimating body composition in HF patients with fluid disturbances is widely debated, and assessment of body composition in HF patients remains controversial. Even in stable, ambulatory patients, cautious data interpretation is warranted and might require specific algorithms for the estimation of BC from BIS and ADP in HF. Large, representative sample sizes should be considered in the future to reveal the potential of the BC techniques assessed here.
In conclusion, detailed BC assessments in a HF population are of significant value for the clinical setting, as the data depth of common anthropometric surrogates such as BMI and WHR is limited in these patients. Especially in comparison to these established anthropometric indices, body density appears to be a useful surrogate for FM. ADP was found to be capable of estimating FM (%) and convincing for FFM [kg] in HF patients. BIS showed mixed, but especially for the estimation of FM (%) in HFrEF and for FFM [kg] in HFpEF patients, acceptable results. Estimation of FM (%) in HFpEF and of FFM [kg] in HFrEF was less convincing. We encourage selection of a suitable method for BC assessment according to the compartment of interest in the HF population.
Acknowledgments
Tobias Daniel Trippel and Julian Lenk contributed equally to the paper and share first authorship. Alexander Stahn and Hans-Dirk Duengen contributed equally to the paper and share senior authorship.
This manuscript complies with the ethical guidelines for authorship and publishing in the Archives of Medical Science Atherosclerotic Diseases. We wish to express our gratitude towards all patients and research personnel for their participation in this trial.
The research leading to these results has partly been funded from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 241558 (SICA-HF) [25] and an unrestricted research grant from Novartis Germany (Novartis Deutschland GmbH, Nürnberg, Germany).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Doehner W, Anker SD. Cardiac cachexia in early literature: a review of research prior to Medline. Int J Cardiol. 2002;85:7–14. doi: 10.1016/s0167-5273(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Mosterd A, Wood DA, et al. The epidemiology of heart failure. Eur Heart J. 1997;18:208–2. doi: 10.1093/oxfordjournals.eurheartj.a015223. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Hart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–63. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 5.Christensen HM, Kistorp C, Schou M, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626–34. doi: 10.1007/s12020-012-9836-3. [DOI] [PubMed] [Google Scholar]
- 6.Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 7.Anker SD, Ponikowski P, Varnes S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–2. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 8.von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers – update 2014. J Cachex Sarcopenia Muscle. 2014;4:261–3. doi: 10.1007/s13539-014-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morley JE, Anker SD. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology – update 2014. J Cachex Sarcopenia Muscle. 2014;4:253–9. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Haehling S, Steinbeck L, Doehner W, et al. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol. 2013;45:2257–65. doi: 10.1016/j.biocel.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 11.Fülster S, Tacke M, Sandek A, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF) Eur Heart J. 2013;34:512–9. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 12.Christensen HM, Kistorp C, Schou M, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626–34. doi: 10.1007/s12020-012-9836-3. [DOI] [PubMed] [Google Scholar]
- 13.Lang T, Streeper T, Cawthon P, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futter FE, Cleland JGF, Clark AL. Body mass indices and outcome in patients with chronic heart failure. Eur J Heart Fail. 2011;12:207–13. doi: 10.1093/eurjhf/hfq218. [DOI] [PubMed] [Google Scholar]
- 15.Guptha PP, Adigopula S, Fonarow GG, et al. Patients with higher fat mass have longer survival in heart failure. J Card Fail. 2014;20:S16. [Google Scholar]
- 16.Shah R, Gayat E, Januzzi JL, Jr, et al. (Global Research on Acute Conditions Team) Network. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–85. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 17.Khan RS, Kato TS, Chokshi A, et al. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: correction after ventricular assist device implantation. Circ Heart Fail. 2012;5:340–8. doi: 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavie CJ, Milani RV, Ventura HO. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “Obesity Paradox”. Mayo Clin Proc. 2010;85:605–8. doi: 10.4065/mcp.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–9. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 21.Loehr LR, Rosamond WD, Poole C, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85:609–17. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trippel T, Stahn A, Tscholl V, et al. Seizing impaired body fluid distribution in heart failure – an application for body impedance spectroscopy? Eur J Heart Fail. 2013;(Suppl. 1):S286. [Google Scholar]
- 25.Von Haehling S, Lainscak M, Doehner W, et al. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) J Cachexia Sarcopenia Muscle. 2010;1:187–94. doi: 10.1007/s13539-010-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hull H, He Q, Thornton J, et al. iDXA, Prrodigy, and DPXL dual-energy x-ray absorptiometry whole-body scans: a cross-calibration study. J Clin Densitom. 2009;12:95–102. doi: 10.1016/j.jocd.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCrory MA, Molé PA, Gomez TD, et al. Body composition by air-displacement plethysmography by using predicted and measured thoracic gas volumes. J Appl Physiol. 1998;84:1475–9. doi: 10.1152/jappl.1998.84.4.1475. [DOI] [PubMed] [Google Scholar]
- 28.Siri WE. Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition. 1995;9:480–91. discussion 480, 492. [PubMed] [Google Scholar]
- 29.Stahn A, Terblanche E, Gunga H.C. Use of bioelectrical impedance: general overview and principles. In: Preedy VR, editor. Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. New York: Springer; 2012. pp. 49–90. [Google Scholar]
- 30.Hanai T. Electrical properties of emulsions. In: Sherman DH, editor. Emulsion Science. London: Academic; 1968. pp. 354–477. [Google Scholar]
- 31.Matthie JR. Second generation mixture theory equation for estimating intracellular water using bioimpedance spectroscopy. J Appl Physiol. 2005;99:780–1. doi: 10.1152/japplphysiol.00145.2005. [DOI] [PubMed] [Google Scholar]
- 32.De LA, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol. 1997;82:1542–58. doi: 10.1152/jappl.1997.82.5.1542. [DOI] [PubMed] [Google Scholar]
- 33.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–33. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 34.Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. 2008;30:1257–69. doi: 10.1016/j.medengphy.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8476:307–10. [PubMed] [Google Scholar]
- 37.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 38.Futter FE, Cleland JGF, Clark AL. Body mass indices and outcome in patients with chronic heart failure. Eur J Heart Fail. 2011;12:207–13. doi: 10.1093/eurjhf/hfq218. [DOI] [PubMed] [Google Scholar]
- 39.Noreen EE, Lemon PW. Reliability of air displacement plethysmography in a large, heterogeneous sample. Med Sci Sports Exerc. 2006;38:1505–9. doi: 10.1249/01.mss.0000228950.60097.01. [DOI] [PubMed] [Google Scholar]
- 40.Cole KS. Dispersion and absorption in dielectrics. I. Alternating current characteristics. J Chem Phys. 1941;9:341–51. [Google Scholar]
- 41.Stahn A, Terblanche E, Gunga HC. Selected applications of bioelectrical impedance analysis: body fluids, blood volume, cell and fat mass. In: Preedy VR, editor. The Handbook of Anthropometry: Physical Measures of Human Form in Health and Disease. New York: Springer; 2012. pp. 415–40. [Google Scholar]
- 42.Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract. 2004;19:433–46. doi: 10.1177/0115426504019005433. [DOI] [PubMed] [Google Scholar]
- 43.Earthman CP. Body composition tools for assessment of adult malnutrition at the Bedside – a tutorial on research considerations and clinical applications. J Parenter Enteral Nutr. 2015;39:787–822. doi: 10.1177/0148607115595227. [DOI] [PubMed] [Google Scholar]
- 44.Oreopoulos A, Kalantar-Zadeh K, Padwal RS, et al. Comparison of direct body composition assessment methods in patients with chronic heart failure. J Card Fail. 2010;16:867–72. doi: 10.1016/j.cardfail.2010.06.416. [DOI] [PubMed] [Google Scholar]
- 45.Chamney PW, Wabel P, Moissl UM, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–9. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 46.Stahn A, Terblanche E, Strobel G. Modeling upper and lower limb muscle volume by bioelectrical impedance analysis. J Appl Physiol. 2007;103:1428–35. doi: 10.1152/japplphysiol.01163.2006. [DOI] [PubMed] [Google Scholar]
- 47.Heymsfield SB, Adamek M, Gonzalez MC, et al. Assessing skeletal muscle mass: historical overview and state of the art. J Cachex Sarcopenia Muscle. 2014;1:9–18. doi: 10.1007/s13539-014-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trippel TD, Anker SD, von Haehling S. The role of micronutrients and macronutrients in patients hospitalized for heart failure. Heart Failure Clin. 2013;9:345–57. doi: 10.1016/j.hfc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Rozentryt P, von Haehling S, Lainscak M, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1:35–42. doi: 10.1007/s13539-010-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ball SD, Altena TS. Comparison of the Bod Pod and dual energy x-ray absorptiometry in men. Physiol Meas. 2004;25:671–8. doi: 10.1088/0967-3334/25/3/007. [DOI] [PubMed] [Google Scholar]
- 51.Ballard TP, Fafara KM, Vukovich MD. Comparison of Bod Pod and DXA in femal collegiate athletes. Med Sci Sports Exerc. 2004;36:731–5. doi: 10.1249/01.mss.0000121943.02489.2b. [DOI] [PubMed] [Google Scholar]