Abstract
Introduction
Alzheimer's disease (AD) is defined by the progressive accumulation of amyloid plaques and neurofibrillary tangles in the brain which precedes cognitive decline by years.
Methods
Using amyloid biomarkers, chemical modeling, mouse behavioral models, and drug development techniques, we investigate the properties of NGP 555, a clinical-stage γ-secretase modulator.
Results
NGP 555 shifts amyloid peptide production to the smaller, nonaggregating forms of amyloid. Our preclinical studies show beneficial effects on amyloid biomarkers, pathology, and cognition. NGP 555 has successfully completed chemistry, pharmacology, toxicity, metabolism, and safety studies.
Discussion
Abundant data support Aβ42 as a target for prophylactic or early-stage intervention therapies in AD. The γ-secretase modulator, NGP 555 is being actively developed in human clinical trials for the prevention of Alzheimer's disease with the overall aim to achieve an appropriate balance of potency/efficacy on reducing the toxic forms of amyloid versus safety.
Keywords: Alzheimer's, Amyloid, Modulator, Biomarkers, Pathology, Prevention, Cognition, Cerebrospinal fluid, Dementia, Gamma-secretase
1. Introduction
Alzheimer's disease (AD) is a progressive and fatal brain disease, currently afflicting as many as 5.4 million Americans and one in eight people over age 65 years. AD will become an even greater public health issue with the aging of the baby boom population. It is estimated that by 2050, 11 to 16 million Americans will suffer from AD dementia [1], [2]. Therapeutic interventions that can effectively treat or prevent AD are urgently needed. AD is a neurodegenerative disease characterized by two distinct pathologies: (1) neurofibrillary tangles, comprised mostly of hyperphosphorylated tau, and (2) amyloid plaques, consisting primarily of amyloid β-peptides (Aβ) [3], [4], [5]. Recent work in autosomal dominantly inherited AD patients indicates that pathologic alterations in the brain and cerebrospinal fluid are evident approximately 10–20 years before dementia can be detected [6], [7]. Notably, several biomarker changes occur in the years before dementia including early reduction in Aβ42 and later increase in tau concentrations in the cerebrospinal fluid (CSF), hippocampal and regional brain atrophy, and brain amyloid plaque deposits [8], [9], [10].
Aβ42 is derived from a larger protein, the amyloid precursor protein (APP) [11], by sequential cleavage steps requiring two enzymes. However, as the aging process occurs or under pressure of specific genetic mutations in APP or Presenilin, the accumulation of the toxic form of amyloid, Aβ42, is increased. Numerous genetic, biological, and clinical investigations suggest that accumulation of Aβ42 is likely one of the earliest factors in the pathogenesis of AD [12], [13], [14], [15], [16], [17].
In an effort to prevent the damaging effects of Aβ42, several lines of drug discovery have focused on inhibiting the formation of Aβ42. Many efforts have been based on the development of γ-secretase inhibitors (GSIs), BACE inhibitors, or the development of anti-Aβ antibodies. Other approaches include NSAID-like γ-secretase modulators (GSMs) and non-NSAID-like GSMs [18], [19], [20], [21], [22].
A number of amyloid therapies to date have failed in clinical studies. The reasons for these failures are that either these agents were tested in patients with disease that was too far advanced or that these agents showed mechanism-based toxicities; for example, GSIs, whose failure was partially attributed to Notch inhibition and APP-carboxy-terminal fragment accumulation [23], [24], [25]. Multiple BACE-1 inhibitors and anti-Aβ antibody trials are now underway in early patient populations, and those results may help to confirm the amyloid hypothesis.
GSMs identified to date can be categorized into three groups and have been reviewed extensively [22], [26], [27], [28], [29], [30]: (1) NSAID-derived GSMs with flurizan and follow-up molecules generally showing lack of potency in the clinic. It is notable that this class of modulators acts with a different mechanism of action (MOA) as compared to NGP 555 [22], [30], [31], (2) Naturally derived molecules with an unknown target such as Satori's GSM (SPI-1865) which showed preclinical renal toxicity (reported via press release, 2012), and (3) Heterocyclic GSMs such as E2012, E2212, and BMS 932,481—molecules which have a similar MOA to NGP 555 and show CNS or peripheral biomarker efficacy in the clinic. However, these molecules failed due to lack of an adequate safety margin/compound structure-based toxicities [21], [22], [32], [33].
NGP 555 has been cited as a first of type GSM (28, 34, US Patent # 7,244,739, 2004) and has a heterocyclic structure but does have several important distinctions that are described below in the results section that are thought to be the necessary improvements to render a safe and effective drug product. To date, no GSM has been adequately tested in the clinic to determine whether the GSM mechanism will translate preclinical findings on Aβ biomarker reductions (Aβ42 CSF levels and brain amyloid plaques) to prevention of cognitive decline with an adequate safety margin.
Herein, we describe our clinical stage small-molecule modulator of Aβ42 production, NGP 555.
2. Methods
2.1. In vitro cell-based assays
SH-SY5Y-APP cells, Tg2576 mixed brain cultures, or C57 mixed brain cultures were treated with various concentrations of NGP 555 or NGP 328, in triplicate wells, for 18 hours. Media was collected and analyzed for Aβ peptides using Meso Scale triplex ELISA (Aβ38 and Aβ42) as previously detailed in [34].
2.2. Aβ Meso Scale assay
The Aβ peptides were quantified using the triplex Meso Scale kit (catalog #K11141A-3 for the human Aβ sequence or K11141E-2 for the rat Aβ sequence) and Meso Scale Sector Imager 6000 for detection after the manufacturer's recommended protocol.
2.3. In vivo studies
All animal care protocols were followed in accordance with the Institutional Animal Care and Use Committee of the university or company at which the studies were conducted.
2.4. Brain and plasma Tg2576 studies
Tg2576 mice (∼3 months) were dosed at indicated dose levels with NGP 328 in 80% PEG/water once-daily for 3 days. Six hours following dosing on the last day, blood was collected from all mice via cardiac puncture under isofluorane anesthesia into heparinized tubes and centrifuged (10,000 × g for 10 minutes) to isolate plasma. Brain hemispheres used for biochemical analyses were extracted in 70% formic acid, neutralized with 2M Tris base, and the amount of Aβ was measured as previously detailed in [34].
2.5. CSF studies
Normal Sprague–Dawley male rats (250–300 g) were administered NGP 555 in 80% PEG orally or vehicle only, n = 10/group. Rats were dosed once-daily for a single-dose or 14 days of dosing. After the final dose, CSF samples were either collected at varying time points or a single time point post-last dose as indicated in Figure legends. Rats were deeply anesthetized with isoflurane, and CSF was collected from the cisterna magna. Samples were tested for Aβ levels using the Meso scale ELISA (K11141E-2 for the rat Aβ sequence).
2.6. Y-maze
Transgenic mice (Tg2576 line expressing the APP-Swe mutation) [25] and non-transgenic age-matched littermates (n = a minimum of 12/group) were treated with vehicle, NGP 555 (25 mg/kg) or semagacestat (a γ-secretase inhibitor at 25 mg/kg) once-daily for 30 consecutive days (starting at 5 months of age). Mice were allowed to lick vehicle or drugs orally in a cherry syrup mixture to avoid adverse effects from chronic oral gavaging, and experimenters confirmed that mice (housed alone) finished each dose. At 6 months, mice were assessed on the Y-maze behavior test. This test included a determination of spontaneous alternation behavior, a measure of spatial working memory, exploratory behavior, and responsiveness to novelty. This behavior test was chosen based on the deficits seen at this age range in the Tg2576 model, and the statistical power to reproduce these deficits with n = 12 per group as determined by previous studies (unpublished findings from Dr. Amanda Roberts at the Mouse Behavioral Assessment Core at Scripps Research Institute (La Jolla, CA). Each mouse received one 5-minute trial during which arm choices, and total numbers of arm entries were recorded. Spontaneous alternation, expressed as percentage, refers to the ratio of arm choices differing from the previous two choices, to the total number of arm entries.
2.7. Morris water maze
Transgenic Tg2576 mice were obtained from Taconic [35] and crossed with Reln fl/fl mice [36]. The Reln fl/fl; Tg2576 line was then crossed with B6.Cg-Tg(CAG-cre/Esr1)5Amc/J mice, referred to as CAG-Cre mice (obtained from Jackson Laboratories [37]). Animals were group-housed in a standard 12-h light cycle and fed ad libitum standard mouse chow. At two months, the resulting Reln fl/fl; Tg2576 mice with or without CAG-Cre (Cre+ and Cre−) were given daily intra-peritoneal injections with 135-mg/kg tamoxifen (Sigma) dissolved in sunflower oil for 5 days. The resulting reelin conditional knockout (cKO) and control mice were then aged for approximately 7 months. At that time, they were treated orally with NGP 555 in cherry syrup or cherry syrup vehicle as described above for 28 days before the start of the Morris Water Maze (MWM) and continued until the completion of behavioral testing. This behavior test was chosen based on the optimal deficits seen in this model.
2.8. Notch in vivo assays
Male and female Crl:CD(SD) rats, approximately 7–8 weeks, were administered vehicle or NGP 555 (150 mg/kg) via oral gavage daily for 14 consecutive days. The number of goblet cells in the jejunum and in the ileum was counted using Alcian blue/PAS stained sections from all animals in the control and 150 mg/kg/day groups. Digital images were captured from scanned slides of the jejunum and ileum using the Hamamatsu slide scanner and NDP software. Image-Pro Plus software was used to measure the length of 10 well-oriented, intact crypt-villus units for each tissue (jejunum and ileum) of each animal [38], and the goblet cells along the left side of the crypt-villus axis were enumerated [39].
3. Results
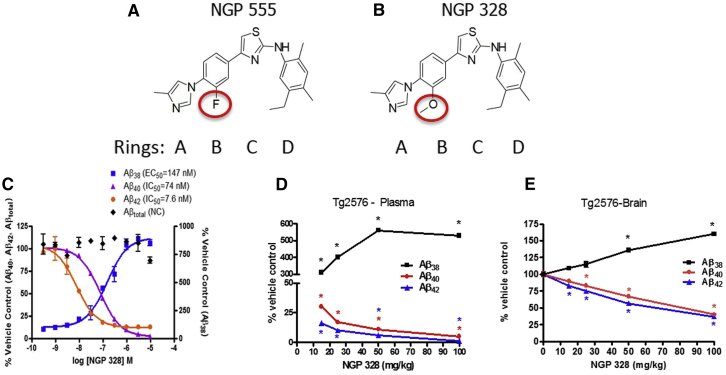
High throughput screening for lowering Aβ42 levels in cells without changes on Notch processing, followed by extensive medicinal chemistry efforts, yielded several lead candidates, including NGP 555 and NGP 328 [40]. Data in Fig. 1 show the chemical structures of two lead compounds, NGP 555 and NGP 328. Panel C shows the in vitro potency profiles for modulating Aβ peptides for NGP 328; the in vivo lowering of Aβ42 and raising of Aβ38 in the plasma (panel D) and brain (panel E) after dosing once daily for 3 days in Tg2576 mice. Similar data for NGP 555 were previously published [34]. Both compounds potently lower Aβ42 in cell cultures (9 nM) while increasing shorter forms of Aβ and have similar in vivo efficacy profiles and potency. However, subsequent rat toxicity studies revealed liver toxicity as a liability of NGP 328 at efficacious doses (10 mg/kg and above). NGP 555 in contrast had markedly higher exposure levels versus efficacious levels (∼20-fold higher) before liver enzymes were increased in the rat studies. In addition for NGP 555, beagle dog toxicity studies revealed no increase in liver enzymes up to and beyond the no-observable adverse effect level (NOAEL). These data led us to the hypothesis that the single differential chemical feature, the inclusion of a methoxy-group on ring B (Panel B) in comparision to the fluoro-group on NGP 555 (Panel A) was a contributing factor in hepatic toxicity. For this reason, NGP 555 was chosen as a clinical candidate.
Fig. 1.
NGP 328 reduce Aβ42 and Aβ40 increase Aβ38 in SH-SY5Y-APP cells and Tg2576 plasma and brain. SH-SY5Y-APP cells were treated with various concentrations of NGP 555 or NGP 328, in triplicate wells, for 18 hours. Media was collected and analyzed for Aβ peptides using Meso Scale triplex ELISA (Aβ38, Aβ40, and Aβ42) and total Aβ ELISA as described in Kounnas, et al. 2010. IC50 or EC50 values determined from nonlinear fitted curves are indicated. For Tg2576 studies, mice were dosed once-daily for 3 days. Six hours after the last dose, brain (then extracted with formic acid and neutralized with 2-M Tris base) and plasma was collected, and Aβ levels were quantified using a Meso Scale triplex kit which simultaneously measured Aβ38, Aβ40, and Aβ42 in a single well. Raw signals were converted to pg/well based on standard peptide curves, Data are shown as percent vehicle control. *P < .05 by one-tailed ANOVA versus vehicle control. Chemical structures for the compounds are shown for NGP 328 and NGP 555 with ring designations indicated. (A) NGP structure; (B) NGP 328 structure; (C) NGP 328 in vitro SH-SY5Y data; (D) NGP 328 Tg2576 plasma data; (E) NGP 328 Tg2576 brain data. NGP 555 data have been previously reported as compound 4 [35].
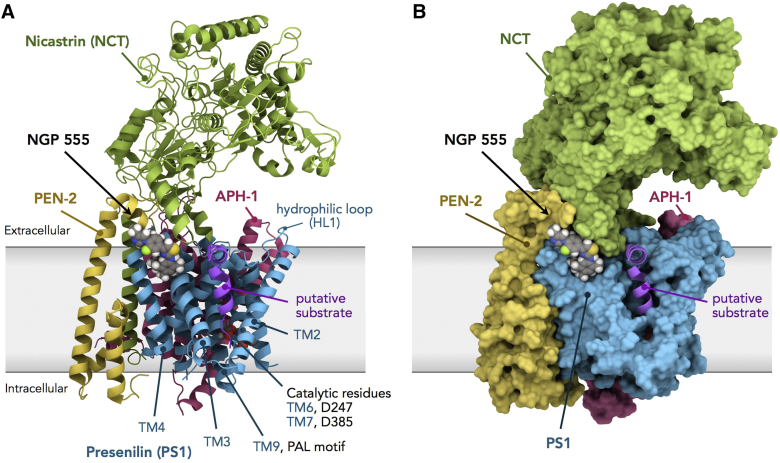
The scope of chemical structures synthesized and tested for brain bioavailability and Aβ42 levels has been presented [40]. The polar arylimidazole end of NGP 555 has been optimal for most series of GSMs reported [28], but most GSM candidates include a methoxy group in the B-ring for increased water solubility. We chose the fluoro group in the B-ring, which makes the imidazole less basic but retains potency and avoids binding at ancillary sites. The methoxy group confers water solubility, but less brain distribution and faster metabolism in the liver, as seen for NGP 328 compared to NGP 555. In support of our findings, liver toxicity is given as the reason for discontinuing several methoxy containing GSMs [28], [33]. A recent review of GSMs [29] noted that NGP 555 has significant potency but suffers from poor calculated “drug-like” properties. In contrast to these calculated properties, NGP 555 has very good brain penetration/absorption, distribution, metabolism, and excretion (ADME) properties; these features are based on in vivo results rather than in vitro or calculated properties and are the basis for NGP 555 being a preferred clinical candidate. Another potential advantage of NGP 555 is its optimal shape for docking at the PEN-2/PS1 site of gamma secretase [41]. This hypothetical docking site was anticipated by binding studies [34], [42] and supported by energy plots and docking studies revealed in Fig. 2. Low-energy rotation models show NGP 555 prefers to have four coplanar rings with A-B-C linear and C-D about 120° (hockey stick shape). The polar A ring docks at PS1/PEN-2, the B and C rings at the PS1/Nicastrin interface, and the lipophilic D ring in the hydrocarbon pocket (F175, F176, and F179) of PS1.
Fig. 2.
Model of the human γ-secretase complex and computational docking of NGP 555. Docking of NGP 555 to γ-secretase was carried out using AutoDock Vina. (A) Ribbon representation of γ-secretase. NGP 555 docks between TM domains 3 and 4 of PS1-NTF and near non-TM alpha helices of PEN-2 and Nicastrin (NCT). The PAL sequence motif, implicated in substrate recognition, resides in TM9, and catalytic residues D247 and D385, in TM6 and TM7, of PS1. (B) Surface representation of γ-secretase. The polar A ring of NGP 555 docks at the PS1/PEN-2 interface, the B and C rings at the PS-1/NCT interface, and the lipophilic D ring in the greasy pocket environment of F175, F176, and F179 of PS1.
Fig. 2 shows a model based on an atomic structure of human γ-secretase [43] and the result of computational docking of NGP 555 using AutoDock Vina, that is, the 1st ranked docking site (as the energy minimum) from repeated docking simulations. Based on these studies, NGP 555 is expected to dock between PEN-2 and transmembrane (TM) domains 3 and 4 of PS1-NTF. Activation of the PS1 catalytic site (Fig. 2A; D247 and D385 catalytic residues residing in TM6 and TM7, respectively) depends on the binding of PEN-2 to TM4 of PS1-NTF, which has an allosteric effect on TM6 of PS1 [41]. In the model, the transmembrane helix of the putative substrate is in close proximity to Nicastrin where NGP 555 docks (Fig. 2B). The alkyl pattern of the D-ring is optimal for this docking [40]. Thus, NGP 555 may modulate γ-secretase by (a) affecting the interaction between PEN-2 and TM4 of PS1-NTF, thereby modulating catalytic activity through an allosteric effect on TM6 of PS1, and/or (b) affecting cleavage through changes in substrate conformation near Nicastrin or the substrate cavity [34], [44].
Previous data demonstrated that NGP 555 efficiently crosses the blood-brain barrier, has a brain:plasma ratio of 0.93 (in Tg2576 mice) and shows in vivo efficacy for lowering the biomarker Aβ42 in rodent studies for brain and plasma. Notably, NGP 555 (aka Compound 4) potently and selectively reduced Aβ42 in neurons derived from human pluripotent stem cells from patients carrying presenilin 1 mutations [45], [46].
Chronic administration of NGP 555 (milled into chow) to Tg2576 mice resulted in a significant reduction of amyloid plaques [34].
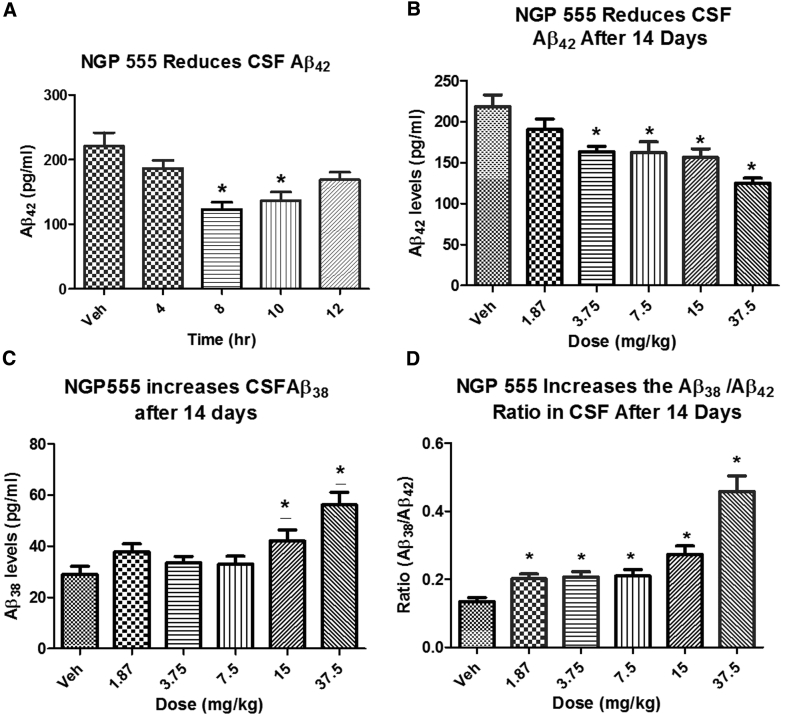
We evaluated the ability of oral NGP 555 to modulate concentrations of Aβ alloforms in CSF from male Sprague–Dawley rats, relative to vehicle-treated control rats. Both a time course at 15 mg/kg (single dose) and dose-response (1.75–37.5 mg/kg) given once-daily for 14 days were conducted.
Fig. 3 reveals NGP 555 significantly lowered Aβ42 in the CSF at time points from 8–10 hours post dose, panel B shows that reduction of Aβ42 levels was significant at 3.75 mg/kg and above, and panel C shows an increase in Aβ38 levels at 15 mg/kg and above. When combining the reduction of Aβ42 with an increase in Aβ38, NGP 555 was effective at raising CSF Aβ38/42 ratio at 1.87 mg/kg and above (panel D).
Fig. 3.
NGP 555 effectively lowers Aβ42 in CSF. (A) NGP 555 lowers Aβ42 in CSF. Normal Sprague–Dawley male rats (250–300 g) were administered a single dose orally with NGP 555 in 80% PEG (15 mg/kg) or vehicle only, n = 10/group. CSF was collected at the indicated time points. All samples were quantified using specific ELISAs to measure Aβ alloforms. *P < .05 by unpaired t test versus vehicle control. (B) NGP 555 lowers Aβ42 in CSF. Normal Sprague–Dawley male rats (250–300 g) were dosed once-daily for 14 days orally with NGP 555 in 80% PEG (0–37.5 mg/kg), n = 10/group. Eight hours later, CSF was collected. All samples were quantified using specific ELISAs to measure Aβ alloforms. *P < .05 by unpaired t test versus vehicle control. (C) NGP 555 raises Aβ38 in CSF. Normal Sprague–Dawley male rats (250–300 g) were dosed once-daily for 14 days orally with NGP 555 in 80% PEG (0–37.5 mg/kg), n = 10/group. Eight hours later, CSF was collected. All samples were quantified using specific ELISAs to measure Aβ alloforms. *P < .05 by unpaired t test versus vehicle control. (D) NGP 555 lowers Aβ42 and raises Aβ38 in CSF. Normal Sprague–Dawley male rats (250–300 g) were dosed once-daily for 14 days orally with NGP 555 in 80% PEG (37.5 mg/kg), n = 10/group. Eight hours later, CSF was collected. All samples were quantified using specific ELISAs to measure Aβ alloforms. *P < .05 by unpaired t test versus vehicle control.
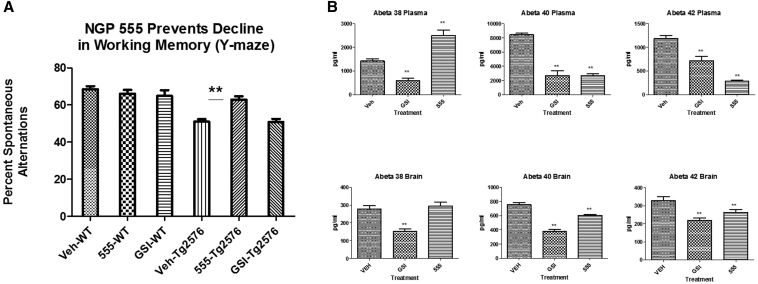
To examine whether chronic administration of NGP 555 (1 month of treatment) could prevent the cognitive deficits observed in Tg2576 mice at 6 months, mice non-Tg and Tg were treated orally once daily (25 mg/kg) for 1 month (from age 5 months to 6 months) then tested in the Y-maze cognition test.
Data for this study are shown in Fig. 4, Panel A. The results show that the Tg vehicle-treated mice showed a 25% decline in performance as compared to non-Tg vehicle-treated mice (P < .001). NGP 555-treated Tg mice showed a significant protection from decline with >65% less decline (P < .005) when comparing the differential of Tg to non-Tg vehicle-treated mice. A control γ-secretase inhibitor, semagacestat, did not demonstrate significant protection from decline as compared to the non-Tg treated vehicle. Fig. 4, Panel B confirms target engagement in the brain and plasma for both compounds. NGP 555 showed the expected profile, revealing statistically significant reductions of Aβ42 and Aβ40 in both brain and plasma while increasing Aβ38 in plasma. Aβ38 in the brain was modestly increased. Semagacestat showed significant reductions of all Aβ alloforms in both brain and plasma.
Fig. 4.
NGP 555 showed beneficial effects in the Y-maze behavioral test in a Tg2576 mouse model. Mice were treated orally once-daily for 1 month with vehicle (cherry syrup), NGP 555 (25 mg/kg), or semagacestat, gamma-secretase inhibitors (25 mg/kg). Tg2576 transgenic (Tg) or non-transgenic littermates (non-Tg) were dosed with each treatment arm. (A) Mice were assessed in the Y-maze (working memory test) with the experimenter blinded to genotype and treatment. Data were collected on the total arm choices and arm choice as compared to the previous choice. Data expressed as % spontaneous alteration (Mean +/− SEM), which is a measure of the ratio of arm choices differing from the previous two choices to the total number of arm entries, was plotted. Statistical analysis was performed using a 2-way ANOVA with genotype and drug as the factors (Graphpad, San Diego, CA). **P < .05 by 2-way ANOVA versus vehicle control. (B) Three hours after final dose, brain (then extracted with formic acid and neutralized with 2-M Tris base) and plasma were collected, and Aβ levels were quantified using a Meso Scale triplex kit which simultaneously measured Aβ38, Aβ40, and Aβ42 in a single well. Raw signals were converted to pg/well based on standard peptide curves, and the data then collated as pg/mL for each group. Data are shown as pg/mL. **P < .05 by one-tailed ANOVA versus vehicle control.
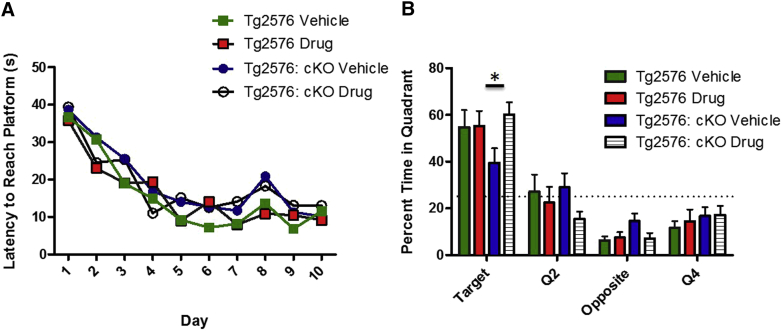
Because mice are remarkably resilient to Aβ accumulation, display little neuronal cell death and show cognitive impairment due to synaptic suppression only after exorbitantly high amyloid accumulation, NGP-555 was tested in a second mouse model, the reelin cKO mouse model, using another behavioral endpoint (Morris Water Maze). Reelin is highly expressed in the brain and is a ligand for ApoER2 and VLDLr, both members of the low-density lipoprotein receptor family. Mice-lacking reelin are exquisitely sensitive to the effect of Aβ both at a synaptic and behavioral level [36]; thus, we hypothesized that treatment with NGP-555 might rescue the learning deficit caused by the moderate amount of synapse suppressing Aβ42 accumulation that would occur in <1-year-old Tg2576 mice. 6–10-month-old Tg2576; reelin cKO and Tg2576 control mice were treated with NGP-555 or vehicle control daily for 28 days then trained on the Morris Water Maze. There was no significant difference in acquisition of the task (Fig. 5A), although a trend towards a deficit was observed in the vehicle-treated Tg2576; reelin cKO mice. As previously shown, the Tg2576; reelin cKO mice treated with vehicle were markedly impaired on the probe trial compared to control mice. This deficit was rescued by treatment with NGP 555 (Fig. 5B) (P < .05).
Fig. 5.
NGP 555 showed beneficial effects in the Morris Water Maze in the Tg2576; reelin conditional knockout model. Mice were treated orally once-daily for 28 days with vehicle (cherry syrup) or NGP 555 (25 mg/kg). They were treated again at the end of behavioral testing each day through the MWM. (n = 8 vehicle-treated Tg2576; n = 9 NGP 555-treated Tg2576; n = 13 vehicle-treated Tg2576; reelin cKO; n = 15 NGP 555-treated Tg2576; reelin cKO). Data represented as mean ± S.E.M. (A) Tg2576; reelin cKO and Tg2576 mice were trained on 4 trials per day over 10 days to find a hidden platform using cues on the wall. 2-way ANOVA revealed a significant effect of time (P < .001) but not of genotype (P > .05) on learning. (B) On day 11, the platform was removed and time spent in the target quadrant over 60 seconds was recorded. 2-way ANOVA revealed a significant effect of time (P < .001) and a significant interaction between time and genotype (P < .05) on learning. Post-hoc analysis revealed a significant difference between vehicle and NGP 555-treated Tg2576; reelin cKO mice in the target quadrant (*P < .05).
The safety, absorption, distribution, metabolism and excretion (ADME) properties of NGP 555 have been studied extensively. NGP 555 shows good oral bioavailability and is brain-penetrant with a brain:plasma ratio of ∼0.93 in mice. NGP 555 was assessed to be negative for cross-reactivity in a Cerep screen against receptors and enzymes and for its potential to inhibit hERG channels. Cardiopulmonary studies in conscious, ambulatory Beagle dogs showed no adverse effects up to 125 mg/kg on cardiovascular and pulmonary endpoints. GLP in vitro genotoxicity studies (bacterial mutagenicity and cytogenetic examination of chromosomal damage) as well as GLP in vivo testing of the clastogenic potential of NGP 555 in rat bone marrow showed negative results for the genotoxic potential of NGP 555. Effects on metabolism were studied, NGP 555 was largely devoid of CYP450 inhibition and induction, and the major metabolite was identified as a carboxylic acid analog of NGP 555.
Repeat-dose toxicology studies in rat (14 days) at 37.5, 75, and 150 mg/kg have established the dose-limiting effects are on liver parameters including liver enzymes, weights, and histology. Routine eye examinations did not reveal toxicity however longer term toxicology studies will be needed to adequately address the potential for optic concerns based on the toxicity observed by another structurally distinct GSM [32]. After 14 days of dosing in male rats, the AUC measured at the NOAEL (37.5 mg/kg) was 25,779 ng·h/mL. compared to the AUC measured at 3.75 mg/kg (the minimally effective dose for lowering CSF Aβ42) of 1092 ng·h/mL. These data indicate a large therapeutic window (>20-fold) between ability to lower CSF Aβ42 with 14 days of dosing versus the NOAEL in rats. Additionally, the potential for known Notch-related effects on the gastrointestinal (GI) tract were evaluated in tissues from rats treated for 14 days with NGP 555 at 150 mg/kg resulting in exposure levels exceeding 100,000 ng·h/mL. Table 1 shows the result of the histologic examination of GI tissue with no differences in goblet cell number and density between groups. This is a significant finding since Notch-related toxicity which manifests as goblet cell metaplasia is a key liability for first generation γ-secretase inhibitors [24].
Table 1.
NGP 555 does not affect the goblet cells in the ileum or jejunum in 150 mg/kg treated rats (14 days dosing). Goblet cells in the ileum or jejunum were counted using Alcian blue/PAS
| Dosage (mg/kg/day) | Males |
Females |
||
|---|---|---|---|---|
| 0 | 150 | 0 | 150 | |
| Mean ratio of Goblet cell number to Crypt-Villus axis length, ileum | ||||
| Mean Ratio | 0.071 | 0.066 | 0.067 | 0.068 |
| STD | 0.006 | 0.007 | 0.007 | 0.008 |
| Mean ratio of Goblet cell number to Crypt-Villus axis length, jejunum | ||||
| Mean Ratio | 0.066 | 0.064 | 0.068 | 0.072 |
| STD | 0.004 | 0.006 | 0.012 | 0.007 |
Abbreviation: STD, Standard deviation.
NOTE. Digital images were captured from scanned slides of the ileum using the Hamamatsu slide scanner and NDP software. Image-Pro plus software was used to measure the length of 10 well-oriented, intact crypt-villus units for each tissue of each animal and data were analyzed by Student t test.
A solid dosage formulation was developed to accomplish beagle dog toxicity studies. Repeat-dose Beagle dog toxicity studies (28 days) revealed lower food consumption and weight loss with reversible adrenal gland necrosis but without effects on the liver at 50 mg/kg and above.
In summary, NGP 555, a small molecule disease-modifying amyloid therapy, has emerged as a compelling candidate for AD treatment based on its different mechanistic profile, preclinical efficacy, safety, and ADME properties.
4. Discussion
NGP 555, a molecule aimed at preventative therapy for AD, has been successful in achieving good oral absorption, brain penetration, CNS activity, and specificity for a lipid-based membrane target preclinically [34].
NGP 555 acts by binding directly to the γ-secretase enzyme complex via Pen-2/PS1-NTFs without inhibiting ε-site proteolysis of amyloid precursor protein (APP), Notch, or E-cadherin. NGP 555 potently inhibits the production of Aβ42 and Aβ40 and causes a concomitant increase in the levels of Aβ38 and Aβ37. NGP 555 crosses the blood-brain barrier and has a brain:plasma ratio of 0.93 (in Tg2576 mice) [34] which demonstrates in vivo efficacy for lowering the biomarker Aβ42 in rodent studies for brain, cerebrospinal fluid (CSF), and plasma while raising Aβ38. Chronic administration (NGP 555 milled into chow) to Tg2576 mice resulted in a significant reduction of amyloid plaques [34]. Studies addressing the effects of chronic administration of NGP 555 on cognition in a transgenic mouse model demonstrated that NGP 555 was able to significantly prevent cognitive deficits in Y-maze performance (a measure of working and spatial memory) and in the Morris water maze (a measure of spatial learning and memory). Our data demonstrate that a relatively modest decrease in CSF Aβ42 (20%–40%) plus the increase in the shorter forms after acute or subchronic dosing in rodents translates into a robust reduction in AD-pathology [34]. NGP 555 has demonstrated protection from cognitive decline in two independent mouse studies using different memory and learning tasks.
The quest to find a GSM with the ideal balance of safety, potency, and efficacy in humans is ongoing. Some groups focus efforts on calculated drug-like properties or water solubility [47], [48], [49], whereas others have demonstrated a translation of empirical ADME properties across preclinical species to humans [50]. We are taking an empirical data-driven approach, using efficacy, Aβ biomarkers, ADME properties, and toxicology/safety profiles preclinically to drive our choice of clinical candidates. The translation of these results and success in the clinic will ultimately determine the best path forward for the GSM field.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and presented data. There are numerous descriptions of amyloid approaches and γ-secretase modulators, and they are clearly cited. The innovation with NGP 555 is the improvement in the molecule's properties including structure, efficacy, and safety margins.
-
2.
Interpretation: In characterizing NGP 555, a modulator of amyloid production, we describe our hypothesis that NGP 555 is a preferred molecule because of its chemical structure, proposed 3-D shape, and its ability to effectively (and selectively) target amyloid biomarkers and pathology while preventing cognitive decline preclinically.
-
3.
Future directions: The article provides key information on the preclinical findings and efficacy profile of NGP 555 and provides the rationale to conduct future clinical studies to test the amyloid hypothesis in humans with a γ-secretase modulator.
Acknowledgments
National Institute of Neurological Disease and Stroke (NINDS) supported this work through the Small Business Innovation Research (SBIR) grant # 1U44NS073133-01A1. J.H. and C.L.D. are supported by grants from the NIH (R37 HL63762 and R01 NS093382 to J.H.; F30 AG047799 to C.L.D.). J.H. was further supported by the Consortium for Frontotemporal Dementia Research and the Bright Focus Foundation. We thank Dr. Amanda Roberts at the Mouse Behavioral Assessment Core at Scripps Research Institute (La Jolla) for conducting the mouse behavioral Y-maze studies and Charisma Desai (UTSW) for assistance during part of the MWM studies. Tamara Terrones, Emily Boyle, Rebekah Hewitt, and Isaac Rocha (all UTSW) provided expert help with animal husbandry. Phuong Nguyen (formerly at TPTX) performed the Aβ ELISAs and pharmacology personnel at TPTX conducted Tg2576 dosing and tissue collection for NGP 328 studies.
Footnotes
W.T.C. and M.Z.K. are employees and shareholders of NeuroGenetic Pharmaceuticals, Inc.
References
- 1.Thies W., Bleile L., Alzheimer's Association 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Hebert L., Weuve J., Scherr P., Evans D. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer A., Stelzmann R., Schnitzlein H., Murtagh F. An English translation of Alzheimer's 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin Anat. 1995;90:429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 4.Kidd M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- 5.Terry R., Gonatas N., Weiss M. Ultrastructural studies in Alzheimer's presenile studies. Am J Pathol. 1964;44:269–287. [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman R., Xiong C., Benzinger T., Fagan A., Goate A., Fox N., Dominantly Inherited Alzheimer Network Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiman E., Quiroz Y., Fleisher A., Chen K., Velez-Pardo C., Jimenez-Del-Rio M. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11:1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw L., Vanderstichele H., Knapik-Czajka M., Clark C., Aisen P., Petersen R., Alzheimer's Disease Neuroimaging Initiative Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe C., Fagan A., Grant E., Hassenstab J., Moulder K., Maue Dreyfus D. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J., Korecka M., Toledo J., Trojanowski J., Shaw L. Clinical Utility and Analytical Challenges in Measurement of Cerebrospinal Fluid Amyloid-β1-42 and τ Proteins as Alzheimer Disease Biomarkers. Clin Chem. 2013;59:903–916. doi: 10.1373/clinchem.2013.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling Y., Morgan K., Kalsheker N. Amyloid precursor protein (APP) and the biology of proteolytic processing : Relevance to Alzheimer's disease. Int J Biochem Cell Biol. 2003;35:1505–1535. doi: 10.1016/s1357-2725(03)00133-x. [DOI] [PubMed] [Google Scholar]
- 12.Younkin S. The role of Abeta 42 in Alzheimer's disease. J Physiol Paris. 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanzi R., Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Hardy J., Selkoe D. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein S., Dupuis N., Lazo N., Wyttenbach T., Condron M., Bitan G. Amyloid-beta oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golde T., Petucell C., Lewis J. Targeting Aβ and tau in Alzheimer's disease, an early interim report. Exp Neurol. 2009;223:252–266. doi: 10.1016/j.expneurol.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen D. Changing the course of Alzheimer's disease: Anti-amyloid disease-modifying treatments of the horizon. J Clin Psychiatry. 2007;9:32–41. doi: 10.4088/pcc.v09n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evin G. γ-secretase modulators: Hopes and setbacks for the future of Alzheimer's treatment. Expert Rev Neurother. 2008;8:1611–1613. doi: 10.1586/14737175.8.11.1611. [DOI] [PubMed] [Google Scholar]
- 19.Tomita T. Secretase inhibitors and modulators for Alzheimer's disease treatment. Expert Rev Neurother. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- 20.Green R., Schneider L., Amato D., Beelen A., Wilcock G., Swabb E., Tarenflurbil Phase 3 Study Group Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;16:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y., Logovinsky V., Schuck E., Kaplow J., Chang M., Miyagawa T. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel γ-secretase modulator, E2212, in healthy human subjects. J Clin Pharmacol. 2014;54:528–536. doi: 10.1002/jcph.249. [DOI] [PubMed] [Google Scholar]
- 22.Crump C.J., Johnson D.S., Li Y.M. Development and mechanism of γ-secretase modulators for Alzheimer's disease. Biochemistry. 2013;52:3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamayev R., D'Adamio L. Inhibition of gamma-secretase worsens memory deficits in a genetically congruous mouse model of Danish dementia. Mol Neurodegener. 2012;7:1–7. doi: 10.1186/1750-1326-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milano J., McKay J., Dagenais C., Foster-Brown L., Pognan F., Gadient R. Modulation of Notch processing by γ-secretase inhibition causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 25.van Es J., van Gijn M., Riccio O., van den Born M., Vooijs M., Begthel H. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe M.S. γ-Secretase as a target for Alzheimer's disease. Adv Pharmacol. 2012;64:127–153. doi: 10.1016/B978-0-12-394816-8.00004-0. [DOI] [PubMed] [Google Scholar]
- 27.Pissarnitski D. Advances in γ-secretase modulation. Curr Opin Drug Discov Devel. 2007;10:392–402. [PubMed] [Google Scholar]
- 28.Gijsen J., Bischoff F. Secretase inhibitors and modulators as a disease modifying approach against Alzheimer's Disease. Annu Rep Med Chem. 2012;47:55–69. [Google Scholar]
- 29.Bursavich M., Harrison B., Blain J. Gamma Secretase Modulators, New Alzheimer's Drug on the Horizon? J Med Chem. 2016;59:7389–7409. doi: 10.1021/acs.jmedchem.5b01960. [DOI] [PubMed] [Google Scholar]
- 30.Borgegard T., Juréus A., Olsson F., Rosqvist S., Sabirsh A., Rotticci D. First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms. J Biol Chem. 2012;287:11810–11819. doi: 10.1074/jbc.M111.305227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kukar T., Ladd T., Bann M., Fraering P., Narlawar R., Maharvi G. Substrate-targeting γ-secretase modulators. Nature. 2008;453:925–930. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakano-Ito K., Fujikawa Y., Hihara T., Shinjo H., Kotani S., Suganuma A. E2012-Induced Cataract and Its Predictive Biomarker. Toxicol Sci. 2014;137:249–258. doi: 10.1093/toxsci/kft224. [DOI] [PubMed] [Google Scholar]
- 33.Soares H., Gasior M., Toyn J., Wang J., Hong Q., Berisha F. Gamma Secretase Modulator, BMS-932481, Modulates Aβ Peptides in the Plasma and CSF of Healthy Volunteers. J Pharmacol Exp Ther. 2016;358:138–150. doi: 10.1124/jpet.116.232256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kounnas M., Danks A., Cheng S., Tyree C., Ackerman E., Zhang X. Modulation of γ-secretase reduces β-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 36.Lane-Donovan C., Philips G., Wasser C., Durakoglugil M., Masuilis I., Upadhaya A. Reelin Protects against amyloid beta toxicity in vivo. Sci Signal. 2015;8:1–26. doi: 10.1126/scisignal.aaa6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi S., McMahon A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 38.Chang J., Chen S., Ma L., Jiang L., Chen J., Chang R. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito H., Satsukawa M., Arai E., Sugiyama K., Kiriyanma S., Morita T. Soluble fiber viscosity affects both goblet cell number and small intestine mucin secretion in rats. J Nutr. 2009;139:1640–1647. doi: 10.3945/jn.109.110171. [DOI] [PubMed] [Google Scholar]
- 40.Cheng S, Mao L, Comer D, Yu C, Pleynet D, Kounnas M, et al. 2-Aminothiazole derivatives as potent Gamma-secretase modulators. 240th ACS National Meeting, MEDI-1 2010.
- 41.Takeo K., Watanabe N., Tomita T., Iwatsubo T. Contribution of the γ-secretase subunits to the formation of catalytic pore of presenilin 1 protein. J Biol Chem. 2012;287:25834–25843. doi: 10.1074/jbc.M111.336347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozdnyakov N., Murrey H.E., Crump C.J., Pettersson M., Ballard T.E., Am Ende C.W. γ-Secretase modulator (GSM) photoaffinity probes reveal distinct allosteric binding sites on presenilin. J Biol Chem. 2013;288:9710–9720. doi: 10.1074/jbc.M112.398602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai X., Yan C., Yang G., Lu P., Ma D., Sun L. An atomic structure of human γ-secretase. Nature. 2015;525:212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bai X., Rajendra E., Yang G., Shi Y., Scheres S. Sampling the conformational space of the catalytic subunit of human γ-secretase. Elife. 2015;4 doi: 10.7554/eLife.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q., Waltz S., Woodruff G., Ouyang J., Israel M., Herrera C. Effect of Potent γ-secretase modulator in human neurons derived from multiple Presenilin 1-Induced pluripotent stem cell mutant carriers. JAMA Neurol. 2014;71:1481–1489. doi: 10.1001/jamaneurol.2014.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao F., Holtzman D. Human Neurons Derived From Induced Pluripotent Stem Cells as a New Platform for Preclinical Drug Screening and Development. JAMA Neurol. 2014;71:1475–1476. doi: 10.1001/jamaneurol.2014.2802. [DOI] [PubMed] [Google Scholar]
- 47.Gijsen H.J., Mercken M. γ-secretase modulators: Can we combine potency and safety? Int J Alzheimers Dis. 2012;2012:295207. doi: 10.1155/2012/295207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner S.L., Tanzi R.E., Mobley W.C., Galasko D. Potential use of γ-secretase modulators in the Treatment of Alzheimer Disease. Arch Neurol. 2012;69:1255–1258. doi: 10.1001/archneurol.2012.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rynearson K.D., Buckle R.N., Barnes K.D., Jason Herr R., Mayhew N.J., Paquette W.D. Design and Synthesis of Aminothiazole Modulators of the Gamma-secretase Enzyme. Bioorg Med Chem Lett. 2016;26:3928–3937. doi: 10.1016/j.bmcl.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 50.Toyn J.H., Boy K.M., Raybon J., Meredith J.E., Robertson A.S., Guss V. Robust Translation of γ-Secretase Modulator Pharmacology across Preclinical Species and Human Subjects. J Pharmacol Exp Ther. 2016;358:125–137. doi: 10.1124/jpet.116.232249. [DOI] [PMC free article] [PubMed] [Google Scholar]