Abstract
BACKGROUND
Primary effusion lymphoma (PEL) is a rare non-Hodgkin lymphoma subtype primarily seen in human immunodeficiency virus (HlV)-infected individuals with low CD4+ cell counts and elevated HIV viral loads. It is always associated with human herpesvirus type-8 (HHV-8) and in 80% of cases is also associated with Epstein Barr Virus (EBV). Less commonly, PEL presents in patients with advanced age and other conditions associated with altered immunity, including malignancy, liver cirrhosis, and immunosuppressive medications. It is a tumor of B-cell lineage; however, it shows a “null” phenotype, rarely expressing pan-B cell surface antigens. It does usually express CD45, CD30, CD38, CD138 and MUM1 and is characterized by lymphomatous effusions in body cavities but not lymphadenopathy. It is an aggressive lymphoma; average median survival time is less than a year. HHV-8-associated large B-cell Lymphoma (HHV-8-LBL) is a second variant of PEL that is both solid and extra-cavitary. It has immunoblastic and/or anaplastic morphological features, a distinct immuno-histochemical staining pattern, and may have a different clinical presentation than classic PEL.
METHODS
We describe the case of a 57-year-old HIV-infected man who presented with a slow growing and asymptomatic abdominal mass. An excisional biopsy showed malignant large cells with prominent cytoplasm that were positive for pan-B cell antigen CD20, HHV-8 and EBV, and negative for CD138, CD10, BCL-6, CD3 and CD30. Ki-67 labeling index was 90%. He was diagnosed with stage IIIA HHV-8-LBL, and he was treated with six cycles of R-EPOCH (rituximab, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) infusion chemotherapy. He remains in complete remission (CR) 12 months post-treatment. We also performed a Medline and Embase search to better understand the clinical findings of this patient and the unique attributes of HHV-8-LBL. Focusing our search on English language articles, we identified 83 cases of HHV-8-LBL without an effusion component. We compared this to 118 reported cases of classic PEL.
RESULTS
The median age of HHV-8-LBL patients was 41 years (range, 24–77) and 96% were HIV-associated vs. 41 years (range, 2686) and 96% HIV–association for patients with classic PEL. Fifty-one percent (31/61) of HHV-8-LBL patients had a pre-existing AIDS diagnosis, and 75% (47/63) were co-infected with EBV. In contrast, 72% (69/96) of classic PEL patients had a pre-existing AIDS diagnosis and 82% (40/49) were co-infected with EBV. The mean CD4+ count of HHV-8-LBL patients was 256 cell/uL (range, 18–1126) compared to 139 cell/uL (range, 2–557) for classic PEL patients. Median survival time for both groups was similar; 5.5 months for patients with HHV-8-LBL (range, 25 days – 25+ months) and 4 months (range, 2 days –113+ months) for those with classic PEL. More patients with HHV-8-LBL were alive at time of the followup (59% vs 18%). The percent of patients achieving a complete remission (CR) was 54% (30/56) and 36% (32/89) for HHV-8-LBL and classic PEL, respectively.
CONCLUSIONS
Our patient’s high CD4+ cell count, lack of pre-existing AIDS diagnosis and excellent response to chemotherapy highlights that HHV-8-LBL may have a distinct clinical picture and possibly a better response to chemotherapy than classic PEL. HHV-8-LBL should be included in the differential diagnosis of HIV patients with solid lesions. It is essential that patients’ CDC HIV clinical status and HIV viral load at time of diagnosis of PEL and HHV-8-LBL be reported and that clinical results include longer-term follow-up. Only then can a more complete clinical picture of this little appreciated and understood PEL variant be defined.
The estimated lifetime prevalence of non-Hodgkin’s Lymphoma (NHL) in people living with HIV/AIDS (PLWHA) is between 5% and 20%1,2. Among this subset of NHL patients, approximately 2–4% will be diagnosed with a rare NHL variant known as primary effusion lymphoma (PEL) or body cavity-based lymphoma2,3. PEL is a B-cell NHL etiologically linked to human herpesvirus type -8 (HHV-8) and most often is diagnosed in the setting of HIV infection. Less commonly it is seen in other immunocompromised groups such as the elderly and solid organ transplant recipients1.
Patients with PEL often present with symptoms referable to the site of effusion accumulation. For example, dyspnea related to malignant pericardial or pleural effusions and abdominal distention secondary to peritoneal fluid accumulation. Imaging studies usually reveal extranodal and malignant effusions located in body cavities involving the pericardial, peritoneal, and pleural spaces; lymphadenopathy is not usually present1.
Within the spectrum of PEL, there is also a rare HHV-8 –associated large B-cell lymphoma (HHV-8-LBL) variant. Unlike classic PEL, HHV-8-LBL presents in extra-cavitary regions such as lymph nodes, gastrointestinal tract and spleen, and without lymphomatous effusions. Median survival for patients with PEL who are treated with combination chemotherapy has been reported to be less than a year, although this may be improving in the highly active antiretroviral therapy (HAART) era. It is unclear whether patients with the HHV-8-LBL PEL variant have a different clinical course with treatment4,5,6,7,8,9.
Etiologically and morphologically, both classic PEL and HHV-8-LBL share many similarities. HHV-8 infection is the main oncogenic driver; presence of HHV-8 is essential for diagnosis of both PEL and HHV-8-LBL. In both variants, approximately 70–80% of tumor cells are co-infected with Epstein Barr Virus (EBV). However, they differ in immunoglobulin expression with the latter subtype more often expressing the pan-B cell antigens CD20 and CD79a8.
We briefly describe the case of an HIV positive man with a slowly progressive but asymptomatic abdominal mass that on biopsy proved to be HHV-8-LBL. He was treated with multi-agent infusional chemotherapy and remains in remission 12 months after treatment completion. To better understand the clinical findings and natural history of HHV-8-LBL, we performed a Medline (Pub Med) and Embase search focusing on English language articles and using the key terms “extra-cavitary primary effusion lymphoma”, “solid primary effusion lymphoma”, “primary effusion lymphoma”, “KSHV- and HHV-8-associated extra-cavitary lymphoma” and “HIV-associated non-Hodgkin’s lymphoma”. We identified 83 cases of HHV-8 LBL and 118 cases of classic PEL. They were reported in individual case reports, medical literature reviews, and retrospective case studies.
Case Report
A 46- year - old homosexual man with exercise-induced rhinorrhea, erectile dysfunction, gastro-esophageal reflux disease, and a five-year history of mild obesity and sleep apnea tested HIV-seropositive in 2006. He did not smoke or drink alcohol. His initial CD4+ count was 326 cells/uL and his HIV viral load was 47,000 copies/mL. He began HAART consisting of a single pill tri- formulation of efavirenz, emtricitabine and tenofovir. With the exception of a chronic and non-productive cough, he remained well with a non-detectable HIV viral load and a CD4+ count typically in excess of 400 cells/uL.
In 2011 he sought further evaluation for persistent cough. His workup included pulmonary function tests, and a computerized tomographic (CT) scan of chest and abdomen. Initial studies proved unremarkable and consistent with a sensitivity to environmental allergens. Although CT scan images through the chest were unremarkable, the abdominal views revealed an incidental finding of a retroperitoneal mass of several centimeters in size. He was followed conservatively and over a three-year period the mass slowly enlarged. During this time the patient remained afebrile and with normal blood studies including sedimentation rate, serum LDH, CD4+ cell count, and a consistently nondetectable HIV viral load. In 2015, he was referred back to medical oncology for reassessment. He was feeling well and was without fevers, night sweats or weight loss. His physical examination was unremarkable for peripheral adenopathy, visceromegaly, elevated neck veins, and abdominal distention and diminished lung sounds. His blood studies, including complete blood count, full chemistry and liver panel, sedimentation rate and serum LDH, were all within the broad range of normal. His CD4+ count was 947 cells/uL, he had a non-detectable HIV viral load and his Centers for Disease Control (CDC) and Prevention HIV stage was A1. Serum HHV-8 viral load by polymerase chain reaction was 9500 copies/mL. A whole body 18F-fluorodeoxyglucose positron emission tomography – CT scan (18FDG-PET-CT) showed the previously identified portacaval mass was enlarged to 43 × 64 mm and now markedly hyper-metabolic with a standardized uptake value (SUV) of 16.5. An additional mildly hyper-metabolic 10 × 13 mm left para-tracheal node with a SUV of 3.0 was identified (Figure 1, and inserts A and B).
Figure 1.
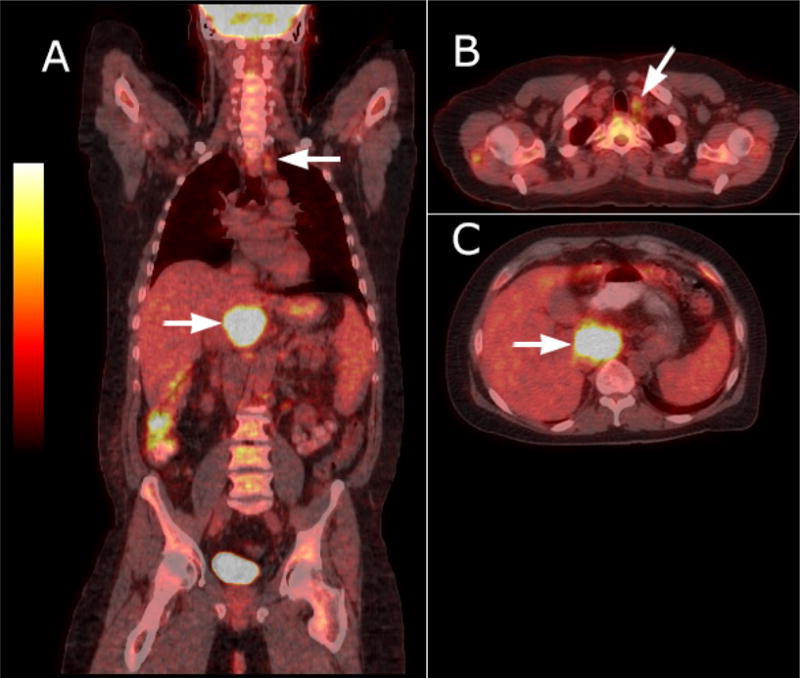
Whole body CT-PET fusion imaging findings: there is focal intense FDG uptake of portacaval lymph node measuring 43 × 64 mm. Max SUV 16.5 (Figure, A). Inserts show fused CT-PET coronal image of hypermetabolic hepatic artery lymph node (Figure, B) and mildly hypermetabolic left superior para-tracheal node measuring 10 ×13 mm. Max SUV 3.1 (Figure, C)
Owing to the now prominent size of the mass and its intense SUV reading, the patient underwent exploratory laparotomy and excisional biopsy of the portacaval mass. The node showed reactive morphological characteristics reminiscent of Castleman’s lymphadenopathy (e.g., onion skinning of mantle zone, small atrophic follicles, increased plasma cells) interspersed with large cells with abundant cytoplasm and prominent and round nuclei. The Ki-67 labeling index was 90%. Immuno-histochemical studies showed that these large cells stained positive for pan-B cell antigen CD20 and negative for CD138, CD10, BCL-6, CD3 and CD30. Malignant cells were also co-infected with HHV-8 and EBV (Figure 2, a–f). He was diagnosed with the rare HHV-8-LBL PEL variant. His bone marrow biopsy showed moderate hypercellularity but no obvious lymphoma.
Figure 2.
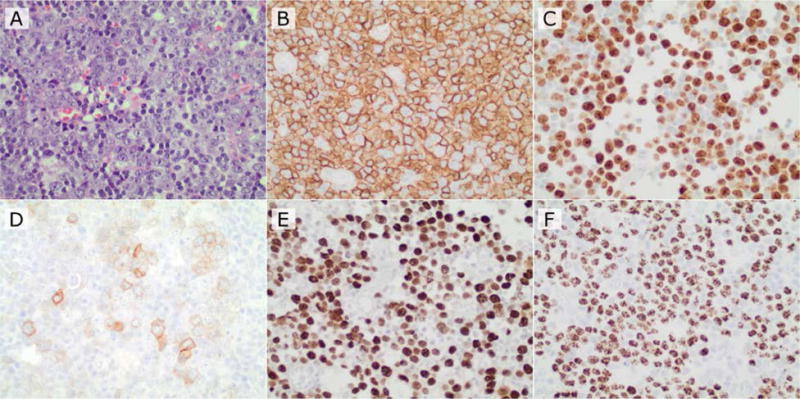
Immuno-histochemical staining of lymph node
A. 400× H&E hepatic artery lymph node biopsy.
B. Immunohistochemistry for CD20 (positive in tumor cells).
C. Immunohistochemistry for Ki67 (positive in approximately 80–90% of tumor nuclei).
D. Immunohistochemistry for CD30 (variable and dimly positive in a subset of tumor cells).
E. In situ hybridization for EBV EBER1 mRNAs (positive in tumor cell nuclei).
F. Immunohistochemistry for HHV- 8 (positive in tumor nuclei).
The patient was treated with R-EPOCH (rituximab, etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) infusional chemotherapy on an AIDS Malignancy Consortium (AMC) clinical trial54. During his first rituximab infusion he had significant rigors, chills and hypotension. Ten days later he was hospitalized with neutropenic fevers and enterovirus –induced gastroenteritis. He received intravenous fluids, empiric antibiotics and granulocyte -colony – stimulating factor and he recovered uneventfully. His next three cycles of chemotherapy were uncomplicated and a repeat 18FDG-PET-CT scan showed a complete radiographic response. He received a total of six rounds of infusional chemotherapy. This was tolerated well with the exception of a moderately severe lower extremity sensorial-motor neuropathy and fingertip paresthesias, which led to the elimination of vincristine during his final two cycles of chemotherapy. He also experienced short – lived dysphonia, which on laryngoscope exam, was felt due to vocal cord atrophy. He responded well to conservative care and voice exercises. At 12-month follow-up he remains in complete remission (CR); his CD4+ count is 665 cells/uL and his HHV-8 and HIV viral loads are both non-detectable.
Discussion
Kaposi’s sarcoma is the most prevalent tumor in HIV-infected individuals and NHL remains the second most prevalent malignancy in the current HAART era2, 10. It is estimated that PEL makes up 2 – 4% of all AIDS-associated NHLs, but precise numbers are not available as large scale epidemiological studies have not specifically included PEL in their analysis2,3,10. The incidence of HHV-8-LBL has not been reported; our search identified 84 cases (Table 1).
Table 1.
Combined Cases
| Reference | Case Number | Median Age (Range,years) | Sex | HIV + | Epstein- Barr Virus encoded RNA | CD4+ Cell Count at Diagnosis (median, cells/μL) | HIV Viral load (copies/mL) | HIV/AIDS Therapy | Prior AIDS Diagnosis | Lymphoma Stage | Chemotherapy regimen | Response | FU (median,months) | Comments | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index Case | 1 | 57 | M | Yes | (+) | 1344 | <50 | EFV/ FTC/ TDF | No | IIIA | R-EPOCH | (CR) | 12+ | Elevated HHV-8 viral load at diagnosis- not detectable at last FU. | |
| Guillet et al.13 | 2–18 | 41 (39–53) |
16- M 1- F |
Yes | NR | 204 (103–377) |
17- NR | 11- HAART 6- NR |
9- Yes 8- NR |
7- Stage IV 10- NR |
CHOP + HDMTX,ASCT |
7(CR) 10(NR) |
120 + | 7/17 patients achieved remission. None of these patients had reccurent lymphoma at time of follow up. | |
| Chadburn et al.7 | 19–26 | 40 (27–51) |
M | Yes | 7(+) 1(−) |
3- 188 (43–714) 5- NR |
2- UD 6- NR |
1- pre-HAART 4- None 3- NR |
2- Yes 1- No 5- NR |
1- III 2- IIIB 2- IV 3- NR |
6-Chemotherapy 1- N/A 1- NR |
3(CR) 1(PD) 4(NR) |
11 +, 25+, and
44+ 5 |
Patient with PD died 5 months following
diagnosis. Individual chemotherapy regimens NR. |
|
| Carbone et al.5 | 27 28 |
75 52 |
M M |
No No |
(−) (+) |
470 501 |
N/A N/A |
N/A N/A |
N/A N/A |
IVB IIIA |
CHOP CEOP |
(SD) 3(PD) |
2+ 8+ |
||
| Carbone et al.4 | 29 30 31 |
39 24 41 |
M F M |
Yes Yes Yes |
(−) (+) (+) |
20 282 104 |
NR 125.155 <50 |
NR NR NR |
Yes No Yes |
IVB IVB IVB |
CHOPLD VCR-BLM CHOPLD |
(PD) (PD) (PD) |
15 25 45 |
All three patients died. | |
| Pan et al.8 | 32–40 | 44 (26–77) |
M | Yes | 8(+) 11(−) |
3- 160 (7–443) 6- NR |
NR | NR | 3- Yes 6- No |
NR | NR | 5(NR) 4(PR) or (CR) |
16 days 6+, 15+, and 33+ |
Cause of death NR. Outcomes were reported for four of the nine patients. |
|
| El-Ayass et al.30 | 41 | 48 | M | Yes | NR | 19 | 431,855 | HAART | Yes | NR | EPOCH | (CR) | 14+ | Individual HAART therapy NR. | |
| Deloose et al.29 | 42–50 | 41 (35–54) |
M | Yes | 8(+) 1(−) |
NR | NR | NR | NR | 8- IV 1- IIE |
NR | (NR) | NR | ||
| Costes et al.27 | 51 52 |
44 41 |
M M |
Yes Yes |
(+) (+) |
<50 NR |
NR NR |
HAART HAART |
Yes Yes |
NR NR |
PCT | (CR) (CR) |
8+ 8+ |
Individual HAART therapy
NR. Specific chemo regimen not reported for either patient. |
|
| Shah et al.49 | 53 | 33 | M | Yes | (+) | 60 | 184,000 | HAART | Yes | NR | R-EPOCH+, IT-MTX, ESHAP ×6 | (CR) | 6+ | Individual HAART therapy
NR. Patient showed CR after four cycles of R- EPOCH. |
|
| Hasegawa et al.34 | 54 | 50 | M | Yes | (−) | 0.044 | 73,000 | HAART | Yes | Yes | CHOP | (CR) | NR | Individual HAART therapy NR. FU data NR; lymphoma lesions disappeared after initiation of HAART and CHOP. |
|
| Oksenhendl er et al.44 | 55 56 57 58 |
NR | NR | Yes | (−) (−) (NR) (−) |
260 686 1126 114 |
334,000 <500 362,000 <50 |
D4T-3TC-RTV AZT-3TC-IDV NR ABC-DDL-RTV-SQV |
No No No Yes |
NR | EDX-VP16 None ACVBP CHOPLD |
(PR) (PD) (CR) (PD) |
5 3 weeks 25+ 7 weeks |
Cause of death for cases 55, 56, and 58 are
NR. Case 57 remains in follow-up. |
|
| Zhang et al.53 | 59 | 46 | M | Yes | (+) | 88 | >100,000 | HAART | Yes | NR | R-C | (PD) | 2 weeks | Patient continued to decline
despite antiretroviral and chemo intervention. Death due to sepsis, worsening acidosis, hepatorenal failure, and hypotension. |
|
| Kim et al.38 | 60 61 62 |
55 42 42 |
M | Yes | (−) (+) (−) |
NR | NR | HAART | NR | NR | NR | (CR) (CR) (NR) |
13+ 25+ NR |
Individual HAART therapy NR. NED at follow-up. | |
| Engels et al.31 | 63–65 | 48 (41–48) |
M | Yes | 2(+) 1(−) |
94 2- NR |
NR | NR | 1-Yes 2-NR |
NR | NR | (NR) | NR | Overall survival or time to progression data NR. | |
| Andrews et al.21 | 66 | 46 | M | Yes | (−) | 159 | 511,000 | HAART | Yes | NR | 1. R-CHOP, IT-MTX, ESHAP
×1; 2. EPOCH, HDMTX ×3 |
(CR) | NR | Individual HAART therapy
NR. Patient switched to second regimen after being diagnosed with CNS disease. Patient continues to experience left sided numbness and facial nerve palsy |
|
| Huang et al.36 | 67 | 49 | M | Yes | (−) | NR | NR | HAART | No | NR | ICE + ASCT ×2 | (CR) | 2+ | Individual HAART therapy NR. | |
| Crane et al.28 | 68 | 59 | M | Yes | (+) | 526 | 93 | HAART | No | NR | None | (PD) | 2 | Patient performance status declined after and he was transferred to comfort care. | |
| Ferry et al.32 | 69 | 59 | M | Yes | (−) | NR | NR | HAART | No | NR | NR | (NR) | 8 days | Individual HAART therapy
NR. Death due to a combination of hypotension, acute renal failure, and metabolic acidosis. |
|
| Navarro et al.42 | 70 | 37 | M | Yes | (+) | 120 | NR | AZT | Yes | IVB | CHOP ×3 | (NR) | 3 | Death due to bilateral pneumonia. | |
| Beaty et al.11 | 71 | 32 | M | Yes | (+) | NR | NR | NR | No | NR | Surgical Resection | (CR) | 5 | NED at last follow-up. | |
| Buske et al.24 | 72 | 35 | M | Yes | (+) | NR | NR | NR | NR | NR | DOX | (PD) | 4 | Death due to septic shock four months after completing chemotherapy. | |
| Aboulafia et al.20 | 73 | 39 | M | Yes | (+) | 30 | 90,000 | 1. ART, 3TC 2.HAART |
Yes | IE | R-CHOP | (NR) | 1 week | Death due to fibril pneumonia. | |
| Giessen et al.33 | 74 | 38 | M | Yes | (+) | 390 | 19,000 | NVP, 3TC, ABC | No | NR | CHOP ×6 | (CR) | NR | FU data NR. | |
| Henao-Martinez et al.35 | 75 | 45 | M | Yes | (+) | 173 | 32,783 | TDF, FTC, RTV, ATV | Yes | NR | EPOCH | (CR) | 12+ | ||
| Pielasinski et al.47 | 76 | 33 | M | Yes | (+) | 288 | 1,078,000 | HAART | No | NR | CHOP | (CR) | 20+ | Individual HAART therapy NR. | |
| Meng-Feng et al.47 | 77 | 31 | M | Yes | (+) | 18 | <200 | NR | Yes | NR | VCR-BLM | (NR) | NR | Death 44 days after diagnosis. | |
| Verma et al.47 | 78 | 38 | F | No | (−) | NR | NR | NR | NR | NR | Surgical resection | (NR) | NR | ||
| Colom et al.47 | 79 | 51 | M | Yes | (+) | NR | NR | NR | NR | NR | NR | (NR) | NR | ||
| Katano et al.9 | 80 | 30 | M | Yes | (+) | 50 | NR | NR | Yes | NR | CV | (PR) or (CR) | NR | Death due to MCD and hemorrhage. | |
| Pantanowitz et al.45 | 81 | 40 | M | Yes | (+) | 300 | 86 | HAART | No | NR | CHOP | (CR) | 39+ | Patient continues to undergo HAART therapy, specific regimen NR. CD4 count remains >300 cells/mm^3. | |
| Mylona et al.54 | 82 | 39 | M | Yes | (NR) | 685 | <50 | TDF, DDL, LPV, RTV | Yes | NR | 1. EPOCH ×6 2. ESHAP ×2 |
(PD) | 24 | Individual HAART therapy NR. Death due to massive haemoptysis. | |
| Morand et al.40 | 83 | 40 | M | Yes | (+) | 10 | NR | NR | Yes | Yes | NR | (PD) | 2 weeks | Death due to infectious complications during chemotherapy. | |
ABC, abacavir; ddl, didanosine; ABVp, doxorubicin, bleomycin, etoposide; ACVBP, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone; ASCT, autologous stem cell transplantation; AZT, zidovudine; CDE, cyclophosphamide, doxorubicin, etopside; CEOP, cyclophosphamide, epidoxorubicin, vincristine, prednisone; CHVp, cyclophosphamide, doxorubicin, etoposide; CHVp-M, cyclophosphamide, doxorubicin, etoposide, high-dose methotrexate; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CHOPLD, cyclophosphamide, doxorubicin, vincristine, predmustine (CHOP) 50% of dose; CHOP-M, cyclophosphamide, doxorubicin, vincristine, prednisone, high-dose methotrexate; CNS, central nervous system; CR, Complete Response; CV, Cyclophosphamide; CVp, cyclophosphamide, etoposide; d4T, stavudine; DOX, doxorubicin Dx, Diagnosis; EFV, Efavirenz; ESHAP, etoposide, cisplatin, high-dose methotrexate; F, Female; FTC, Emtricitabine; FU, Follow Up; HAART, highly active antiretroviral therapy; ICE, ifosfamide, carboplatin, etopside; IDV, indinavir; IT-MTX, intrathecal methotrexate; KS, Kaposi’s Sarcoma; LPV, lopinavir; M, Male; MCD, Multicentric Castleman’s Disease; N/A, Not applicable; HDMTX, High-dose methotrexate; NED, No Evidence of Disease; NHL; non-hodgkins lymphoma; NR, No Reported; NVP, Nevirapine; PCT, Polychemotherapy; PD, Progressive Disease; PR, partial response; R-C, rituximab, cyclophosphamide; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; RTV, ritonavir; SD, stable disease; SQV, saquinavir; Tx, treatment; VCR-BLM, vincristine, bleomycin; TDF, Tenofovir, vinblastine; 3TC, lamivudine;
Based on our review of the literature, we found several differences between classic PEL and HHV-8-LBL. HHV-8-LBL expresses pan-B cell antigens and surface receptor CD138 more often than PEL, and less frequently expresses CD45. Unlike classic PEL, HHV-8-LBL is more likely to be focally positive for T-cell antigen CD3 (Table 2). Despite these different cellular surface receptor expression patterns immuno-histochemical studies show that HHV-8-LBL expresses all key proteins found in the classic PEL secretome, suggesting both these malignancies are genetically linked11.
Table 2.
Comparison of clinical features, immunohistochemistry, and outcomes of HHV-8-LBL and classic PEL
| HHV-8-LBL | PEL | |
|---|---|---|
| Age (years) | 41 | 41 |
| Sex (M) | 96% | 99% |
| HIV+ | 95% | 96% |
| Antecedent AIDS diagnosis | 47% | 72% |
| CD4+ count | 256 cell/ul | 139 cell/ul |
| EBV+ | 80% | 82% |
| CD3+ | 26% | 6% |
| CD20+ | 18% | 5% |
| CD79a+ | 26% | 5% |
| CD30+ | 64% | 76% |
| CD138+ | 72% | 38% |
| Median Survival | 5.5 months | 4.0 months |
| % Achieve Complete Remission | 37 | 36 |
HIV+ = Human Immunodeficiency Virus positive, EBV+ = Epstein Barr Virus positive, AIDS = Acquired Immune Deficiency Syndrome, M=Male
The diagnosis of HHV-8-LBL is made challenging by its similar clinical profile to other lymphoproliferative disorders, and its typical lack of pan B-cell antigens, and because it is rarely included in clinical or pathological differential diagnosis8. Without the proper set of immuno-histochemical studies, HHV-8-LBL could be misdiagnosed as anaplastic large cell lymphoma (ALCL), plasmablastic lymphoma (PBL), large B-cell lymphoma-multicentric Castleman’s disease (LBL-MCD), plasmablastic plasma cell myeloma (PCM), ALK1+ large B-cell lymphoma (LBL), or diffuse large B-cell lymphoma with immunoblastic morphology (Table 3).
Table 3.
HHV-8-LBL and related solid-lymphomas
| HHV-8-LBL Differential Diagnosis | HHV-8 + LBL | PBL | ALCL, Null-cell Type | Plasma-blastic, PCM | LBL-MCD | ALK1 + DLBCL | DLBCL, Immunoblastic |
|---|---|---|---|---|---|---|---|
| Clinical Features | |||||||
| Common location | Nodal, extranodal | Oral cavity | Lymph node | Bone Marrow | Lymph node, spleen | Lymph node | Nodal, extranodal |
| HIV | + | + | − | − | + | − | − |
| EBV | + +++ | + ++ | − | − | − | − | − |
| HHV-8 | + | −/+ | − | − | + | − | − |
| Paraprotein-emia | − | − | − | + | − | − | − |
| Immuno-globulin | − | Primarily IgG | − | IgG, IgA, and IgD | IgM | Primarily IgA | − |
| Morphology | Anaplastic, immunoblastic, Plasmablastic | Plasmablastic, immunoblastic | Sinusoidal Anaplastic | Plasma-blastic | Plasma-blastic, immuno-blastic | Sinusoidal, Immuno-blastic, Plasma-blastic | Immunoblastic |
| Immunostaining | |||||||
| CD45 | + ++ | + | −/+ | − | − | + ++ | + |
| CD20 | + | − (5%) | − | − | +/− | −/+ | + |
| CD79a | + | + | − | +/− | − | −/+ | + |
| PAX-5 | − | − | − | − | − | − | + |
| CD2 | − | − | − | − | − | − | − |
| CD3/CD5/CD7 | − | − | − | − | − | − | − |
| CD138 | + ++ | + +++ | − | + (100%) | − | ++++ (100%) | − |
| MUM1 | ++++ (100%) | ++++ (100%) | − | ++++ (100%) | − | ++++ (100%) | +/− |
| EMA | + ++ | +++ | + | ++++ (100%) | − | ++++ (100%) | − |
| CD30 | ++ | − | + | − | − | −/+ | − |
| ALK1 | − | − | + | − | − | ++++ (100%) | − |
| Ig light chain | − | − | − | + | + | + | + |
| other stains | − | − | TIA+, perforin+, granzyme+ | − | vIL-6+ | CD4++ | − |
| Molecular Studies | |||||||
| IGH@ Rearrange-ment | + | + | − | + | − | + ++ | + |
| TRG@ Rearrange-ment | − | − | + | − | − | − | − |
| ALK@ Rearrange-ment | − | − | +/− | − | − | + | − |
ALCL = anaplastic large cell lymphoma; ALK 1= anaplastic lymphoma kinase 1; DLBCL= diffuse large B-cell lymphoma; EBV=Epstein Barr Virus; EMA=epithelial membrane antigen; Ig, immunoglobulin; HHV-8= Human Herpesvirus type-8; HIV=Human Immunodeficiency Virus; LBL= large B-cell l lymphoma; MUM I= multiple myeloma oncogene-1; PAX= paired box gene·S; PCM= plasma cell myeloma; TIA=T-cell intracellular antigen; v L·6= viral interleukin-6. Table modified from Pan et al Table 522
HHV-8-LBL can be distinguished from ALCL; although both entities may express the T-cell antigen CD3, molecular studies reveal that only HHV-8-LBL will show IGH gene rearrangements while ALCL will show T-cell receptor gene rearrangements. PBL is morphologically similar to HHV-8-LBL and it too can be localized to extranodal sites and be associated with EBV. PBL will also express EMA, MUM1, and CD138. However, B-cell antigen expression is less commonly seen in PBL than HHV-8-LBL cases and, unlike HHV-8-LBL, PBL expresses CD45 less frequently and only rarely is HHV-8 positive. LBL-MCD is also associated with HHV-8, is common in HIV-infected individuals, and lacks B-cell antigen expression; but, HHV-8-LBL is more frequently associated with EBV, CD138, and rarely expresses cytoplasmic IgM. PCM is not associated with HIV, HHV-8 or EBV. Finally, neither ALK+ LBL nor DLBCL are associated with HHV-88.
Clinically, there are important distinctions between HHV-8-LBL and PEL. Patients with HHV-8-LBL present with solid lesions in lymph nodes or extracavitary tissues. Whereas patients with classic PEL are apt to present with signs or symptoms of congestive heart failure or shortness of breath, as the pleural cavity is the most common site of effusion accumulation12. In addition, HHV-8-LBL patients may be less immunocompromised than classic PEL patients as reflected by the finding that HHV-8-LBL is associated with a higher median CD4+ cell count (256 cells/uL vs 139 cell/uL, P=0.024). A previous diagnosis of AIDS appears to be more common in classic PEL than in HHV-8-LBL (72% vs 51%, P=.007). Our patient’s clinical presentation highlights these distinctions as he too had a normal CD4+ cell count, a non-detectable HIV viral load, no body-cavity-based effusions and no antecedent AIDS diagnosis.
Whether prognosis differs between classic PEL and HHV-8-LBL is unclear. One comparative study looking at 8 cases of HHV-8-LBL and 29 cases of classic PEL suggested a better prognosis for HHV-8-LBL (11 month vs 3 month median survival rate), despite the majority of both groups being treated with multi-agent anthracycline-based chemotherapy7. Only 1 patient out of 21 classic PEL cases with follow-up information was alive at three months, whereas three of the five HHV-8-LBL cases were alive at 12+, 21+, and 44+ months, respectively. Interpretation of these results, however, is hampered by the fact that some of these patients were cared for in the pre- HAART era, and not all patients were reliably taking their HIV medications at time of lymphoma diagnosis. In contrast, another retrospective review involving 51 patients from a single institution (17 HHV-8-LBL and 34 classic PEL) treated with comparable anthracycline-based chemotherapy13 found that 64% of patients with classic PEL had a CR in contrast to 41% of patients with HHV-8-LBL13. However, all seven (100%) HHV-8-LBL patients who did achieve a CR remained disease free at five years follow up. Among the classic PEL cohort 62% (13/21) of patients who had previously achieved a CR, had relapsed at a median time of 25 months.
In aggregate and among the case reports that we reviewed, the median survival of HHV-8-LBL was 5.5 months (range, 25 days – 25+ months) and for classic PEL 4 months (range, 2 days – 113+ months). Although median survival time was similar for both groups, more HHV-8-LBL patients than PEL patients were alive at time of follow up (23/39 [59%] vs. 10/55[18%], P<.0001) and a higher percentage achieved CR (30/56 [54%] vs 32/89 [36%], P<.0001)
Several factors are linked to favorable outcomes in PEL patients. These include good compliance with HAART prior to PEL diagnosis, a normal serum LDH, and a CD4+ cell counts> 200 cells/uL at time of NHL diagnosis14, 15. In addition, lymphomatous effusion in only one body cavity, or having a pericardial effusion was linked to better outcomes12. The protective effect of having a pericardial effusion could possibly be due to less disease burden and possibly earlier clinical symptoms compared to involvement of the larger body cavities such as the peritoneum. Other clinical factors that may prove to be prognostic but for which data has been inconsistently reported include: HIV status (including HIV viral load at time of diagnosis and CD4+ cell count), age at PEL diagnosis, gender, lymphoma stage at diagnosis, serum LDH, treatment received, response to treatment, and survival status at two years.
The National Cancer Institute-funded AMC is conducting a retrospective analysis of clinical and pathological materials to ascertain whether certain genes among the 51 that are differentially expressed between PEL cells and B cells and other AIDS-NHLs are associated with better or worse clinical outcomes16. Furthermore, the future of PEL, HHV-8 LBL and other HHV-8 lymphoma treatments may be improved by the recent elucidation of the molecular steps in HHV-8 driven oncogenesis. This could provide molecular targeting therapies for proteasomes, NF- κB, cytokines and surface antigens17.
Due to its rarity, there is little epidemiological or detailed clinical information regarding patients with PEL. Treatment is left to professional judgment and there is no standard best treatment option. For patients with AIDS-associated DLBCL the most common treatment historically has been R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy and concurrent HAART. R-EPOCH, with or without concomitant HAART, has also emerged as an alternative to R-CHOP and in the context of HIV infection may be associated with a slightly higher response rate17. The use of rituximab has improved outcomes in all studied subsets of B-cell lymphoma and is an important component of modern multi-agent chemotherapy. Because PEL tumor cells rarely express CD20, rituximab has traditionally not been included in treatment algorithms. Regardless of treatment regimen, this immunocompromised population is at risk for opportunistic infections. Therefore, it is essential that they receive prophylactic antibiotics and, when the risk of febrile neutropenia exceeds 20%, adjuvant granulocyte-colony-stimulating factor. Additionally, evolving knowledge of drug interactions with HAART, particularly protease inhibitors and chemotherapy, is important when creating a treatment plan17.
Studies using cultured PEL cells and xenograft mouse models show that PEL cells are highly sensitive to irradiation18. Radiation therapy’s potential efficacy is further supported by a report of its successful use in a chemotherapy-refractory PEL patient with solid pleural and chest masses who had remained disease free at 12 months follow-up19. Thus, in addition to effective HAART and intensive chemotherapy, radiation therapy may be an important additional option for those with PEL and persistent localized effusions and where autologous stem cell transplantation is not a viable option. Whether HHV-8-LBL patients respond better to chemotherapy than classic PEL patients is unclear. The findings that HHV-8-LBL patients achieve a more long-lasting CR, and that a greater percentage were reported to be alive at time of follow-up suggest HHV-8-LBL may be more responsive to treatment. This could be, in part, related to their greater CD4+ cell count at time of lymphoma diagnosis and their corresponding lesser incidence of pre-existing AIDS. An additional important modern variable may be that due to a higher expression of pan-B cell antigen CD20, a greater number of HHV- 8-LBL patients have the option of rituximab-based treatment.
There are weaknesses inherent in our literature review. Findings are limited by incomplete and non-uniform reporting of case reports and case series and the inherent selection biases by which cases are reported in the medical literature. Most importantly, precise clinical variables such as HIV/AIDS CDC classification and patient age and lymphoma-related variables such as stage and serum LDH, were inconsistently reported. Data overlap may also be present in our analysis; the largest case series which included 17 cases of HHV-8-LBL and 34 cases of classic PEL was reported from a single institution which did not report individual case data. The authors of that review have published other case reports that were included in our data collection so duplication of data may contribute to additional inaccuracies. Also problematic is that the findings of this large series have yet to be published in a peer-reviewed journal.
In summary, PEL is a rare malignancy that is primarily restricted to patients with HIV. HHV-8-LBL is a variant of PEL that can be distinguished by its unique clinical presentation and cell surface antigen expression. HHV-8-LBL appears to be the manifestation of PEL in more immune competent patients, and may have a less aggressive clinical course and better prognosis. While rare, HHV-8-LBL should be included in the differential diagnosis of HIV-infected patients with solid mass lesions in order for appropriate and timely treatment for this aggressive lymphoma.
References
- 1.Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12(5):569–576. doi: 10.1634/theoncologist.12-5-569. [DOI] [PubMed] [Google Scholar]
- 2.Little RF, Gutierrez M, Jaffe ES, Pau A, Horne M, Wilson W. HIV-associated non-hodgkin lymphoma: Incidence, presentation, and prognosis. JAMA. 2001;285(14):1880–1885. doi: 10.1001/jama.285.14.1880. [DOI] [PubMed] [Google Scholar]
- 3.Ammari ZA, Mollberg NM, Abdelhady K, Mansueto MD, Massad MG. Diagnosis and management of primary effusion lymphoma in the immunocompetent and immunocompromised hosts. Thorac Cardiovasc Surg. 2013;61(4):343–349. doi: 10.1055/s-0033-1333897. [DOI] [PubMed] [Google Scholar]
- 4.Carbone A, Gloghini A, Vaccher E, et al. Kaposi’s sarcoma associated Herpesvirus/Human herpesvirus type 8-positive solid lymphomas: A tissue-based variant of primary effusion lymphoma. J Mol Diagn. 2005;7(1):17–27. doi: 10.1016/S1525-1578(10)60004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone A, Volpi CC, Caccia D, et al. Extracavitary KSHV-positive solid lymphoma: A large B-cell lymphoma within the spectrum of primary effusion lymphoma. Am J Surg Pathol. 2013;37(9):1460–1461. doi: 10.1097/PAS.0b013e31829caada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone A, Gloghini A. KSHV/HHV8-associated lymphomas. Br J Haematol. 2008;140(1):13–24. doi: 10.1111/j.1365-2141.2007.06879.x. [DOI] [PubMed] [Google Scholar]
- 7.Chadburn A, Hyjek E, Mathew S, Cesarman E, Said J, Knowles DM. KSHV-positive solid lymphomas represent an extra-cavitary variant of primary effusion lymphoma. Am J Surg Pathol. 2004;28(11):1401–1416. doi: 10.1097/01.pas.0000138177.10829.5c. [DOI] [PubMed] [Google Scholar]
- 8.Pan ZG, Zhang QY, Lu ZB, et al. Extracavitary KSHV-associated large B-cell lymphoma: A distinct entity or a subtype of primary effusion lymphoma? study of 9 cases and review of an additional 43 cases. Am J Surg Pathol. 2012;36(8):1129–1140. doi: 10.1097/PAS.0b013e31825b38ec. [DOI] [PubMed] [Google Scholar]
- 9.Katano H, Suda T, Morishita Y, et al. Human herpesvirus 8- associated solid lymphomas that occur in AIDS patients take anaplastic large cell morphology. Mod Pathol. 2000;13:77–85. doi: 10.1038/modpathol.3880012. [DOI] [PubMed] [Google Scholar]
- 10.Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol. 2015;42(2):247–257. doi: 10.1053/j.seminoncol.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Beaty MW, Kumar S, Sorbara L, Miller K, Raffeld M, Jaffe ES. A biophenotypic human herpesvirus 8– associated primary bowel lymphoma. Am J Surg Pathol. 1999;23(8):992–994. doi: 10.1097/00000478-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Castillo JJ, Shum H, Lahijani M, Winer ES, Butera JN. Prognosis in primary effusion lymphoma is associated with the number of body cavities involved. Leuk Lymphoma. 2012;53(12):2378–2382. doi: 10.3109/10428194.2012.694075. [DOI] [PubMed] [Google Scholar]
- 13.Primary Effusion Lymphoma and HIV infection: 51 Patients from a Single Institution. Guillet S, Meignin V, Agbalika F, Cuccuini W, Galicier L, Oksenhendler E. topics in Antiviral Medicine. 2014;22:e-1. (362). Date of Publication: Apr 2014. [Google Scholar]
- 14.Boulanger E, Gerard L, Gabarre J, et al. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23(19):4372–4380. doi: 10.1200/JCO.2005.07.084. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, Marley E, Naina HV. Prognostic factors and outcomes in primary effusion lymphoma in HIV-infected patients: A single center experience. Blood. 2014;124(21):5415–5415. [Google Scholar]
- 16.ClinicalTrials.gov. Identifier: NCT 01092182 Phase II Study of Dose-Adjusted EPOCH-Rituximab in Adults with Untreated Burkitt Lymphoma and c-MYC+ Diffuse Large B-Cell Lymphoma [Google Scholar]
- 17.Okada S, Goto H, Yotsumoto M. Current status of treatment for primary effusion lymphoma. Intractable Rare Dis Res. 2014;3(3):65–74. doi: 10.5582/irdr.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiraishi Y, Gotoh K, Towata T, Shimasaki T, Suzu S, Kojima A, Okada S. Therapeutic effects of gamma-irradiation in a primary effusion lymphoma mouse model. Exp Ther Med. 2010;1:79–84. doi: 10.3892/etm_00000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassoni A, Ali U, Cave J, Edwards SG, Ramsay A, Miller RF, Lee SM. Remission after radiotherapy for a patient with chemotherapy-refractory HIV-associated primary effusion lymphoma. J Clin Oncol. 2008;26:5297–5299. doi: 10.1200/JCO.2008.18.3350. [DOI] [PubMed] [Google Scholar]
- 20.Aboulafia DM. HHV-8- and EBV-associated nonepidermotrophic large B-cell lymphoma presenting as a foot rash in a man with AIDS. AIDS Patient Care STDS. 2002;16:139–145. doi: 10.1089/10872910252930830. [DOI] [PubMed] [Google Scholar]
- 21.Andrews JR, Cho-Park YA, Ferry J, Abramson JS, Robbins GK. Kaposi’s Sarcoma-Associated Herpesvirus-Related Solid Lymphoma Involving the Heart and Brain. AIDS Res Treat. 2011;2011:729854. doi: 10.1155/2011/729854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulanger E, Agbalika F, Maarek O, et al. A clinical, molecular and cytogenetic study of 12 cases of human herpesvirus 8 associated primary effusion lymphoma in HIV-infected patients. Hematol J. 2001;2:172–179. doi: 10.1038/sj.thj.6200096. [DOI] [PubMed] [Google Scholar]
- 23.Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224–233. doi: 10.1002/cncr.22691. [DOI] [PubMed] [Google Scholar]
- 24.Buske C, Hannig H, Hiddemann W, et al. Human herpesvirus-8 (HHV-8) DNA associated with anaplastic large cell lymphoma of the B-cell type in an HIV-1-positive patient. Int J Cancer. 1997;73:303–304. doi: 10.1002/(sici)1097-0215(19971009)73:2<303::aid-ijc23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 25.Carbone A, Vaccher E, Gloghini A, et al. Diagnosis and management of lymphomas and other cancers in HIV-infected patients. Nat Rev Clin Oncol. 2014;11(4):223–238. doi: 10.1038/nrclinonc.2014.31. [DOI] [PubMed] [Google Scholar]
- 26.Carbone A, Gloghini A, Vaccher E, et al. KSHV/HHV-8 associated lymph node based lymphomas in HIV seronegative subjects. Report of two cases with anaplastic large cell morphology and plasmablastic immunophenotype. J Clin Pathol. 2005;58:1039–1045. doi: 10.1136/jcp.2005.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costes V, Faumont N, Cesarman E, et al. Human herpesvirus-8-associated lymphoma of the bowel in human immunodeficiency virus-positive patients without history of primary effusion lymphoma. Hum Pathol. 2002;33:846–849. doi: 10.1053/hupa.2002.126184. [DOI] [PubMed] [Google Scholar]
- 28.Crane GM, Ambinder RF, Shirley CM, et al. HHV-8-positive and EBV-positive intravascular lymphoma: An unusual presentation of extra-cavitary primary effusion lymphoma. Am J Surg Pathol. 2014;38(3):426–432. doi: 10.1097/PAS.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deloose ST, Smit LA, Pals FT, et al. High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia. 2005;19:851–855. doi: 10.1038/sj.leu.2403709. [DOI] [PubMed] [Google Scholar]
- 30.El-Ayass W, Yu EM, Karcher DS, Aragon-Ching JB. Complete response to EPOCH in a patient with HIV and extracavitary primary effusion lymphoma involving the colon: A case report and review of literature. Clin Lymphoma Myeloma Leuk. 2012;12(2):144–147. doi: 10.1016/j.clml.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Engels EA, Pittaluga S, Whitby D, et al. Immunoblastic lymphoma in persons with AIDS-associated Kaposi’s sarcoma: a role for Kaposi’s sarcoma-associated herpesvirus. Mod Pathol. 2003;16:424–429. doi: 10.1097/01.MP.0000056629.62148.55. [DOI] [PubMed] [Google Scholar]
- 32.Ferry JA, Sohani AR, Longtine JA, et al. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol. 2009;22:618–626. doi: 10.1038/modpathol.2009.36. [DOI] [PubMed] [Google Scholar]
- 33.Giessen C, Di Gioia D, Huber B, et al. Primary effusion lymphoma (PEL) without effusion: A patient case report of a PEL solid variant. J Clin Pathol. 2012;65(2):189–190. doi: 10.1136/jclinpath-2011-200279. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa H, Katano H, Tanno M, et al. BCL-6-positive human herpesvirus 8-associated solid lymphoma arising from liver and spleen as multiple nodular lesions. Leuk Lymphoma. 2004;45:2169–2172. doi: 10.1080/10428190410001723241. [DOI] [PubMed] [Google Scholar]
- 35.Henao-Martinez AF, Gray JM, Gonzalez-Fontal GR, Reves R. Intracardiac extra-cavitary primary effusion lymphoma in an HIV-infected patient. Int J STD AIDS. 2013;24(1):78–79. doi: 10.1258/ijsa.2012.012178. [DOI] [PubMed] [Google Scholar]
- 36.Huang Q, Chang KL, Gaal KK, et al. KSHV/HHV8-associated lymphoma simulating anaplastic large cell lymphoma. Am J Surg Pathol. 2004;28:693–697. doi: 10.1097/00000478-200405000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Juskevicius D, Dietsche T, Lorber T, et al. Extracavitary primary effusion lymphoma: Clinical, morphological, phenotypic and cytogenetic characterization using nuclei enrichment technique. Histopathology. 2014;65(5):693–706. doi: 10.1111/his.12478. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y, Leventaki V, Bhaijee F, Jackson CC, Medeiros LJ, Vega F. Extracavitary/solid variant of primary effusion lymphoma. Ann Diagn Pathol. 2012;16(6):441–446. doi: 10.1016/j.anndiagpath.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Lim ST, Rubin N, Said J, Levine AM. Primary effusion lymphoma: Successful treatment with highly active antiretroviral therapy and rituximab. Ann Hematol. 2005;84(8):551–552. doi: 10.1007/s00277-005-1040-6. [DOI] [PubMed] [Google Scholar]
- 40.Morand P, Buisson M, Collandre H, et al. Human herpesvirus 8 and Epstein Barr-virus in a cutaneous B-cell lymphoma and a malignant cell line established from the blood of an AIDS patient. Leuk Lymphoma. 1999;35:379–387. doi: 10.3109/10428199909145743. [DOI] [PubMed] [Google Scholar]
- 41.Nador RG, Cesarman E, Chadburn A, et al. Primary effusion lymphoma: A distinct clinicopathologic entity associated with the kaposi’s sarcoma-associated herpes virus. Blood. 1996;88(2):645–656. [PubMed] [Google Scholar]
- 42.Navarro JT, Ribera JM, Junca J, et al. Anorectal lymphoma without effusion associated with human herpesvirus-8 and type 1 Epstein-Barr virus in an HIV-infected patient. Hum Pathol. 2003;34:630. doi: 10.1016/s0046-8177(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 43.Navarro WH, Kaplan LD. AIDS-related lymphoproliferative disease. Blood. 2006;107(1):13–20. doi: 10.1182/blood-2004-11-4278. [DOI] [PubMed] [Google Scholar]
- 44.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 45.Pantanowitz L, Wu Z, Dezube BJ, et al. Extracavitary primary effusion lymphoma of the anorectum. Clin Lymphoma Myeloma. 2005;6:149–152. doi: 10.3816/CLM.2005.n.044. [DOI] [PubMed] [Google Scholar]
- 46.Patel S, Xiao P. Primary effusion lymphoma. Arch Pathol Lab Med. 2013;137(8):1152–1154. doi: 10.5858/arpa.2012-0294-RS. [DOI] [PubMed] [Google Scholar]
- 47.Pielasinski U, Santonja C, Rodriguez-Pinilla SM, Requena L. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: A case report and literature review. J Cutan Pathol. 2014;41(9):745–753. doi: 10.1111/cup.12368. [DOI] [PubMed] [Google Scholar]
- 48.Pinzone MR, Berretta M, Cacopardo B, Nunnari G. Epstein-barr virus- and kaposi sarcoma-associated herpesvirus-related malignancies in the setting of human immunodeficiency virus infection. Semin Oncol. 2015;42(2):258–271. doi: 10.1053/j.seminoncol.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 49.Shah NN, Harrison N, Stonecypher M, Frank D, Amorosa V, Svoboda J. Extracavitary primary effusion lymphoma initially presenting with hemophagocytic lymphohistocytosis. Clin Lymphoma Myeloma Leuk. 2014;14(5):e157–60. doi: 10.1016/j.clml.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Simonelli C, Spina M, Cinelli R, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single- institution study. J Clin Oncol. 2003;21:3948–3954. doi: 10.1200/JCO.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Wang HY, Fuda FS, Chen W, et al. Notch1 in primary effusion lymphoma: a clinicopathological study. Mod Pathol. 2010;23:773–780. doi: 10.1038/modpathol.2010.67. [DOI] [PubMed] [Google Scholar]
- 52.Yiakoumis X, Pangalis GA, Kyrtsonis MC, et al. Primary effusion lymphoma in two HIV-negative patients successfully treated with pleurodesis as first-line therapy. Anticancer Res. 2010;30(1):271–276. [PubMed] [Google Scholar]
- 53.Zhang H, Yang XY, Hong T, et al. Kaposi sarcoma-associated herpesvirus (human herpesvirus type 8)-associated extracavitary lymphoma: report of a case in an HIV-positive patient with simultaneous Kaposi sarcoma and a review of the literature. Acta Haematol. 2010;123:237–241. doi: 10.1159/000314347. [DOI] [PubMed] [Google Scholar]
- 54.Mylona E, Baraboutis IG, Georgiou O, et al. Solid variant of primary effusion lymphoma in successfully treated HIV infection: a case report. Int J STD AIDS. 2008;19:570–572. doi: 10.1258/ijsa.2007.007285. [DOI] [PubMed] [Google Scholar]


