According to the specified primary objective, a regimen of cisplatin, paclitaxel and bevacizumab was tolerable in Japanese patients and demonstrated encouraging activity in this small single-arm study.
Keywords: bevacizumab, cervical cancer, anti-angiogenic, Japanese
Abstract
Background
Adding bevacizumab to chemotherapy for recurrent, persistent or metastatic cervical cancer significantly improved overall survival (primary endpoint), progression-free survival and overall response rate in the randomized Phase III GOG-0240 trial. However, data for bevacizumab-containing therapy are scarce in Japanese patients with advanced cervical cancer.
Methods
The primary objective of the single-arm multicenter Phase II JO29569 study was to evaluate the tolerability of paclitaxel (135 mg/m2 over 24 h or 175 mg/m2 over 3 h), cisplatin (50 mg/m2) and bevacizumab (15 mg/kg), administered every 3 weeks until disease progression or unacceptable toxicity in Japanese patients with stage IVB, persistent or recurrent cervical cancer.
Results
The seven treated patients received a median of nine (range 7–12) bevacizumab cycles and six (range 4–12) chemotherapy cycles. None of the predefined adverse events occurred during the tolerability evaluation period. The most common all-grade adverse events were alopecia, hypertension, decreased appetite, nausea and peripheral sensory neuropathy. There were no cases of fistula. The most common grade ≥3 adverse events were hypertension, neutrophil count decreased and neutropenia. Only one patient experienced febrile neutropenia. The overall response rate was 86% (95% confidence interval, 42–100%), including a complete response in one patient. At data cutoff, disease had progressed in one patient; bevacizumab therapy was ongoing in the remaining six.
Conclusions
According to the specified primary objective, a regimen of cisplatin, paclitaxel and bevacizumab was tolerable in Japanese patients and demonstrated encouraging activity in this small single-arm study. Further study is warranted to confirm the safety and effectiveness of bevacizumab in Japanese patients with cervical cancer.
Introduction
Globally, cervical cancer is the fourth most common cancer in women, and the seventh overall (1). In 2012 there were an estimated 528 000 new cases of cervical cancer and 266 000 deaths from this disease, representing 7.5% of all deaths from cancer in women. Corresponding figures specifically for Japan in 2012 were 9390 new cases and 3645 deaths, making cervical cancer the fifth most common cancer in Japanese women after breast, colorectal, gastric and lung cancers. The incidence of cervical cancer and associated mortality in Japan have been increasing since 1990 (2), particularly in younger women. Among women aged <50 years, mortality from cervical cancer has doubled during the past three decades (3). A recently reported cancer registry study of age-specific mortality rates from cervical cancer in the Kanagawa population indicated increasing trends for women aged 20–29 and 30–49. The particularly low rate of cervical screening in young Japanese women is likely to contribute to this discrepancy with Western countries; furthermore, proactive recommendations for human papilloma virus vaccination have been suspended in Japan (4,5).
For patients with advanced cervical cancer, one of the most important recent advances has been the introduction of bevacizumab. This monoclonal antibody targets vascular endothelial growth factor (VEGF), the key mediator of tumor angiogenesis. Bevacizumab has demonstrated efficacy in a wide range of solid tumor types, including advanced cervical cancer. In the Gynecologic Oncology Group (GOG)-0240 randomized Phase III trial, the addition of bevacizumab to chemotherapy (either cisplatin plus paclitaxel or topotecan plus paclitaxel) for recurrent, persistent or metastatic cervical cancer significantly improved overall survival (primary endpoint), progression-free survival (secondary endpoint) and the overall response rate (secondary endpoint) (6). These significant efficacy benefits were not associated with any significant detrimental effect of bevacizumab on health-related quality of life (7). Bevacizumab was associated with a significantly increased incidence of grade ≥2 hypertension (25% versus 2% in patients receiving chemotherapy alone), but no patients discontinued bevacizumab because of this side effect. Grade ≥3 gastrointestinal and genitourinary fistulas were also more common in patients receiving bevacizumab-containing therapy compared with chemotherapy alone, driven by an elevated incidence of gastrointestinal-vaginal fistulae [8.3% versus 0.9%, respectively (8)]. However, incidences of other typical bevacizumab-associated adverse events were similar to those reported for other tumor types and there was no difference in the incidence of fatal adverse events between the bevacizumab and non-bevacizumab treatment arms.
The GOG-0240 trial was conducted predominantly in the United States, with a small number of additional participating sites in Spain. Data in Japanese patients treated with bevacizumab for cervical cancer are extremely limited. Therefore, we conducted the present single-arm study to assess the tolerability, safety and activity of bevacizumab-containing therapy in a Japanese population of patients with advanced cervical cancer.
Patients and methods
Study design
JO29569 was a multicenter single-arm phase II study evaluating the combination of paclitaxel, cisplatin and bevacizumab in Japanese patients with stage IVB, persistent or recurrent cervical cancer. The target sample size for the study was six patients, based on the fact that tolerability is typically evaluated in 3–6 patients per cohort in most studies with the primary objective of evaluating the tolerability of a given treatment.
The study protocol was approved by the Independent Review Board of each participating site.
Patients
Eligible patients were aged ≥20 years and had GOG performance status 0 or 1, radiologically confirmed stage IVB, persistent or recurrent cervical cancer that was not amenable to curative treatment with surgery or radiation therapy (including concurrent chemoradiotherapy), and a life expectancy of ≥3 months. Patients had to have adequate hematologic, renal and hepatic function. Patients were ineligible if they had received any prior chemotherapy (except with concurrent radiation) for recurrent disease, any prior anti-VEGF or anti-VEGF receptor therapy, prior surgery for cervical cancer within the preceding 4 weeks, radiation (without concurrent chemotherapy) within the preceding 3 weeks, chemoradiation within the preceding 6 weeks, major surgery within the preceding 4 weeks or anticipated during the course of the study, or biopsy within 1 week before study entry. Patients were not eligible if they had: clinical signs or symptoms of intestinal obstruction requiring parenteral hydration or nutrition; a serious non-healing wound, ulcer or bone fracture; a history of abdominal fistula, gastrointestinal perforation or intra-abdominal abscess within 6 months before study entry; bilateral hydronephrosis that could not be alleviated by ureteral stent or percutaneous drainage; complications or history of symptomatic cerebrovascular accident within 6 months before study entry; ongoing grade ≥2 peripheral neuropathy according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03; central nervous system metastases that were symptomatic or required treatment; or complications or history of NCI CTCAE grade ≥2 hemoptysis within 1 month before study entry. Patients were also excluded if they were considered to be at high risk of bleeding, or if they were pregnant or nursing. All patients provided written informed consent.
Treatment
All patients received cisplatin 50 mg/m2 combined with paclitaxel given either at 135 mg/m2 infused over 24 h or 175 mg/m2 infused over 3 h (choice of paclitaxel regimen at the investigator's discretion). In addition, all patients received bevacizumab 15 mg/kg. Treatment with all three agents was repeated every 3 weeks until disease progression or unacceptable adverse events. If a patient discontinued chemotherapy because of an adverse event, bevacizumab could be continued as a single agent provided the adverse event had not been caused by bevacizumab.
Study objectives
The primary objective was to evaluate the tolerability of the bevacizumab, cisplatin and paclitaxel combination in Japanese patients with advanced or recurrent cervical cancer. The evaluation period for tolerability was from day 1 to day 21 of cycle 1. The regimen was considered tolerable if no more than 34% of patients evaluable for tolerability experienced any of the following adverse events (graded according to NCI CTCAE version 4.03) considered by the investigator to be related to any of the study drugs: grade 4 neutrophil count decrease persisting for ≥7 days; febrile neutropenia; grade 4 platelet count decrease or grade 3 platelet count decrease requiring platelet transfusion; grade 4 hypertension; grade ≥3 diarrhea, nausea, vomiting or rash that could not be controlled with appropriate medical management; grade ≥3 liver function abnormality; or other grade ≥3 non-hematologic toxicity, excluding transient electrolyte abnormality. A ≥3-week delay in administration of cycle 2 because of drug-related adverse events was also considered as a qualifying adverse event.
Secondary objectives were to evaluate the safety (frequency, severity and time to onset of adverse events) and efficacy (tumor response according to Response Evaluation Criteria in Solid Tumors [version 1.1], confirmed at least 42 days after the first recorded response) of the triplet regimen. The following adverse events were considered to be of special interest for bevacizumab and were reported according to grouped preferred terms: any grade of non-gastrointestinal fistula or abscess; any grade of gastrointestinal perforation; grade ≥3 bleeding; grade ≥3 congestive heart failure or left ventricular systolic dysfunction; febrile neutropenia; grade ≥3 hypertension; grade ≥3 proteinuria; any grade of arterial thromboembolic event; grade ≥3 venous thromboembolic event; grade ≥3 wound-healing complication; or any grade of posterior reversible encephalopathy syndrome. Adverse events were classified as serious adverse events if they met any of the following criteria: fatal; life threatening; requiring or prolonging inpatient hospitalization; resulting in persistent or significant disability or incapacity; a congenital anomaly or birth defect in a neonate or infant born to a mother exposed to study drug; or a significant medical event in the investigator's judgment.
The primary analysis was prespecified when the last enrolled patient completed cycle 1 observation. The follow-up analysis reported here was not prespecified.
Results
Patient population
Between 10 January 2015, and 14 April 2015, eight patients were enrolled from six centers in Japan. One patient discontinued before receiving treatment because her condition was considered by the investigator to be too poor to start investigational treatment (poor renal function, elevated blood pressure); the remaining seven patients received study treatment and had measurable disease, and were thus included in the tolerability, safety and efficacy analyses. Baseline characteristics are shown in Table 1, with further details including the selected treatment schedule provided in Supplementary data, Table S1 (online only).
Table 1.
Patient characteristics at baseline
| Characteristic | JO29569 (n = 7) |
|---|---|
| Median age, years (range) | 61 (34–69) |
| GOG performance status, n (%) | |
| 0 | 6 (86) |
| 1 | 1 (14) |
| Histologic subtype, n (%) | |
| Squamous cell carcinoma | 3 (43) |
| Adenocarcinomaa | 2 (29) |
| Adenosquamous | 1 (14) |
| Small cell carcinoma | 1 (14) |
| Disease status, n (%) | |
| Stage IVB | 3 (43) |
| Recurrent | 4 (57) |
| Persistent | 0 |
| Prior platinum therapy, n (%) | 2 (29)b |
| Prior radiotherapy to pelvis, n (%) | 3 (43) |
GOG, Gynecologic Oncology Group.
aMucinous adenocarcinoma (endocervical type) in one patient, adenocarcinoma unspecified in one patient.
bWith concurrent chemoradiation.
Treatment exposure
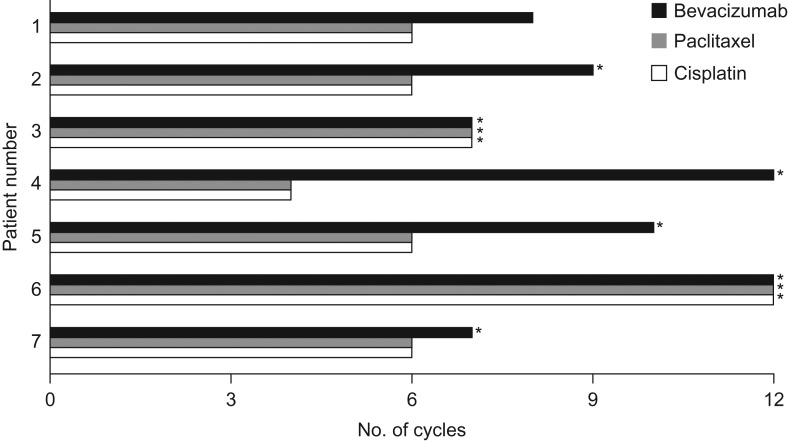
The data cutoff for the primary analysis was 7 May 2015, when the last patient completed cycle 1 observation. We report an updated analysis with a data cutoff of 14 October 2015. As of this date, the median number of bevacizumab treatment cycles delivered was nine (range 7–12). The median number of cycles of paclitaxel and cisplatin was six (range 4–12). These medians represent 6.5 months of bevacizumab exposure (range 5.1–8.7 months) and 3.7 months of chemotherapy exposure (range 2.1–8.4 months). Treatment exposure is summarized in Fig. 1.
Figure 1.
Summary of treatment administered by patient. *Treatment ongoing.
Five patients continued bevacizumab as a single agent after discontinuation of chemotherapy. The median duration of single-agent bevacizumab was three cycles (range 1–8). At the time of data cutoff, one patient had discontinued study therapy because of disease progression. The remaining patients were still receiving at least one component of study therapy.
Tolerability
None of the seven treated patients experienced any of the adverse events predefined for the primary endpoint during the tolerability evaluation period. Therefore, according to the specified primary objective, the regimen was considered tolerable in Japanese patients.
Safety
All seven treated patients experienced at least one adverse event of grade ≥3 intensity, including grade 4 adverse events in three patients (43%), but there were no fatal adverse events. The most common adverse events (all grades) were alopecia, hypertension, decreased appetite, nausea and peripheral sensory neuropathy (Table 2). There were no cases of fistula. The most common grade ≥3 adverse events were hypertension, neutrophil count decreased and neutropenia; however, only one patient experienced febrile neutropenia (Table 2).
Table 2.
Adverse events
| Adverse event, n (%) | All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| All events | 7 (100) | 7 (100) | 7 (100) | 7 (100) | 3 (43) |
| Alopecia | 7 (100) | 0 | 7 (100) | 0 | 0 |
| Hypertension | 6 (86) | 0 | 3 (43) | 3 (43)a | 0 |
| Decreased appetite | 6 (86) | 3 (43) | 3 (43) | 0 | 0 |
| Nausea | 6 (86) | 4 (57) | 2 (29) | 0 | 0 |
| Peripheral sensory neuropathy | 6 (86) | 3 (43) | 3 (43) | 0 | 0 |
| Malaise | 4 (57) | 2 (29) | 2 (29) | 0 | 0 |
| Dysgeusia | 4 (57) | 2 (29) | 2 (29) | 0 | 0 |
| Neutrophil count decreased | 3 (43) | 0 | 1 (14) | 1 (14) | 1 (14) |
| Stomatitis | 3 (43) | 1 (14) | 2 (29) | 0 | 0 |
| Upper respiratory tract infection | 3 (43) | 0 | 3 (43) | 0 | 0 |
| Constipation | 3 (43) | 1 (14) | 2 (29) | 0 | 0 |
| Nail discoloration | 3 (43) | 3 (43) | 0 | 0 | 0 |
| Flushing | 3 (43) | 3 (43) | 0 | 0 | 0 |
| Neutropenia | 2 (29) | 0 | 0 | 1 (14) | 1 (14) |
| Anemia | 2 (29) | 1 (14) | 0 | 1 (14) | 0 |
| Dehydration | 2 (29) | 0 | 1 (14) | 1 (14) | 0 |
| Proteinuria | 2 (29) | 0 | 1 (14) | 1 (14)a | 0 |
| Blood creatinine increased | 2 (29) | 0 | 2 (29) | 0 | 0 |
| Vomiting | 2 (29) | 1 (14) | 1 (14) | 0 | 0 |
| Fatigue | 2 (29) | 1 (14) | 1 (14) | 0 | 0 |
| Back pain | 2 (29) | 1 (14) | 1 (14) | 0 | 0 |
| Hemorrhoids | 2 (29) | 2 (29) | 0 | 0 | 0 |
| Infusion-site pain | 2 (29) | 2 (29) | 0 | 0 | 0 |
| Insomnia | 2 (29) | 2 (29) | 0 | 0 | 0 |
| Febrile neutropenia | 1 (14) | 0 | 0 | 0 | 1 (14)a |
| Diarrhea | 1 (14) | 0 | 0 | 1 (14) | 0 |
| Hyponatremia | 1 (14) | 0 | 0 | 1 (14) | 0 |
| Gingivitis | 1 (14) | 0 | 0 | 1 (14) | 0 |
| Pyelonephritis | 1 (14) | 0 | 0 | 1 (14) | 0 |
| Infusion-site phlebitis | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Wound infection | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Herpes zoster | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Gamma glutamyltransferase increased | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Ingrowing nail | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Urinary tract infection | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Weight decreased | 1 (14) | 0 | 1 (14) | 0 | 0 |
| Pruritus | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Rash maculopapular | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Abdominal pain upper | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Infusion-site edema | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Edema | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Oral herpes | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Alanine aminotransferase increased | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Blood pressure increased | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Arthralgia | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Myalgia | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Hot flush | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Pharyngeal inflammation | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Periodontal disease | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Abdominal discomfort | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Fecal incontinence | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Chills | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Face edema | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Infusion-site induration | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Infusion-site swelling | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Edema peripheral | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Periodontitis | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Tinea infection | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Headache | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Dizziness | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Cough | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Contusion | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Excoriation | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Fall | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Anxiety | 1 (14) | 1 (14) | 0 | 0 | 0 |
| Tinnitus | 1 (14) | 1 (14) | 0 | 0 | 0 |
aAdverse event of special interest for bevacizumab.
The majority of adverse events occurred during the concurrent chemotherapy and bevacizumab treatment phase. Among the five patients who received single-agent bevacizumab after discontinuation of chemotherapy, three experienced a grade 3 adverse event (one case each of hypertension, proteinuria and hyponatremia). However, these adverse events were manageable with appropriate treatment or temporary interruption of bevacizumab. No serious adverse events were observed after discontinuation of chemotherapy (Supplementary data, Table S2, online only).
Apart from the three cases of grade ≥3 hypertension, one case of grade 4 febrile neutropenia and one case of grade 3 proteinuria mentioned above, none of the other predefined adverse events of special interest for bevacizumab was observed. Adverse events led to discontinuation of paclitaxel in five patients (71%; peripheral sensory neuropathy in four patients; malaise and decreased appetite in one patient) and discontinuation of cisplatin in five patients (71%; fatigue in two patients; malaise and decreased appetite in one patient; nausea in one patient; increased blood creatinine in one patient) (Table 3). None of the patients discontinued bevacizumab because of adverse events.
Table 3.
Summary of efficacy and reason for treatment discontinuation by patient
| Patient number | Best overall response | PFS, months | Reason for treatment discontinuation | ||
|---|---|---|---|---|---|
| Paclitaxel | Cisplatin | Bevacizumab | |||
| 1 | PR | 5.6 | Grade 1 peripheral sensory neuropathy | Grade 1 fatigue | Disease progression |
| 2 | PR | 5.4a | Grade 2 peripheral sensory neuropathy | Grade 1 fatigue | – |
| 3 | PR | 4.8a | – | – | – |
| 4 | PR | 7.6a | Grade 2 peripheral sensory neuropathy | Grade 2 blood creatinine increased | – |
| 5 | CR | 5.9a | Grade 2 malaise, grade 2 decreased appetite | Grade 2 malaise, grade 2 decreased appetite | – |
| 6 | SD | 7.4a | – | – | – |
| 7 | PR | 5.8a | Grade 1 peripheral neuropathy | Grade 1 nausea | – |
PFS, progression-free survival; PR, partial response; CR, complete response; SD, stable disease.
aCensored observation, treatment ongoing.
Bevacizumab was temporarily interrupted because of adverse events in all seven patients. Proteinuria and hypertension each led to temporary interruption of bevacizumab in two patients, as did grade 2 neutropenia/neutrophil count decreased and grade 2 blood creatinine increased. Other causes of bevacizumab interruption, each occurring in only one patient and all considered unrelated to bevacizumab, were: grade 2 peripheral neuropathy, grade 3 hyponatremia, pyelonephritis (two episodes in one patient), herpes zoster infection and anemia. All cases resolved following bevacizumab interruption. In five patients, the chemotherapy dose was reduced or treatment was interrupted because of adverse events.
Two patients experienced adverse events that met the criteria for serious adverse events (prolonging hospitalization): grade 4 febrile neutropenia in one patient and grade 3 pyelonephritis (considered unrelated to study treatment) in another patient.
Efficacy
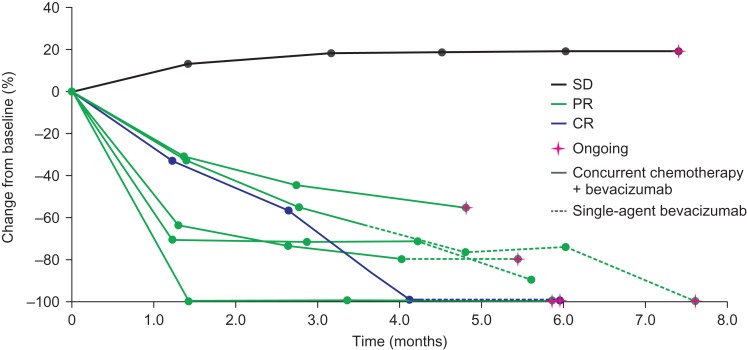
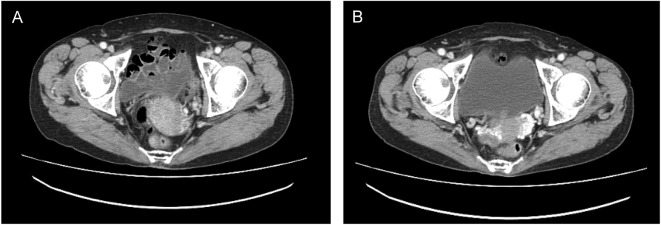
The overall response rate was 86% (95% confidence interval, 42–100%), comprising a complete response in one patient (14%) and partial responses in five patients (71%). The remaining patient had a best response of stable disease. Change in tumor volume from baseline over time is shown in Fig. 2. In three patients, including the patient with a complete response, all target legions disappeared; however, in two patients evaluation of non-target lesions did not meet the criteria for complete response, therefore, these patients were assessed as partial responders. Computed tomography scans from one patient before and after bevacizumab treatment are shown in Fig. 3.
Figure 2.
Change in tumor volume from baseline. CR, complete response; PR, partial response; SD, stable disease.
Figure 3.
Patient case: computed tomography: (A) before treatment (screening) and (B) after treatment (cycle 7).
Progression-free survival results are immature as six patients remained on treatment with sustained disease control at the time of data cutoff.
Discussion
In this small prospective clinical study designed to assess the tolerability, safety and activity of bevacizumab-containing therapy in Japanese patients with advanced or recurrent cervical cancer, the combination of bevacizumab, cisplatin and paclitaxel was tolerable. Generally, the safety profile was consistent with previous reports of Japanese populations receiving bevacizumab in other disease settings (9, 10) and the safety profile observed in the bevacizumab-containing arm of the GOG-0240 randomized Phase III trial in cervical cancer (6). Importantly, there were no cases of fistula.
The chemotherapy schedules administered in JO29569 were identical to the chemotherapy options available to patients in the cisplatin plus paclitaxel arm of the GOG-0240 trial. However, in GOG-0240, continuation of bevacizumab was not specified if patients experienced adverse events necessitating discontinuation of chemotherapy. In the present study, patients could continue to receive bevacizumab as a single agent if chemotherapy was discontinued because of adverse events unrelated to bevacizumab. Five of the seven patients in JO29569 received single-agent bevacizumab after discontinuing chemotherapy and this treatment strategy raised no safety concerns. There is speculation in the literature about the potential additional benefits that may be gained from maintenance bevacizumab in cervical cancer (11). Adverse events during the maintenance bevacizumab phase were infrequent and were manageable with appropriate treatment or temporary interruption of bevacizumab. Furthermore, as four of the six patients still receiving treatment with sustained clinical benefit are receiving bevacizumab as a single agent without chemotherapy, our observations seem to lend support to the sustained disease control potentially offered by a maintenance bevacizumab approach.
A striking difference between the population of patients enrolled in the present study and those in GOG-0240 is their age: median age in the Japanese study was 61 years compared with 47 years in the Phase III trial. Only one patient in the Japanese study was aged <55 years. This is slightly surprising given the reported preponderance of younger women presenting with cervical cancer in Japan, and may reflect some degree of selection bias for the present study. On the other hand, all but the youngest patient in JO29569 had GOG performance status 0 whereas in GOG-0240, almost half of the patients had GOG performance status 1.
The median duration of study treatment was nine cycles in the present study compared with seven cycles in patients receiving bevacizumab plus chemotherapy in GOG-0240. Most patients (six of seven) were still receiving bevacizumab at the time of data cutoff for the present report, and all of these still appeared to be deriving clinical benefit, showing maintained disease stabilization, partial response and even complete response in one patient.
Interestingly, no cases of fistula, including gastrointestinal-vaginal fistula, were observed in the present study in Japanese patients. In the GOG-0240 trial, an exploratory analysis of the clinico-pathologic characteristics associated with development of fistulae revealed that all of the patients who developed gastrointestinal-vaginal fistulae had received prior pelvic irradiation (8). In JO29569, three of the seven patients had received pelvic irradiation. Although the lack of gastrointestinal-fistula events, even in patients with prior pelvic irradiation, is reassuring, it is impossible to draw firm conclusions about this particular adverse event in Japanese patients from such a small study. In addition, these findings should be interpreted with a degree of caution given the duration of exposure and ongoing bevacizumab therapy in six patients. In GOG-0240, the median time of fistula appearance was cycle 5, and fistula events were observed as late as cycle 10 or 12 in some patients (6).
The incidence of grade ≥2 hypertension in the present study (86%) appears to be considerably higher than that reported in GOG-0240 (25%). This apparent difference may be explained in part by the different versions of the NCI CTCAE used: in GOG-0240, adverse events were graded according to version 3.0 whereas in the present study we used the more recent version 4.03, which has more stringent criteria for hypertension. Consistent with GOG-0240, none of the patients treated in the present study required discontinuation of bevacizumab because of hypertension (or indeed any adverse event). Interestingly, grade ≥3 thromboembolic events were more common with bevacizumab-containing therapy than chemotherapy alone in GOG-0240, but we observed no thromboembolic events among Japanese patients treated with bevacizumab in JO29569.
Although the safety and efficacy data in Japanese patients with cervical cancer are limited, being based on only seven patients treated in the present study, the results reported here together with results from the GOG-0240 trial give no reason to anticipate unacceptable toxicity in Japanese patients receiving bevacizumab-containing therapy for cervical cancer. Efficacy results should be interpreted with caution, especially in a single-arm study. Nevertheless, the 86% response rate provides a reassuring suggestion of activity.
To the best of our knowledge, the only other reported data on bevacizumab-containing therapy for cervical cancer in Japanese patients come from a series of 12 patients treated with bevacizumab, paclitaxel and carboplatin with or without the multikinase inhibitor sorafenib (12). However, as the dose and schedule of both bevacizumab and chemotherapy were quite different from those administered in our study, and seven patients also received sorafenib, it is difficult to draw any conclusion about safety from the limited data reported.
Further investigation of bevacizumab plus chemotherapy will be needed in Japanese patients with cervical cancer given the paucity of data in this setting. The ongoing CECILIA study will provide additional data on the tolerability and activity of bevacizumab in populations from geographic regions other than the US and Spain, as it is recruiting in South America, South Africa and Europe (13). However, no sites from Asia are involved in this study. Furthermore, the CECILIA study is designed to assess the safety of bevacizumab not only in different geographic regions with differing healthcare systems and standards, but also to answer the important question concerning whether bevacizumab can be combined with a carboplatin-based chemotherapy backbone rather than the cisplatin/paclitaxel regimen evaluated both in GOG-0240 and in the present Japanese study. In many countries, carboplatin may be preferred to cisplatin. Use of a carboplatin chemotherapy backbone in Japanese patients is supported by results of the recently published Japanese JCOG0505 randomized phase III trial, which demonstrated non-inferior overall survival with carboplatin plus paclitaxel versus conventional cisplatin plus paclitaxel in patients with metastatic or recurrent cervical cancer. In exploratory subgroup analyses, overall survival was more favorable with carboplatin plus paclitaxel in patients previously treated with platinum-containing therapy, whereas in platinum-naïve patients, cisplatin plus paclitaxel was associated with improved overall survival (14). To date, no safety data are available for this alternative bevacizumab-containing regimen but there is no reason to expect a difference in safety profile, particularly as a regimen of paclitaxel, carboplatin and bevacizumab has been evaluated extensively in other tumor types including ovarian cancer (15,16).
In summary, the GOG-0240 regimen of bevacizumab, cisplatin and paclitaxel was shown to be tolerable with a predictable and manageable safety profile in Japanese patients with advanced or recurrent cervical cancer, albeit in a small single-arm study. The combination of bevacizumab plus chemotherapy seems to be an appropriate treatment option for Japanese patients with advanced or recurrent cervical cancer.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Funding
This work was supported by Chugai Pharmaceutical Co. Ltd. Third-party medical writing support was funded by Chugai.
Conflict of interest statement
Toru Sugiyama and Nobuhiro Takeshima have received consultancy fees/honoraria from and served as members of an advisory board for Chugai Pharmaceutical Co. Ltd. Eisuke Ueda and Kosei Tajima are employees of Chugai Pharmaceutical Co. Ltd. Mika Mizuno, Yoichi Aoki, Manabu Sakurai and Tadaaki Nishikawa have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2013; (6 April 2016, date last accessed).http://globocan.iarc.fr. [Google Scholar]
- 2.Katanoda K, Hori M, Matsuda T, et al. . An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol 2015;45:390–401. [DOI] [PubMed] [Google Scholar]
- 3.Motoki Y, Mizushima S, Taguri M, et al. . Increasing trends in cervical cancer mortality among young Japanese women below the age of 50 years: an analysis using the Kanagawa population-based Cancer Registry, 1975–2012. Cancer Epidemiol 2015;39:700–6. [DOI] [PubMed] [Google Scholar]
- 4.Egawa-Takata T, Ueda Y, Tanaka Y, et al. . Mothers’ attitudes in Japan regarding cervical cancer screening correlates with intention to recommend cervical cancer screening for daughters. Int J Clin Oncol 2016[Epub ahead of print]. DOI: 10.1007/s10147-016-0970-4. [DOI] [PubMed] [Google Scholar]
- 5.Hanley SJ, Fujita H, Yokoyama S, et al. . HPV self-sampling in Japanese women: a feasibility study in a population with limited experience of tampon use. J Med Screen 2016;23:164–70 [DOI] [PubMed] [Google Scholar]
- 6.Tewari KS, Sill MW, Long HJ III, et al. . Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penson RT, Huang HQ, Wenzel LB, et al. . Bevacizumab for advanced cervical cancer: patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240). Lancet Oncol 2015;16:301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roche Avastin Summary of Product Characteristics 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000582/WC500029271.pdf (6 April 2016, date last accessed).
- 9.Hatake K, Doi T, Uetake H, Takahashi Y, Ishihara Y, Shirao K. Bevacizumab safety in Japanese patients with colorectal cancer. Jpn J Clin Oncol 2016;46:234–40. [DOI] [PubMed] [Google Scholar]
- 10.Aogi K, Masuda N, Ohno S, et al. . First-line bevacizumab in combination with weekly paclitaxel for metastatic breast cancer: efficacy and safety results from a large, open-label, single-arm Japanese study. Breast Cancer Res Treat 2011;129:829–38. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander ML. Commentary on the clinical trial reported by: Tewari KS, Sill M, Long HJ III, et al. Incorporation of bevacizumab in the treatment of recurrent and metastatic cervical cancer: a phase III randomized trial of the Gynecologic Oncology Group. J Clin Oncol 2013;(suppl; abstr 3). Chin Clin Oncol 2014;3:6. [DOI] [PubMed]
- 12.Kikuchi Y, Takano M, Goto T, et al. . Effects of weekly bevacizumab and paclitaxel/carboplatin with or without sorafenib on heavily pretreated patients with recurrent or persistent cervical cancer. J Clin Oncol 2011;29 (suppl; abstr 5085). [Google Scholar]
- 13.Redondo A, Colombo N, McCormack M, et al. CECILIA: an open-label global safety study evaluating bevacizumab, carboplatin and paclitaxel therapy in patients with metastatic, recurrent or persistent cervical cancer. In: International Meeting of the European Society of Gynaecological Oncology; October 24–27, 2015, Nice, France (abstract 1078).
- 14.Kitagawa R, Katsumata N, Shibata T, et al. . Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized phase III trial JCOG0505. J Clin Oncol 2015;33:2129–35. [DOI] [PubMed] [Google Scholar]
- 15.Burger RA, Brady MF, Bookman MA, et al. ; Gynecologic Oncology Group Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473–83. [DOI] [PubMed] [Google Scholar]
- 16.Perren TJ, Swart AM, Pfisterer J, et al. . ICON7 Investigators A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. [DOI] [PubMed] [Google Scholar]