Abstract
Mycobacterium africanum lineage (L) 6 is an important pathogen in West Africa, causing up to 40% of pulmonary tuberculosis (TB). The biology underlying the clinical differences between M. africanum and M. tuberculosis sensu stricto remains poorly understood. We performed ex vivo expression of 2179 genes of the most geographically dispersed cause of human TB, M. tuberculosis L4 and the geographically restricted, M. africanum L6 directly from sputa of 11 HIV-negative TB patients from The Gambia who had not started treatment. The DosR regulon was the most significantly decreased category in L6 relative to L4. Further, we identified nonsynonymous mutations in major DosR regulon genes of 44 L6 genomes of TB patients from The Gambia and Ghana. Using Lebek's test, we assessed differences in oxygen requirements for growth. L4 grew only at the aerobic surface while L6 grew throughout the medium. In the host, the DosR regulon is critical for M. tuberculosis in adaptation to oxygen limitation. However, M. africanum L6 appears to have adapted to growth under hypoxic conditions or to different biological niches. The observed under expression of DosR in L6 fits with the genomic changes in DosR genes, microaerobic growth and the association with extrapulmonary disease.
Keywords: Mycobacterium africanum, Mycobacterium tuberculosis, Hypoxia, Sputum, Gene expression, DosR
1. Introduction
Mycobacterium africanum lineages (L) 5 and 6 and M. tuberculosis sensu stricto (L1-L4, L7) belong to the M. tuberculosis complex (MTBC) and co-evolved with distinct human populations [1], [2], [3], [4]. Whereas the global spread of M. tuberculosis lineages 1–4 was associated with urbanization and population expansion, the two lineages of M. africanum, for reasons still unknown, primarily remain geographically restricted to West Africa. Interestingly, M. tuberculosis is relatively more virulent than M. africanum L6 as evidenced by significantly faster progression, in contacts of infectious cases, to active tuberculosis (TB) disease [5]. M. africanum L6 more commonly causes disease in persons with HIV infection, older age and malnutrition, implying a more opportunistic pathogen [6], [7], [8]. Furthermore, M. africanum lineages grow markedly slower than M. tuberculosis [9], [10]. Taken together, clear phenotypic contrasts exist between the lineages of M. africanum and M. tuberculosis, yet the biology underlying the observed differences is still poorly understood.
Lacking an environmental reservoir, the proliferation of the MTBC relies on successful transmission from a diseased to a susceptible host via inhalation of aerosols containing bacilli. The bacteria maintain a complex life cycle enhancing transmission. Upon inhalation, bacilli reaching the lungs are engulfed by alveolar macrophages. Subsequently, infected macrophages induce immune responses leading to the recruitment of further immune cells and the formation of a granuloma. If the host is able to control the pathogen, the bacilli persist within the host for prolonged periods without causing disease. At this noninfectious stage, central metabolism and replication are shut down with bacilli moving into a state of dormancy until conditions for active replication, such as immunosuppression, return [11]. During active TB, when the host is contagious, granuloma maturation occurs leading to the release of bacilli into airways and subsequent expectoration as infectious aerosols [11]. To overcome this infection-to-transmission bottleneck, the bacterium adapted to all stages in its life cycle and developed numerous strategies, including the ability to rapidly adjust to changes from extracellular aerobic environments to hypoxic and nutrient-limited conditions commonly encountered during intracellular survival.
Mycobacterium tuberculosis, although classified as an obligate aerobe, survives during anaerobiosis. In the Wayne model, where cultures undergo gradual self-generated oxygen depletion and shift through microaerobic to anaerobic conditions, bacilli stop growing but survive [12]. The well-studied M. tuberculosis DosR regulon is crucial in this regard, as it controls the adaptation to oxygen limitation and has also been linked to virulence [13], [14]. A recent study described roles for a number of metabolic pathways in the reference strain H37Rv (M. tuberculosis L4) by demonstrating regulatory networks were built around DosR (DevR) and Rv0081, indicating they are the major sensors orchestrating the switch from aerobic to anaerobic survival [15]. Further, dosR (devR) was upregulated during hypoxia and downregulated upon reaeration, a switch that can occur within 5 min [14], [15]. In vitro, dosR is overexpressed in Beijing strains (lineage 2) [16], [17]. However, little is known about the state and expression of genes within the DosR regulon of M. africanum and its response to hypoxia, although early literature indicated a preference for microaerobic growth [18].
Previous studies suggest a different intracellular survival strategy of M. africanum L6 when compared to M. tuberculosis evidenced by non-synonymous mutations in 5 of 7 operons essential for intracellular survival within host macrophages [10]. We here present results from an exploratory analysis in which we compared the ex vivo expression of 2179 genes of M. tuberculosis L4 and M. africanum L6 directly in sputa of HIV-negative TB patients. The DosR regulon was the most significantly differentially expressed category, with lower expression in M. africanum L6 relative to M. tuberculosis L4. Moreover, in a phenotypic analysis using Lebek's test for oxygen preference, we confirmed that M. africanum L6 grew microaerobically while M. tuberculosis L4 grew best aerobically. We also identified sequence polymorphisms in the differentially expressed genes, as well as related genes, in 44 M. africanum L6 genomes. Our results suggest that M. africanum L6 is less dependent on the DosR regulon and more adapted to a microaerobic lifestyle.
2. Materials and methods
2.1. Patients
Adults with new sputum smear positive TB in The Gambia were recruited between 2006 and 2009 and isolates were genotyped as previously described [19]. The study was approved by ethical committees in the Gambia, Stanford, and New York University and all patients provided written informed consent. For this analysis on strain differences, gene expression analysis on M. tuberculosis L4 and M africanum L6 from sputum was only performed if patients had not yet initiated treatment and were HIV negative. Eleven sputa from 5 M. africanum L6 and 6 M. tuberculosis L4 infected individuals were consecutively selected for analysis from a total of 27 patients with RNA of sufficient quality and quantity. The HIV status of patients infected with M. africanum L6 could only be confirmed for 3 of the 5 patients. Therefore, although gene expression results were available for the two patients with unconfirmed HIV status (Supplemental Table S1, Supplementary Material online), we excluded these from further analysis.
2.2. Sputum collection and gene expression
Spontaneously expectorated sputum was collected in guanidine thiocyanate (GTC) and resuspended in Trizol for RNA isolation and extraction using previously described methods [20]. We assayed expression of 2179 selected M. tuberculosis genes (54% of the genome) via multiplex quantitative RT-PCR (TaqMan) with a LightCycler 480 (Roche, Indianapolis, Indiana). Genotyping was performed on parallel sputum cultures as described previously [19].
Gene expression data was normalized using a median approach [15], a method appropriate for unpaired data with low levels of non-detection [21]. An unpaired, equal variance t-test was used to identify differential expression between M. africanum L6 and M. tuberculosis L4 strains. A modified Fisher's Exact test [22], [23] was then performed on differentially expressed genes (p-value < 0.05) on TB specific categories [20] with Bonferroni multiple testing correction. Predicted gene functional annotations used in the supplemental tables were derived from MycoBASE and Tuberculist [22], [24].
2.3. Detection of Single Nucleotide Polymorphisms
Single Nucleotide Polymorphisms (SNPs) within and between genes have the potential to affect gene expression. To ascertain if there were lineage specific mutations within genes of M. africanum L6, and whether the primers designed for L4 could bind L6, we compared SNPs in 44 M. africanum L6 strains isolated from TB patients both from The Gambia and Ghana with a reference dataset of SNPs in other lineages (Coscolla et al. in preparation). We used Burrows-Wheeler Aligner (BWA) to map Illumina reads against the MTBC reference genome described in Ref. [4]. BWA outputs were analyzed with SAMtools [25], [26] to detect variable positions with respect to the reference genome. We applied heuristic filters to remove problematic positions. Filtering criteria were: Phred-scaled probability scores <20 and with read depth more than double the average read depth of the genome. Ambiguous base calls (i.e. more than one nucleotide called) were excluded. SNP lists for individual strains were combined into a single non-redundant database, and were annotated with ANNOVAR [27] using H37Rv annotation as a reference. SNPs in repeat-containing genes (REP13E12), family protein PE/PPE/PGRS, integrase, transposase resolvase, maturase, or phage were excluded, and the final high-confidence list of SNPs was used to recover the corresponding base call for each genome.
2.4. Culture of M. africanum and M. tuberculosis in Lebek's medium
To determine if oxygen requirements for growth differed between M. tuberculosis L4 and M. africanum L6, Lebek's test for oxygen preference was carried out as described previously [18], except that the test was conducted in polypropylene-rather than glass tubes to comply with biosafety requirements. Briefly, a 2 mg/ml bacterial suspension was mixed with liquid agar based medium before it solidified, followed by incubation at 37 °C.
3. Results
3.1. DosR regulon gene expression in M. africanum L6
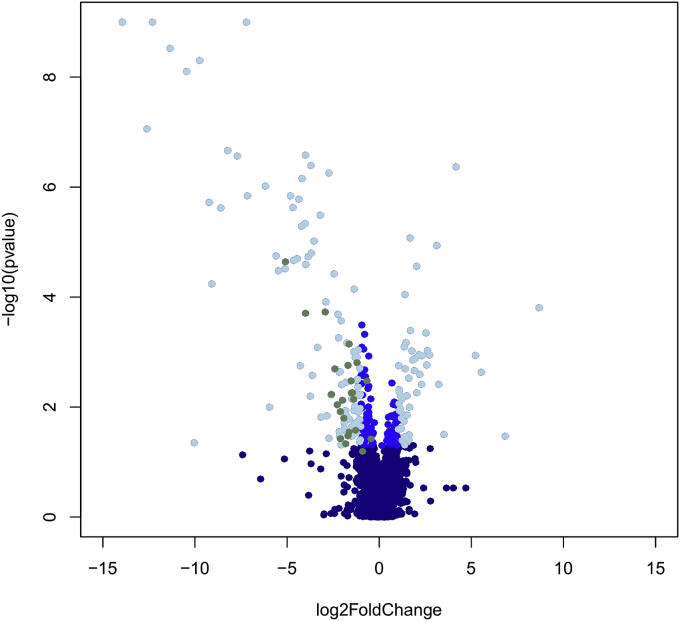
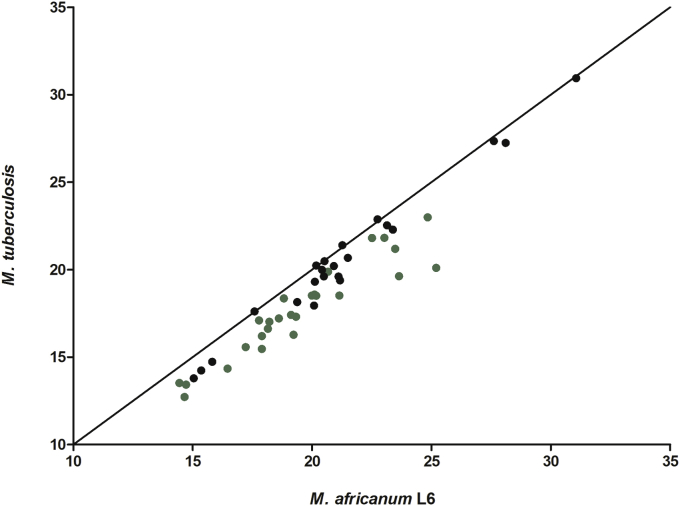
From all 2179 mycobacterial genes tested on sputa, the DosR regulon genes were the most differentially regulated category expressed between M. africanum L6 and M. tuberculosis L4 before and after multiple testing correction (Fig. 1, Supplemental Tables S1 and S2, Supplementary Material online). During the course of infection, M. tuberculosis encounters oxygen limitation. Under such conditions, the bacteria respond by switching on DosR, which is crucial for hypoxic response regulation in M. tuberculosis. As indicated by higher cycle threshold values, DosR regulated genes had lower expression in M. africanum L6 when compared to M. tuberculosis L4 (Fig. 2). On average, M. tuberculosis L4 had 2.5-fold higher expression of DosR regulon genes than M. africanum L6. The under expression of half (n = 26) of these genes was on average approximately 4 fold lower in M. africanum L6 and statistically significant (Supplemental Tables S2 and S3, Supplementary Material online). These included the main regulators encoded within the regulon, Rv0081 and DosR itself, as well as several conserved hypotheticals, implying a relatively lower requirement of these genes by M. africanum L6. Additionally, expression of the DosR-regulated nitrate transporter, narK2 was significantly lower.
Fig. 1.
Volcano plot of all genes tested visualizing fold change in expression and statistical significance. Dark blue dots indicate genes with no significant difference in expression between M. africanum L6 and M. tuberculosis L4. Mid-dark blue shows genes with unadjusted p value less than 0.05 and log 2 Fold change between −1 and 1. Light blue describes genes with unadjusted p value less than 0.05 and log 2 Fold change greater than (−)1. DosR genes significantly under expressed in M. africanum L6 relative to M. tuberculosis L4 following Bonferroni correction are shown in green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Median qPCR cycle threshold values of the 48 dormancy regulon genes of M. africanum L6 and M. tuberculosis L4. Genes with significantly lower expression in M. africanum L6 relative to M. tuberculosis L4 are shown in green (same genes as the green ones in Fig. 1). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Detection of single nucleotide polymorphisms in major hypoxia response genes in M.africanum L6
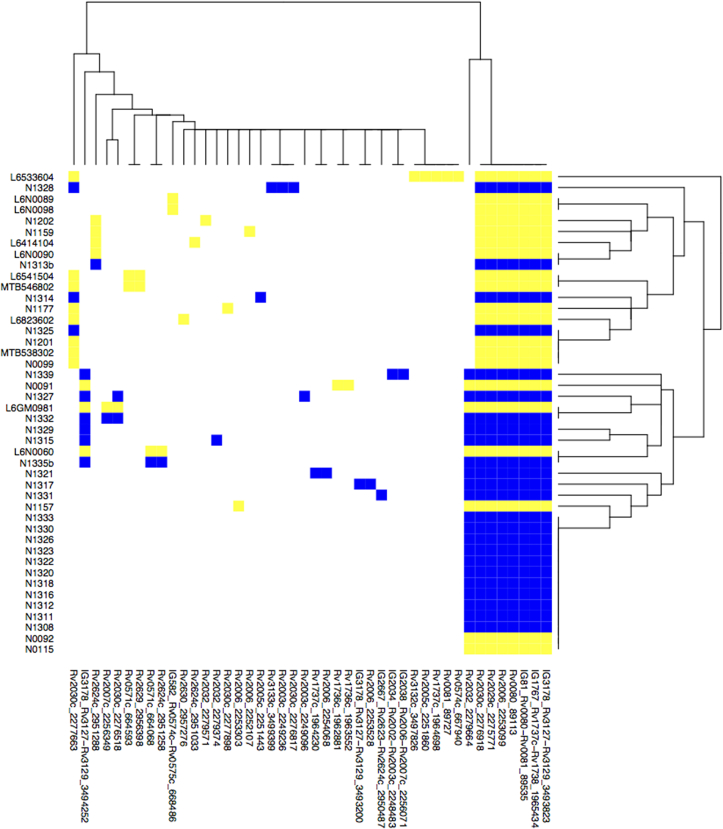
Nucleotide polymorphisms within genes can affect gene function and potentially influence gene regulation when found in intergenic regions. The under expression of a significant number of DosR regulon genes in M. africanum L6 from sputa led us to assess the whole genome sequences of a collection of 44 M. africanum L6 strains for lineage-specific mutations within these genes. Notably, in all 44 strains, specific nonsynonymous SNPs were detected in Rv0080 including the intergenic region of Rv0080 and the gene encoding the regulatory hub in M. tuberculosis during hypoxia, Rv0081 (Fig. 3). Although not under expressed in M. africanum L6 relative to M. tuberculosis L4, lineage-specific nonsynonymous SNPs were found in the dosT gene of all M. africanum L6 and also in phoP/R as described previously [28].
Fig. 3.
Single Nucleotide Polymorphisms detected in 44 M. africanum L6 strains from Gambia and Ghana relative to H37Rv in under expressed genes. Blue and yellow indicate a SNP in Gambian and Ghanaian isolates respectively and white indicates wildtype. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. M. africanum L6 preferentially grew microaerobically while M. tuberculosis L4 grew aerobically
In Lebek's medium, classically used to assess oxygen preference between strains, we compared the growth of a clinical M. tuberculosis L4 strain, two M. africanum L6 clinical strains, and the M. tuberculosis reference strain H37Rv (L4). Both M. africanum L6 strains showed anaerobic growth below the surface while the clinical L4 strain and H37Rv showed growth only at the aerobic surface (Fig. 4). The results shown were confirmed in a technical replicate.
Fig. 4.
Lebek test for oxygen preference. Test for oxygen preference in Lebek medium for the reference strain H37Rv (L4), a clinical L4 strain and 2 clinical M. africanum strains (L6). From left to right, tube 1, Negative control; tube 2, M. tuberculosis H37Rv; tube 3, L4 M. tuberculosis clinical strain (991508); tube 4, L6 M. africanum clinical strain (112287); tube 5, L6 M. africanum clinical strain (133158), showing diffused growth by M. africanum L6 compared to surface growth by M. tuberculosis.
4. Discussion
Evidence points to important differences between the in vitro physiologic state of M. tuberculosis and the state of the bacteria in the human host [20], [29], [30]. Sputum is a valuable source to investigate the physiologic state of bacteria in the lung during disease. A recent study indicates that M. tuberculosis transcription in sputum mirrors M. tuberculosis transcription in the lungs [30]. In the present study, we identified 2.5-fold lower expression of all DosR regulon genes in M. africanum L6 relative to M. tuberculosis L4 from sputa of HIV negative patients with TB disease. Key genes activated by M. tuberculosis in response to hypoxia, dos R-S and regulatory hub gene Rv0081, were significantly less expressed in M. africanum L6. Moreover, in all L6 strains sequenced we detected lineage-specific mutations in Rv0080 and the intergenic region (possibly within the upstream promoter region) between Rv0080 and Rv0081. The importance of Rv0080 and the Rv0080-Rv0081 intergenic region was shown in a previous study [31] in which DosR demonstrated binding to the intergenic region between Rv0080 and Rv0081. Further, Rv0081 was found to bind an inverted repeat element located in its own upstream region. Our findings are significant and serve as a prelude to future studies because it is well known how M. tuberculosis uses the DosR regulon to quickly adjust to hypoxic stress, commonly encountered during intracellular survival within the macrophage and granuloma [11], [12], [32]. However, the state of DosR in M. africanum lineages is underexplored. Adaptation to all stages of a complex life cycle and versatility in the ability to switch between the different metabolic states through regulation of DosR could be a basis for higher pathogenicity of M. tuberculosis. Indeed, the constitutive over expression of DosR in M. tuberculosis W/Beijing (L2) is thought to contribute to the high virulence and transmissibility associated with this lineage [17].
As the DosR regulon has been linked to virulence, the observed under expression might contribute to the previously described differences between M. africanum L6 and M. tuberculosis lineages in clinical phenotype and disease progression [5]. Different animal models have been used to study TB infection and pathogenesis. In guinea pigs and rabbits, where hypoxic lesions develop [33], and in mice, dosR-S was required for full virulence. dosR-S mutants showed a growth defect and slower replication marked by lower counts of colony forming units in both lungs and spleen [34]. In a recent study in macaques, the closest experimental model to humans, Mehra and colleagues also reported the loss of clinical signs of TB, fever, progressive weight loss and radiographic lesions, in animals infected with dosR mutants [13]. Despite being TST positive, macaques infected with dosR mutants failed to develop clinical disease within the study period while those infected with wild type and complemented strains developed early TB. A statistically significant difference in survival between the wild type/complemented infected group and the group infected with mutants was reported. These observations confirm a major role for the full activity of DosR in conferring virulence within the host. Moreover, they bear striking similarity with the M. africanum L6 clinical phenotype which, when compared to M. tuberculosis, is attenuated, favors immunocompromised hosts, multiplies at a slower rate and is associated with slower progression to active disease [5], [8], [10]. The relative under expression of the DosR regulon in M. africanum L6 might thus be one possible explanation for the attenuated M. africanum L6 clinical phenotype.
The DosR regulon is controlled by the response regulator DosR and sensor kinases DosS (DevS) and DosT. The activation of DosR depends on both DosS and DosT. Honaker and colleagues showed the individual and collective importance of both sensors [35]. DosT, the first of the two sensors to respond to hypoxia and to activate DosR, is sensitive to oxygen and is a hypoxia sensor while DosS plays a role as a redox sensor. Therefore, it was suggested that DosT could be more important than DosS during early phase hypoxia particularly during the shift from an aerobic to hypoxic environment [36]. The response of DosT is short lived and quickly diminishes following the drop in oxygen levels. At this point DosS, also a member of the 48-gene DosR regulon, takes over and keeps DosR induction sustained and further induced [35], [36]. In DosS and DosT single mutants, induction of the DosR regulon reduced by 45% showing that both DosT and DosS are essential for full induction of the DosR regulon during oxygen limitation [36]. In addition to the significant under expression of dosS, the mutated dosT gene in all M. africanum L6 strains analyzed could together contribute to the overall under expression of the DosR regulon in M. africanum L6.
dosR is regulated by phoP [15], [37]. Mutations in the PhoPR system reportedly deactivate or interfere with proper functioning of the system and contribute to loss of virulence [38]. When the PhoR mutation common to M. bovis and M. africanum L6 was introduced in H37Rv, the ΔphoPR::phoPR-bovis mutant was marked by reduced bacillary load in human primary macrophages and severely impaired replication in immunocompetent BALB/c mice, which were reversed by complementation of the intact PhoPR gene. In the same study, the RD8 deletion, shared by M. bovis and M. africanum L6, was shown to ‘rescue’ the ESX-1 secretion system, which had been abrogated in the H37Rv ΔphoPR mutant [28]. In the present study, we confirmed nonsynonymous mutations in phoP/R. Mutations in phoR were detected in all Lineage 6 strains. Although no differential expression of phoP, phoR, or genes of the ESX-1 system was observed between M. tuberculosis L4 and M. africanum L6, based on the evidence that the M. bovis and M. africanum L6 PhoR mutation abrogated ESX-1 signaling [28], the reduced expression of the DosR regulon in M. africanum L6 is likely another downstream effect of the mutated PhoPR system.
Besides the clinical consequences, DosR regulon expression differences could reflect the respective bacterial physiologies and lifestyles. Increased DosR activity in M. tuberculosis might indicate greater requirement for genes associated with the switch to hypoxic survival. In contrast, it appears that M. africanum is naturally more capable of growing in hypoxic environments and does not rely on the regulatory switch to the same extent as M. tuberculosis. This assumption was further confirmed by our growth experiments, in which M. africanum L6 was able to grow aerobically and anaerobically throughout the whole Lebek medium (a test for oxygen preference), whereas M. tuberculosis L4 only grew on the surface of the slope, supporting the traditional classification of M. africanum L6 as microaerophilic [9], [39], [40], [41]. The preference for microaerobic growth has been further supported in a study from 1973 in which paraffin embedded culture medium was used to show cross-sections of M. africanum colonies, which, unlike M. tuberculosis colonies that remain strictly at the surface, grew in the depth of the medium, explaining the umbilicated colony morphology [42]. The relative reduced responsiveness of the DosR regulon together with the observed preference for microaerobic growth of M. africanum L6 could either imply a preference for intracellular growth or adaptation to a fundamentally different biological niche within the host.
For instance, another key enzyme regulated by DosR during anaerobiosis in M. tuberculosis is a nitrate transporter encoded by narK2 [43], [44], [45]. We also found that narK2 mRNA is less abundant in M. africanum L6 when compared to M. tuberculosis L4. Traditional biochemical, microbiological assays are in line with our findings and showed that only a minority of M. africanum strains isolated from Ghana and Dakar were positive for intracellular nitrate, indicative of a general lack of NarK2 activity in M. africanum [18]. Our findings support both older and more recent MTBc speciation data describing M. africanum L6 as nitrate reductase negative [9], [46].
Limitations of the present study include the fact that gene expression is averaged across all bacterial populations in sputum, and that the relatively small sample size and correction for multiple testing only allowed to detect sizeable differences in global expression levels. However, the detected differences between M. africanum L6 and M. tuberculosis L4 were still very significant, supporting the magnitude of the difference in expression of the DosR regulon between M. tuberculosis L4 and M. africanum L6. We detected additional differentially expressed genes in M. africanum L6 relative to M. tuberculosis L4 that did not reach statistical significance, possibly as a result of correcting for multiple testing using the more conservative Bonferroni method (Supplemental Table S2, Supplementary Material online). Sample quantities also did not permit mRNA analyses of host genes.
Although we show that M. africanum L6 is more capable of growth under hypoxic conditions reflected by microaerobic growth in Lebek's medium and the under expression of DosR regulon genes relative to M. tuberculosis L4, future studies need to demonstrate whether both pathogens would show these same differences in expression under identical hypoxic conditions. In a recent study where H37Rv (MTBC Lineage 4) was grown in vitro under hypoxic conditions in the Wayne model and subsequently subjected to gene expression analysis, dormancy regulon genes including dosR and narK2 were overexpressed during non-replicating persistence-1 (NRP-1). Interestingly, narK2 remained highly expressed even through NRP-2, emphasizing the importance of the dormancy regulon in MTBC Lineage 4 [47]. Previous gene expression studies in the in vitro Wayne model with M. tuberculosis also reported similar findings [48], [49]. Investigating the expression of M. africanum L6 genes following growth under the Wayne model for instance should provide further insights into differences in the response of M. africanum L6 and M. tuberculosis lineages to low oxygen.
Taken together, our results indicate that M. africanum L6 is less reliant on DosR signaling, and might pursue a different survival strategy within the human host than M. tuberculosis L4. Assuming that M. africanum L6 and M. tuberculosis L4 both infect the same host tissues, a loss of DosR regulon activity could be due to a DosR-independent adaptation and overall preference of M. africanum L6 to hypoxic, or even, anaerobic growth. Supporting this is a recent study that described an association of M. africanum L6 and extrapulmonary disease, reflective of an anaerobic niche [8]. Given that transmission to new hosts depends on the development of pulmonary disease, the evolutionary advantage of extrapulmonary disease is not clear. While M. africanum L6 is as transmissible as M. tuberculosis L4 from pulmonary TB patients to their contacts [5], we postulate that a relatively larger reservoir of latent and/or extrapulmonary infection by M. africanum L6 may offer a degree of protection against re-infection with the more virulent M. tuberculosis L4, maintaining M. africanum L6 endemicity in West Africa.
5. Conclusion
Using ex vivo sputum expression data, we show for the first time directly in sputum samples from patients with TB that the DosR regulon was significantly less expressed in M. africanum L6 compared to M. tuberculosis L4. We describe a clinically relevant lineage, M. africanum L6, which appears to have adapted to growth under hypoxic conditions or different biological niches. We provide gene expression, phenotypic and sequencing data supporting this. M. africanum L6 permits to study factors that have contributed to the virulence and success of the MTBc, which could be exploited to target further attenuation. Such studies will improve understanding of additional biologically relevant differences between M. tuberculosis and M. africanum.
Such a comparison is also justified by the consideration of the DosR regulon as potential vaccine- and drug target, aiming to curtail mycobacterial survival. Over the last decade, M. tuberculosis respiration and energy metabolism has been targeted in TB drug discovery with success. Roles for the DosR regulon in the mechanism of action of drugs and phenotypic drug tolerance have been reported [50], [51]. If we are to make greater strides in controlling this well adapted human pathogen, whether through novel therapies or vaccines, it will be essential to acquire deeper insight into the role the DosR regulon plays under different stimuli and the full spectrum of influence it has on metabolism and disease development by all the different MTBc lineages. The imminent question is whether the DosR regulon will be a viable target in all MTBc strains. This remains to be answered. Understanding strain differences in more detail will facilitate the development of improved therapies useful in all TB endemic settings.
Acknowledgments
The authors thank patients and colleagues at MRC for their contribution to the present analysis. This work was supported by the European Research Council-INTERRUPTB starting grant nr.311725 (to BdJ, BO, FG, MA, CM).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.tube.2017.03.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Gagneux S. Host-pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2012;367(1590):850–859. doi: 10.1098/rstb.2011.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gagneux S., DeRiemer K., Van T., Kato-Maeda M., de Jong B.C., Narayanan S. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103(8):2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershberg R., Lipatov M., Small P.M., Sheffer H., Niemann S., Homolka S. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6(12):e311. doi: 10.1371/journal.pbio.0060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comas I., Coscolla M., Luo T., Borrell S., Holt K.E., Kato-Maeda M. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45(10):1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong B.C., Hill P.C., Aiken A., Awine T., Antonio M., Adetifa I.M. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis. 2008;198(7):1037–1043. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asante-Poku A., Yeboah-Manu D., Otchere I.D., Aboagye S.Y., Stucki D., Hattendorf J. Mycobacterium africanum is associated with patient ethnicity in Ghana. PLoS Neglect. Trop Dis. 2015;9(1):e3370. doi: 10.1371/journal.pntd.0003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong B.C., Adetifa I., Walther B., Hill P.C., Antonio M., Ota M. Differences between tuberculosis cases infected with Mycobacterium africanum, West African type 2, relative to Euro-American Mycobacterium tuberculosis: an update. FEMS Immunol Med Microbiol. 2010;58(1):102–105. doi: 10.1111/j.1574-695X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A., Bloss E., Heilig C.M., Click E.S. Tuberculosis caused by Mycobacterium africanum, United States, 2004–2013. Emerg Infect Dis. 2016;22(3):396–403. doi: 10.3201/eid2203.151505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong B.C., Antonio M., Gagneux S. Mycobacterium africanum–review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis. 2010;4(9):e744. doi: 10.1371/journal.pntd.0000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehre F., Otu J., DeRiemer K., de Sessions P.F., Hibberd M.L., Mulders W. Deciphering the growth behaviour of Mycobacterium africanum. PLoS Negl Trop Dis. 2013;7(5):e2220. doi: 10.1371/journal.pntd.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gengenbacher M., Kaufmann S.H. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol Rev. 2012;36(3):514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne L.G., Sohaskey C.D. Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 13.Mehra S., Foreman T.W., Didier P.J., Ahsan M.H., Hudock T.A., Kissee R. The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am J Respir Crit Care Med. 2015;191(10):1185–1196. doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voskuil M.I., Schnappinger D., Visconti K.C., Harrell M.I., Dolganov G.M., Sherman D.R. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198(5):705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galagan J.E., Minch K., Peterson M., Lyubetskaya A., Azizi E., Sweet L. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499(7457):178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallow A., Domenech P., Reed M.B. Strains of the East Asian (W/Beijing) lineage of Mycobacterium tuberculosis are DosS/DosT-DosR two-component regulatory system natural mutants. J Bacteriol. 2010;192(8):2228–2238. doi: 10.1128/JB.01597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed M.B., Gagneux S., Deriemer K., Small P.M., Barry C.E., 3rd The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol. 2007;189(7):2583–2589. doi: 10.1128/JB.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattyn S.R., Portaels F., Spanoghe L., Magos J. Further studies on African strains of Mycobacterium tuberculosis: comparison with M. bovis and M. microti. Ann Soc Belg Med Trop Parasitol Mycol. 1970;50(2):211–227. [PubMed] [Google Scholar]
- 19.Kamerbeek J., Schouls L., Kolk A., van Agterveld M., van Soolingen D., Kuijper S. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35(4):907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter N.D., Dolganov G.M., Garcia B.J., Worodria W., Andama A., Musisi E. Transcriptional adaptation of drug-tolerant Mycobacterium tuberculosis during treatment of human tuberculosis. J Infect Dis. 2015;212(6):990–998. doi: 10.1093/infdis/jiv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia B., Walter N.D., Dolganov G., Coram M., Davis J.L., Schoolnik G.K. A minimum variance method for genome-wide data-driven normalization of quantitative real-time polymerase chain reaction expression data. Anal Biochem. 2014;458:11–13. doi: 10.1016/j.ab.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia B.J., Datta G., Davidson R.M., Strong M. MycoBASE: expanding the functional annotation coverage of mycobacterial genomes. BMC Genomics. 2015;16:1102. doi: 10.1186/s12864-015-2311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosack D.A., Dennis G., Jr., Sherman B.T., Lane H.C., Lempicki R.A. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4(10):R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew J.M., Kapopoulou A., Jones L.M., Cole S.T. TubercuList–10 years after. Tuberc (Edinb) 2011;91(1):1–7. doi: 10.1016/j.tube.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Li H., Durbin R. Fast and accurate short read alignment with Burrows Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16) doi: 10.1093/nar/gkq603. e164–e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalo-Asensio J., Malaga W., Pawlik A., Astarie-Dequeker C., Passemar C., Moreau F. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci USA. 2014;111(31):11491–11496. doi: 10.1073/pnas.1406693111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garton N.J., Waddell S.J., Sherratt A.L., Lee S.M., Smith R.J., Senner C. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5(4):e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia B.J., Loxton A.G., Dolganov G.M., Van T.T., Davis J.L., de Jong B.C. Sputum is a surrogate for bronchoalveolar lavage for monitoring Mycobacterium tuberculosis transcriptional profiles in TB patients. Tuberc (Edinb) 2016;100:89–94. doi: 10.1016/j.tube.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He H., Bretl D.J., Penoske R.M., Anderson D.M., Zahrt T.C. Components of the Rv0081-Rv0088 locus, which encodes a predicted formate hydrogenlyase complex, are coregulated by Rv0081, MprA, and DosR in Mycobacterium tuberculosis. J Bacteriol. 2011;193(19):5105–5118. doi: 10.1128/JB.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boon C., Dick T. How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Future Microbiol. 2012;7(4):513–518. doi: 10.2217/fmb.12.14. [DOI] [PubMed] [Google Scholar]
- 33.Via L.E., Lin P.L., Ray S.M., Carrillo J., Allen S.S., Eum S.Y. Tuberculous granulomas are hypoxic in Guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76(6):2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Converse P.J., Karakousis P.C., Klinkenberg L.G., Kesavan A.K., Ly L.H., Allen S.S. Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun. 2009;77(3):1230–1237. doi: 10.1128/IAI.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honaker R.W., Leistikow R.L., Bartek I.L., Voskuil M.I. Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy. Infect Immun. 2009;77(8):3258–3263. doi: 10.1128/IAI.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T., He L., Deng W., Xie J. The Mycobacterium DosR regulon structure and diversity revealed by comparative genomic analysis. J Cell Biochem. 2013;114(1):1–6. doi: 10.1002/jcb.24302. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalo-Asensio J., Mostowy S., Harders-Westerveen J., Huygen K., Hernandez-Pando R., Thole J. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One. 2008;3(10):e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung A.S., Tran V., Wu Z., Yu X., Alexander D.C., Gao G.F. Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics. 2008;9 doi: 10.1186/1471-2164-9-413. 413–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frothingham R., Strickland P.L., Bretzel G., Ramaswamy S., Musser J.M., Williams D.L. Phenotypic and genotypic characterization of Mycobacterium africanum isolates from West Africa. J Clin Microbiol. 1999;37(6):1921–1926. doi: 10.1128/jcm.37.6.1921-1926.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niemann S., Richter E., Rusch-Gerdes S. Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J Clin Microbiol. 2000;38(1):152–157. doi: 10.1128/jcm.38.1.152-157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons L.M., Brosch R., Cole S.T., Somoskovi A., Loder A., Bretzel G. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol. 2002;40(7):2339–2345. doi: 10.1128/JCM.40.7.2339-2345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Medeiros D., Castets M., Diop S. Comparative morphological aspects of Mycobacterium tuberculosis, classical type, and Mycobacterium africanus (preliminary note) Bull Soc Med Afr Noire Lang Fr. 1973;18(4):477–484. [PubMed] [Google Scholar]
- 43.Sohaskey C.D. Regulation of nitrate reductase activity in Mycobacterium tuberculosis by oxygen and nitric oxide. Microbiology. 2005;151(Pt 11):3803–3810. doi: 10.1099/mic.0.28263-0. [DOI] [PubMed] [Google Scholar]
- 44.Sohaskey C.D., Wayne L.G. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol. 2003;185(24):7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Wang H., Xie J. Genes and regulatory networks involved in persistence of Mycobacterium tuberculosis. Sci China Life Sci. 2011;54(4):300–310. doi: 10.1007/s11427-011-4134-5. [DOI] [PubMed] [Google Scholar]
- 46.Goh K.S., Rastogi N. Simple and rapid method for detection of nitrate reductase activity of Mycobacterium tuberculosis and Mycobacterium canettii grown in the Bactec MGIT960 system. J Microbiol Methods. 2010;81(2):208–210. doi: 10.1016/j.mimet.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Iona E., Pardini M., Mustazzolu A., Piccaro G., Nisini R., Fattorini L. Mycobacterium tuberculosis gene expression at different stages of hypoxia-induced dormancy and upon resuscitation. J Microbiol. 2016;54(8):565–572. doi: 10.1007/s12275-016-6150-4. [DOI] [PubMed] [Google Scholar]
- 48.Muttucumaru D.G., Roberts G., Hinds J., Stabler R.A., Parish T. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberc (Edinb) 2004;84(3–4):239–246. doi: 10.1016/j.tube.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Voskuil M.I., Visconti K.C., Schoolnik G.K. Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberc (Edinb) 2004;84(3–4):218–227. doi: 10.1016/j.tube.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 50.de Keijzer J., Mulder A., de Beer J., de Ru A.H., van Veelen P.A., van Soolingen D. Mechanisms of phenotypic Rifampicin tolerance in Mycobacterium tuberculosis Beijing genotype strain B0/W148 revealed by proteomics. J Proteome Res. 2016;15(4):1194–1204. doi: 10.1021/acs.jproteome.5b01073. [DOI] [PubMed] [Google Scholar]
- 51.Koul A., Vranckx L., Dhar N., Gohlmann H.W., Ozdemir E., Neefs J.M. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat Commun. 2014;5:3369. doi: 10.1038/ncomms4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.