Abstract
Significance: Cachexia is defined as a complex metabolic syndrome that is associated with underlying illness and a loss of muscle with or without loss of fat mass. This disease is associated with a high incidence with chronic diseases such as heart failure, cancer, chronic obstructive pulmonary disease (COPD), and acquired immunodeficiency syndrome (AIDS), among others. Since there is currently no effective treatment available, cachectic patients have a poor prognosis. Elucidation of the underlying mechanisms is, therefore, an important medical task.
Recent Advances: There is accumulating evidence that the diseased organs such as heart, lung, kidney, or cancer tissue secrete soluble factors, including Angiotensin II, myostatin (growth differentiation factor 8 [GDF8]), GDF11, tumor growth factor beta (TGFβ), which act on skeletal muscle. There, they induce a set of genes called atrogenes, which, among others, induce the ubiquitin-proteasome system, leading to protein degradation. Moreover, elevated reactive oxygen species (ROS) levels due to modulation of NADPH oxidases (Nox) and mitochondrial function contribute to disease progression, which is characterized by loss of muscle mass, exercise resistance, and frailty.
Critical issues: Although substantial progress was achieved to elucidate the pathophysiology of cachexia, effectice therapeutic strategies are urgently needed.
Future Directions: With the identification of key components of the aberrant inter-organ communication leading to cachexia, studies in mice and men to inhibit ROS formation, induction of anti-oxidative superoxide dismutases, and upregulation of muscular nitric oxide (NO) formation either by pharmacological tools or by exercise are promising approaches to reduce the extent of skeletal muscle wasting. Antioxid. Redox Signal. 26, 700–717.
Keywords: : angiotensin, autophagy, cachexia, exercise, skeletal muscle, ubiquitin
Introduction
Besides the nervous system, the cardiovascular system represents the second major communication route of the body, allowing the exchange of signals between remote organs. This inter-organ communication is important for systemic coordination and integration of organ functions. There is substantial evidence that not only the classical endocrine glands use this path to send hormones to remote organs but also many other organs, including the heart, skeletal muscle, and adipose tissue, secrete humoral factors (e.g., “myokines” and “adipokines,” respectively) to modulate processes such as inflammation, metabolism, and function on remote targets.
Under many pathological conditions, a primary dysfunction in one organ may lead to aberrant inter-organ communication, causing dysfunction of a remote organ and the development of comorbidities. Examples for the coordinated development of comorbidities include cardiac cachexia, cancer cachexia, the cardio-renal syndrome, and a.o. (9, 49, 112, 141). On the other hand, short-term skeletal muscle ischemia induces a protective effect in the heart, leading to reduced infarct size, a phenomenon called remote ischemic preconditioning (46). This effect appears to be mediated by factors released from ischemic skeletal muscle, which are able to protect multiple organs from ischemic insults. Moreover, exercise causes release of trans-acting factors from skeletal muscle (58), which regulate other organs and may exert a positive effect on wasting states.
Obviously, there exists extensive organ-organ crosstalk, which substantially contributes not only to morbidity but also to adaptation under pathological conditions. In this review, we will focus primarily on skeletal muscle interaction in the context of different wasting states, including cachexia and sarcopenia, and we will underscore the impact of nitric oxide (NO)/reactive oxygen species (ROS) as mediators of organ crosstalk.
Interorgan Communication in Skeletal Muscle Wasting
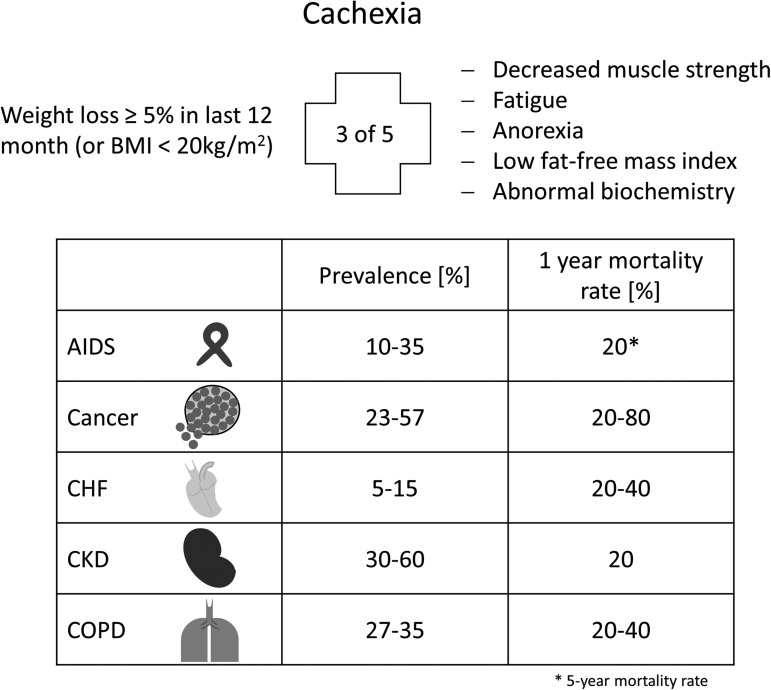
Skeletal muscle wasting can be classified as sarcopenia and cachexia, respectively. Sarcopenia is the aging-associated loss of muscle mass, whereas cachexia represents a more general wasting state, which is associated with chronic disease. According to the official definition, cachexia is a “complex metabolic syndrome associated with underlying illness and a loss of muscle with or without loss of fat mass” (45). Cachexia is, therefore, the consequence of an underlying chronic disease, such as cancer, heart failure, kidney disease, chronic obstructive pulmonary disease (COPD), chronic infection, sepsis, or acquired immunodeficiency syndrome (AIDS) (Fig. 1).
FIG. 1.
Definition, prevalence, and mortality rate of cachexia. General parameters to diagnose cachexia and the variability in prevalence and 1-year mortality of cachexia-associated chronic diseases (AIDS*, cancer, CHF, CKD, and COPD). *5-Year mortality rate. CHF, chronic heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease.
Clinical features of cachexia include weight loss, anorexia, inflammation, insulin resistance, hypogonadism, and anemia. In particular, muscle wasting plays an important role in cachectic patients, as it is mainly responsible for the weight loss, weakness, and fatigue of patients. The clinical definition of cachexia can be found elsewhere (45).
The treatment of cachexia is currently rather limited, mainly relying on treating the underlying chronic disease. Even parenteral nutrition does not prevent the ongoing loss of muscle mass; hence, once wasting is diagnosed, it has a very poor prognosis (8).
General Mechanisms in Skeletal Muscle Wasting
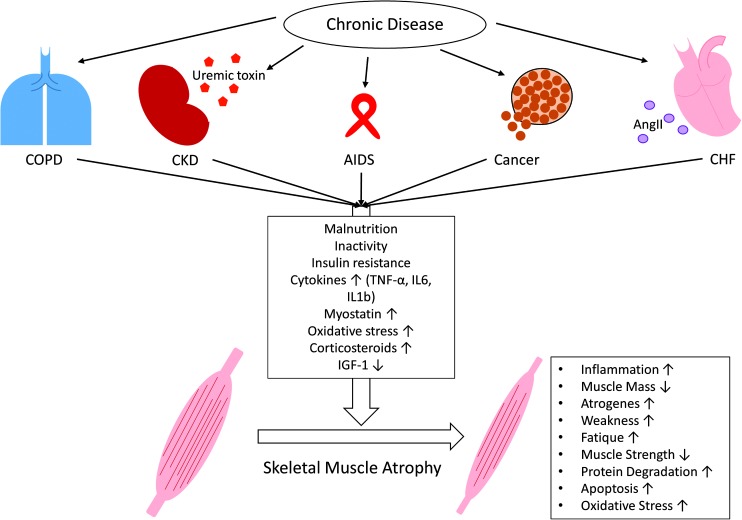
Loss of tissue mass in general results from an imbalance of anabolic and catabolic states due to altered substrate utilization, protein degradation, and synthesis. Although different chronic diseases may trigger cachexia via the release of a variety of different humoral factors from primary diseased organs, there seem to be some general pathogenic mechanisms underlying the cachectic phenotype (93). Until now, it remains uncertain whether loss of skeletal muscle is induced by only a primary diseased organ or via the involvement of other organs. At least, Angiotensin II (AngII), cytokines (e.g., interleukin 6 [IL6]), and growth factors of the tumor growth factor beta (TGFβ) family (e.g., myostatin) seem to be involved in the induction of muscle wasting (20, 29) (Fig. 2).
FIG. 2.
Skeletal muscle wasting induced by chronic diseases. Chronic diseases, such as COPD, CKD, AIDS, cancer, and CHF, share common metabolic changes (malnutrition; inactivity; insulin resistance; increased levels of cytokines [TNFα, IL6, IL1β], myostatin, and corticosteroids; increased oxidative stress; and decreased levels of IGF-1), which lead to skeletal muscle atrophy. Besides these common features, some disease-specific factors further contribute to muscle wasting, for example, uremic toxins in CKD or AngII in CHF. All these metabolic changes induce a catabolic program in skeletal muscles that is indicated by increased inflammation, weakness, fatigue, protein degradation, atrogene expression, apoptosis, and oxidative stress as well as decreased muscle mass and muscle strength. AngII, angiotensin II; IGF-1, insulin-like growth factor 1; IL, interleukin; TNFα, tumor necrosis factor alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Independent of the mode of induction, it is clear that skeletal muscle wasting in cachexia is due to a disturbed protein synthesis and degradation, primarily through activation of the ubiquitin–proteasome pathway. There is also evidence for activation of the autophagy/lysosomal proteolytic pathway, but its causal role in development of muscle wasting remains controversial.
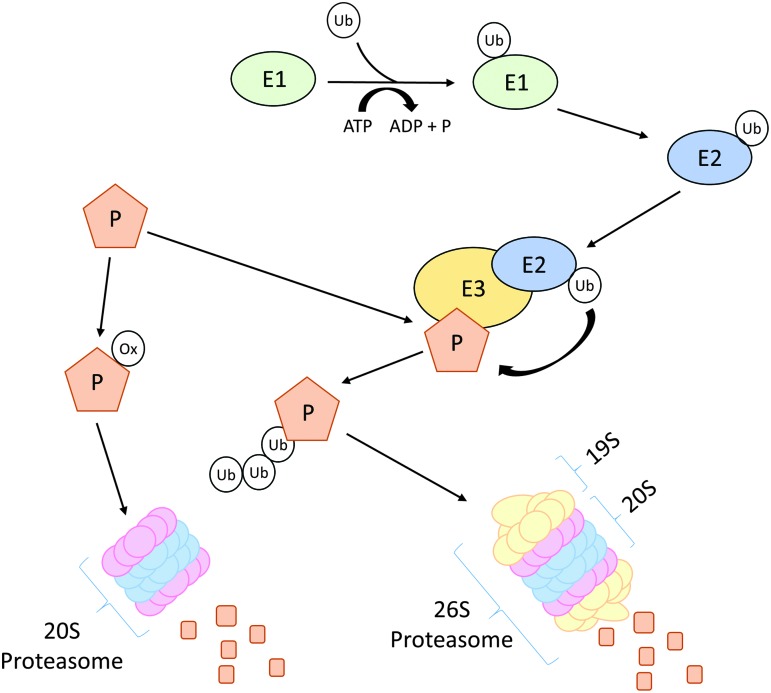
The proteasome system degrades proteins labeled by polyubiquitination (Fig. 3). Three types of enzymes E1, E2, and E3 execute different steps of the ubiquitination process. After activation of ubiquitin by E1 enzymes, activated ubiquitin is transferred to E2, which then delivers ubiquitin to E3 enzymes. The latter act as ubiquitin ligases, which covalently attach ubiquitin to the ɛ-amino groups of lysine residues of target proteins (131). Ubiquitinated proteins are then transferred to the 26S proteasome for degradation.
FIG. 3.
Schematic overview of ubiquitin–proteasome pathway. A protein can be marked for degradation by either ubiquitination or oxidation. Activated ubiquitin binds to E1 and is then transferred to E2, the ubiquitin-conjugating enzyme. The loaded E2 delivers the ubiquitin to ubiquitin ligases (E3), which covalently attach ubiquitin to a lysine residues of target proteins (P). Poly-ubiquitinated proteins are then degraded by the 26S proteasome consisting of a 19S regulatory and a 20S core subunit. If a protein gets oxidized (ox), it will be directly degraded via the 20S proteasome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
A prominent target of the proteasome system in muscle appears to be myosin, which was reduced in C2C12 myocytes under conditions of oxidative stress (79). Loss of myosin may explain reduced muscle mass and, on the other hand, the reduced muscle strength associated with the wasting states. More than thousand different E3 enzymes are present in the human genome, opening the way to a precise temporal and cell type-specific control of protein degradation. In skeletal muscle, the two most prominent players of the ubiquitin–proteasome pathway are the E3 ligases Muscle RING Finger 1 (MuRF1) and Muscle Atrophy F-box (MAFbx also known as Atrogin1) (111). Both these ubiquitin E3 ligases, which target many muscle proteins to degradation, are highly upregulated at the transcriptional level in nearly every model of muscle wasting (16).
Stimulation of the ubiquitin–proteasome pathway is frequently accompanied by increased oxidative stress (94). Experiments involving C2C12 myocytes have clearly shown that ROS induces E3-ubiquitin ligases (57), suggesting enhanced degradation of proteins by the 26S proteasome. However, in cachexia, the 20S proteasome is also upregulated. This proteasome subtype degrades oxidized proteins without prior modification by ubiquitination, which underscores the notion that elevated levels of ROS in cachectic muscle are involved in the pathology (96, 126).
Myocardial infarction induced atrophy of plantaris muscles in rats, which was accompanied by an increase of NADPH oxidases (Nox), activation of nuclear factor “kappa-light-chain-enhancer” of activated B-cells (NF-κB) and p38MAP kinase, and finally, elevated levels of 26S and 20S proteasomes. Almost all of these changes were reverted by apocynin, providing a clear link between elevated ROS formation and enhanced proteasomal activity in cardiac cachexia (14).
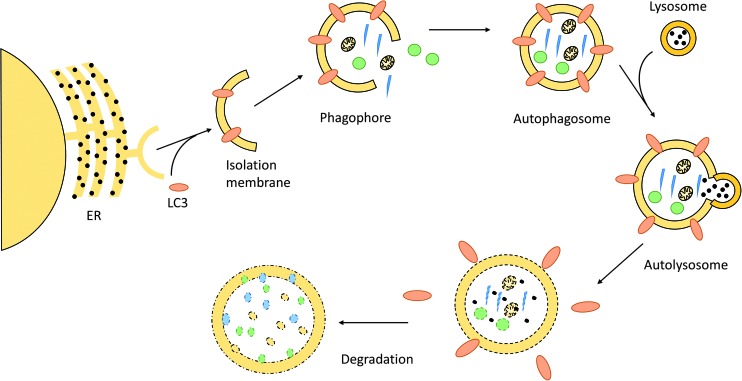
In contrast to the ubiquitin–proteasome pathway, which is responsible for clearing short-lived protein, the autophagy/lysosomal proteolytic pathway removes long-living proteins, protein aggregates, and organelles, including mitochondria (68, 89, 99) (Fig. 4).
FIG. 4.
Schematic overview of autophagy. Autophagy is initiated by Microtubule-associated protein 1A/1B-LC3 conjugation to the nascent phagophore, called isolation membrane—a membrane part derived from the endoplasmic reticulum (ER). While the phagophore expands, it engulfs degradable substrates and finally forms the autophagosome. The autophagosome fuses with a lysosome, which releases its hydrolytic enzymes into the newly formed autolysosome. The content thereby gets degraded and can be recycled by the cell. LC3, microtubule-associated protein 1 light chain 3. To see this illustration in color the reader is referred to the web version of this article at www.liebertpub.com/ars
A hallmark of autophagy is the formation of a specific double membrane compartment, the autophagosome. The autophagosomal membrane originates from the endoplasmic reticulum (ER). Via elongation, the isolation membrane or phagophore grows to form the autophagosome. All of these processes are accompanied by the binding of many proteins expressed from autophagy-related genes, which not only coordinate the assembly process but also represent ligands for the recognition and internalization of cellular components destined to degradation. On fusion of the autophagosome with lysosomes, the degradation of engulfed material and recycling of its components occurs.
Experimental and clinical data demonstrate an upregulation of many genes, contributing to the regulation of autophagy and building of the autophagosomes in skeletal muscle, including mouse models of cancer cachexia as well as patients suffering from different types of cancer or heart failure (103, 104). Although there is a strong association with the loss of skeletal muscle mass, induction of autophagy may be secondary to cellular damage induced during wasting states. In this context, autophagy is important in clearing cells from damaged macromolecular complexes or organelles, which may improve the homeostasis and mitigate additional damage. For example, because damaged mitochondria may contribute to enhanced ROS production and further cell damage, mitophagy, that is, the degradation of damaged mitochondria by autophagosomes, may rather represent a protective mechanism than a cause of muscle wasting (99).
In line with this assumption, several papers describe augmented cell damage occurring with different pathological states when components of the autophagic machinery are inactivated (61, 84, 85, 97, 108).
Humoral Factors Influencing Cachexia
The induction of skeletal muscle wasting by diseased remote organs implies that circulating factors derived from the diseased tissues act on skeletal muscle to elicit catabolism in skeletal muscle.
Cytokines
Chronic diseases (e.g., COPD, heart failure, cancer, etc.) are often associated with tissue infiltration of inflammatory cells, leading to the enhanced release of pro-inflammatory cytokines by activated immune cells and/or tissue cells. These cytokines may travel through the circulation to reach remote organs, such as skeletal muscle (Fig. 5).
FIG. 5.
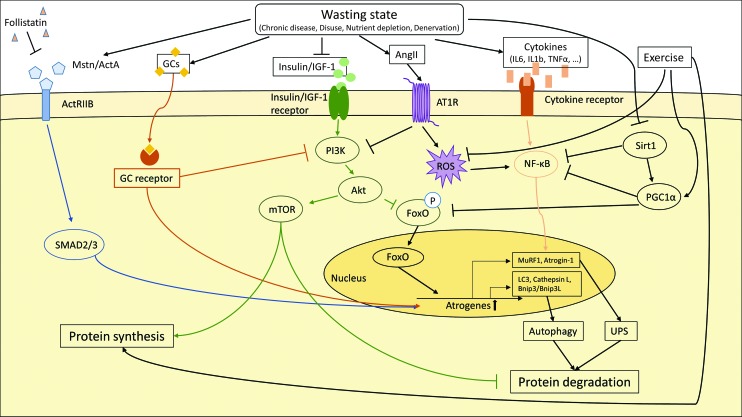
Molecular pathways involved in skeletal muscle protein degradation. The induction of atrogenes in catabolic muscle is driven by various molecular pathways. Upregulation of atrogenes leads to skeletal muscle protein degradation via the ubiquitin–proteasome system (UPS) and autophagy. Specific transcription factors such as FoxO proteins, NF-κB and SMAD2/3, as well as GCs activate the transcription of atrogenes. The transcription factors themselves are activated by external stimuli–myostatin (Mstn), activin A (ActA), GCs, insulin, IGF-1, and cytokines. In addition, the anabolic PI3K–AKT–mTOR activity is suppressed, which decreases skeletal muscle protein synthesis and leads to accelerated skeletal muscle protein degradation. Exercise, on the other hand, promotes protein synthesis, blocks ROS production, and enhances PGC1α gene expression. PI3K, phosphoinositide 3; AKT, V-Akt Murine Thymoma Viral Oncogene Homolog 1; mTor, mammalian target of rapamycin; MuRF1, Muscle RING Finger 1; Sirt1, NAD-dependent protein deacetylase sirtuin 1; IL1b/IL6, interleukin 1b/6; TNFα, tumor necrosis factor α; LC3, microtubule-associated protein 1 light chain 3; Bnip3/Bnip3l, BCL2 interacting protein 3/ligand; AT1R, Angiotensin II receptor type 1; ROS, reactive oxygen species; ActRIIb, activin receptor IIb; FoxO, forkhead box protein O; GCs, glucocorticoids; NF-κB, nuclear factor kappa B; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Several pro-inflammatory cytokines, including IL1, IL2, IL6, interferon γ, and tumor necrosis factor alpha (TNFα), are found to be upregulated in patients suffering from a variety of chronic diseases. Indeed, TNFα was first named cachectin, because it was identified as a factor secreted by macrophages that was able to modulate adipose cell metabolism (138). It is of note that TNFα and some other cytokines also affect muscle protein synthesis (60), the release of cortisol and catecholamines from the adrenal gland (124), affect lipolysis and lipid oxidation (114)—all leading to loss of body weight and negative energy balance.
Finally, members of the TGFβ superfamily of cytokines, including TGFβ, myostatin/growth differentiation factor 8 (GDF8), activin, and GDF11, induce the ubiquitin-proteasome system, which reduces muscle mass by increased protein degradation and promotes skeletal muscle dysfunction and weakness (144) (Fig. 5).
Also, glucocorticoids play an especially important role in sepsis as well as in cancer-induced cachexia (19, 66), characterized by an elevated transcription of ubiquitin proteasome-related genes in muscle (147), inhibition of protein synthesis, and induction of gluconeogenesis. Also, the parathyroid hormone-related peptide (PTHrP) and AngII are related to cancer and cardiac cachexia as well as to skeletal muscle wasting due to kidney failure (27, 72).
Insulin-like growth factor 1 (IGF-1) is a classic anabolic hormone that has a well-known positive effect on muscle bulk and strength (13). There is now evidence that IGF-1 plays a protective role in cachexia, and many of its actions are executed by the phosphoinositide 3-kinase (PI3K) AKT-pathway. Pro-cachectic factors such as AngII may reduce IGF-1 levels; hence, a decrease of IGF-1 levels could be an indicator of malnutrition as well as cachexia (34, 117).
AngII Induces Skeletal Muscle Wasting and Fatigue
Among the circulating factors known to induce cachexia, AngII, the active component of the renin-angiotensin-aldosterone system, is the best characterized in terms of its ability to induce skeletal muscle wasting (27, 44, 120).
Heart failure is associated with a substantial neuro-humoral activation, including a sympathetic overflow as well as stimulation of the renin angiotensin system. In the cardiovascular system, AngII may contribute to hypertension, cardiac myocyte and vascular smooth muscle cell hypertrophy, endothelial dysfunction, and insulin resistance. In skeletal muscle, AngII induces a wasting state and antagonizes the anabolic action of growth factors, such as IGF-1 (21).
As reported for many cell types, AngII induces the production of ROS in skeletal muscle (120, 132). ROS not only possess signaling functions but may also induce oxidative damage of proteins, lipids, and so on. In skeletal muscle, AngII enhances ROS generation, most likely via Nox (Fig. 6).
FIG. 6.
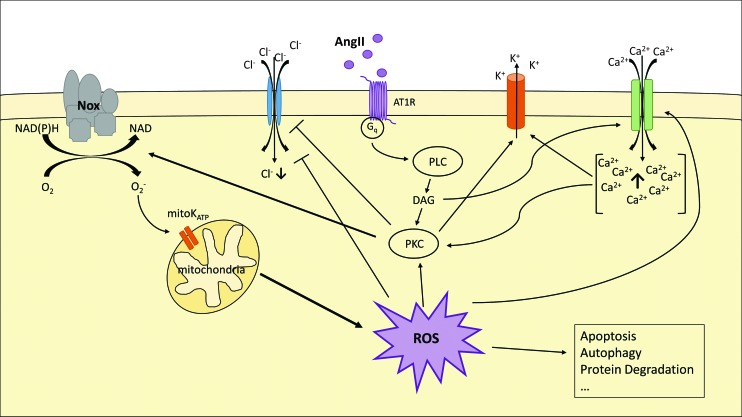
Angiotensin II-induced ROS production via NADPH oxidase. AngII binds to its receptor (AT1R) and induces PKC via the Gq-PLC pathway—which is dependent on DAG as well as on increased cytosolic Ca2+ ions. PKC then activates Nox, blocks Cl− influx, and induces the K+ export. Nox produces superoxide anions (O2·), which open mitochondrial ATP-dependent potassium channels (mitoKATP). This, subsequently, leads to increased mitochondrial ROS production, which gets released in the cytosol. The increasing amount of ROS further induces PKC and the Ca2+ influx and blocks Cl− import. ROS is associated with catabolic mechanism, such as apoptosis, autophagy, and protein degradation. DAG, diacylglycerol; Nox, NADPH oxidase; PKC, proteinkinase C; PLC, phospholipase C. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Wei et al. demonstrated that AngII induces an impairment of insulin sensitivity in L6 skeletal myoblasts concurrent with a reduced GLUT4 translocation to the plasma membrane (145). Small interfering RNA (siRNA), directed against the p47phox subunit of Nox, prevented this effect. Further analysis revealed that a reduced tyrosine phosphorylation of insulin receptor substrate 1 (IRS1) and the protein kinase AKT led to a concerted downregulation of the insulin signaling pathway. This was associated with a reduced translocation of GLUT4 to the plasma membrane, which may underlie the reduced glucose uptake by skeletal muscle cells. Serine phosphorylation of IRS1 also reduces its activity. This inhibitory phosphorylation could result from a feedback inhibition via activated S6 kinase or due to a direct phosphorylation by stress kinases, such as c-Jun N-terminal kinase (JNK).
Thus, reduced insulin sensitivity may contribute to the imbalance between muscle anabolic and catabolic processes in muscle wasting. In this context, it is important to note that there appears to exist a marked antagonism of AngII and IGF-1 (21, 27, 69, 127, 128).
A clinically important hallmark of cachexia is fatigue, which may be the result of degradation of muscle protein. Recent data demonstrate that AngII stimulates the Nox2 isoform via the canonical AT1-receptor—Gαq—phospholipase C (PLC)—diacylglycerol (DAG)—proteinkinase C (PKC)-dependent pathway in skeletal muscle. As a consequence, elevated ROS lead to the oxidation of critical cysteine residues of the ClC-1 chloride channel (35), which leads to reduced Cl− -current and the activation of BK potassium channels. This remodeling of resting ion currents could contribute to fatigue of skeletal muscle observed in different wasting states.
Oxidative stress in skeletal muscle induces proteolysis and, therefore, atrophy via different mechanisms. First, ROS promote transcription of the genes encoding the ubiquitin ligases MuRF1 and Atrogin-1. The basic helix-loop-helix (bHLH) transcription factor EB (TFEB) was identified as a key transcription factor in this response. TFEB binds to specific E-boxes located in the MuRF1 promoter. The transcription-enhancing properties of TFEB can be blocked by histone deacetylases 5 (HDAC5) in the nucleus. An AngII-dependent ATI receptor activation leads to induction of protein kinase D1, which phosphorylates HDAC5 and, hence, causes a cytoplasmic localization of HDAC5. As a result, derepressed TFEB activates MuRF1 expression and paves the way to muscle atrophy (44).
Moreover, activation of calcium-activated proteases, such as calpain as well as the 20S proteasome, may occur in response to AngII stimulation, favoring muscle catabolism (113, 115). Both inhibition of Nox and treatment with antioxidants prevented protein degradation in myotubes. Similarly, upregulation 20S proteasome activity and muscle wasting are sensitive to a genetic or pharmacological inhibition of Nox, indicating the importance of Nox activity in skeletal muscle wasting.
AngII also increases mitochondrial ROS production with respiratory chain complexes I and III as major sources. Other sites, including complex II, have also been identified to be the source of ROS (17). At complexes I and II, electrons are usually transferred to ubiquinone (coenzyme Q), resulting in the formation of ubiquinol, which acts as an electron donor for complex III. However, part of the electrons (0.15%–2% of total mitochondrial oxygen consumption depending on conditions) can be transferred to oxygen, resulting in the formation of O2−· (63, 133).
A large part of O2−· is released from complexes I-III to the mitochondrial matrix, where it is scavenged by the mitochondrial manganese superoxide dismutase (MnSOD; SOD2). This protects Fe-S clusters of aconitase, complex I, and complex II from oxidation-induced inactivation. Under ischemic conditions, complex III may release O2−· anions to the intermembrane space (95), from where they may travel to the cytoplasm via voltage-dependent anion channels (62). This opens the possibility for mitochondria-derived O2−· in modulating cell signaling in the cytosol.
In endothelial cells, the AngII-dependent activation of mitochondrial ROS release is triggered by an initial stimulation of Nox2 via a PKC-dependent phosphorylation of p47phox (40) and an src-mediated stimulation of Ras-related C3 (Rac1) (139). The elevated O2−· levels act on and open mitoKATP channels and decrease mitochondrial membrane potential (mΨ), which is associated with mitochondrial permeability transition pore opening and an enhanced release of ROS to the cytoplasm. This has been shown to further increase oxidative stress, since Nox2 is among the targets for mitochondria-derived ROS, leading to a feed-forward mechanism of oxidative stress development between mitochondrial and Nox-derived ROS [for details, see the review by Schulz et al. (119)].
Overexpression of SOD2 (mitochondrial MnSOD) or the application of mitoTEMPO (a mitochondrially targeted antioxidant) could inhibit AngII-induced mitochondrial dysfunction (38), supporting the relevance of the Nox-mitochondrial crosstalk. In cardiac myocytes, a similar relationship appears to be involved in preconditioning (71). In this context, AngII via Nox stimulation and release of mitochondrial O2−· appears to lead to a ROS-mediated activation of the apoptosis signal regulating kinase 1 (ASK1), which is well known to be regulated by the cellular redox status (18, 67).
Although these mechanisms have been studied extensively in the cardiovascular system, only few studies have addressed the possible contribution of AngII-dependent mechanism in skeletal muscle wasting.
Semprun-Prieto et al. investigated the role of Nox in AngII-mediated skeletal muscle wasting by using p47phox-deficient mice and pharmacological blockade of Nox by a specific inhibitor and antioxidant apocynin (120). They showed that AngII-induced ROS production was blocked in skeletal muscle in both models. Moreover, the elevated 20S proteasome activity and muscle proteolysis were reduced in p47phox-/- mice treated with AngII compared with wild-type mice. In contrast to the mechanism reported for endothelial cells and cardiac myocytes, the mitochondria-targeted antioxidant Mito-TEMPO was not effective in attenuating AngII-induced muscle wasting. Therefore, Nox-dependent stimulation of mitochondrial O2−· release appears not to be a prerequisite for AngII-induced muscle wasting (135).
Cabello-Verrugio et al. and Morales et al. demonstrated in C2C12 myoblasts that AngII-induced ROS production is mediated by an angiotensin I receptor (AT1R)-p38MAPK-Nox signaling cascade, which leads to increased TGFβ expression (26, 27, 92), suggesting a contribution of TGFβ to skeletal muscle wasting and wasting-associated fibrosis.
The effect of ROS not only depends on the expression of pro-oxidative enzymes, such as xanthine oxidase (XO) and Nox, but is also modulated by detoxifying mechanisms (SOD, catalase, and glutathione peroxidase [GPX]).
Although many studies demonstrate a connection between induction of Nox and AngII-mediated protein degradation, oxidative stress in cachexia may be the result of an imbalance between ROS-generating and -degrading systems. Sullivan-Gunn et al. demonstrated a 40% increase of superoxide levels in cachectic muscles (133). Surprisingly, Nox2, p40phox, and p67phox were downregulated in these muscles. Concomitantly, levels of antioxidant proteins, for example, SOD1, SOD2, and GPX, were also downregulated, which most likely resulted in a net increase of oxidative stress. Unfortunately, other ROS-producing systems, such as mitochondrial ROS production, were not evaluated.
Involvement of the TGFβ Superfamily in Cachexia
There exists now ample evidence that members of the TGFβ superfamily of cytokines are involved in the induction of cachexia. TGFβ and its relatives bone morphogenic proteins (BMPs), activins, and myostatin are a family of promiscuous growth factors, which share, in part, their receptors and downstream signal transducers (SMAD family of transcription factors) (118). Signaling involves the formation of protein complexes of class I and class II dimeric receptors, resulting in an active hetero-tetrameric transmembrane receptor.
Tumor growth factor beta receptor II (TGFβRII) receptors together with ALK5 type I receptors are mainly activated by TGFβ itself, leading to downstream activation of Smad2/Smad3/Smad4 complexes. BMPs signal through distinct receptor complexes activating Smad1, Smad5, and Smad8 family members together with Smad4 (11). The cytokine myostatin binds to receptor complexes consisting of the Activin Receptor IIb (ActRIIb) and ALK4 or ALK5 type I receptors, respectively. Signaling then occurs through an Smad2/Smad3 complex.
Myostatin, also known as growth/differentiation factor 8, is the first identified myokine of the TGFβ superfamily. Its role in skeletal muscle is illustrated by the fact that inactivation of the myostatin gene (knockout) results in extensive skeletal muscle hypertrophy in mice, cattle, and humans (43, 86, 87). Myostatin is one of the very prominent examples of a secreted growth factor that is involved in the development of cardiac cachexia. It is generated predominantly in muscle and acts as a major suppressor of muscle growth. Interestingly, cardiomyocyte-specific deletion of the myostatin gene in mice attenuates skeletal muscle wasting in response to pressure overload (20).
Besides myostatin, TGFβ also plays an important role in cachexia. Studies in C2C12 myocytes and in mice revealed that TGFβ-induced muscle wasting was associated with upregulation of E3 ubiquitin ligases (MuRF), and it led to reduced diameter of myotubes and myosin heavy chain degradation (2). These effects were reversible by apocynin or N-acetylcysteine, indicating the participation of ROS in the TGFβ effects. However, it was not investigated as to which Nox isoform caused myocyte shrinkage.
A recent study contributed additional important insights into the role of TGFβ in muscle wasting. Waning and Guise and Waning et al. demonstrated that TGFβ is a principal factor inducing skeletal muscle weakness in the context of cancer cachexia involving NAPDH oxidase Nox4 as a key element (143, 144). Many types of cancer are associated with bone metastases, where the activity of bone-degrading osteoclasts is elevated. Bone matrix, however, is rich with stored TGFβ, which is released during the osteolytic action of tumor cell-stimulated osteoclasts. The released TGFβ induces Smad2/3/4 signaling in muscle, which, in turn, elevates expression of Nox4. The Nox4 isoform of Nox predominantly produces H2O2 (90%) and little O2−· (10%) (100, 121), and its activity is mainly regulated on the transcriptional level.
Nox4 activity depends on binding to the p22phox subunit, but it is independent from other “classic” Nox subunits. Nox4 is localized to cardiac and limb muscle mitochondria and to the sarcoplasmic reticulum (6, 134). Nox4-dependent oxidation of sarcoplasmic calcium release channel ryanodine receptor 1 (Ryr1) acts as an oxygen sensor in skeletal muscle. High pO2 increases oxidation of a subset of Ryr1 thiols by Nox4, leading to Ryr1 activation. Exercise induces molecular alterations of Ryr1 complex, including hyper-nitrosation and loss of calstabin binding, which is a Ryr1-stabilizing protein. The consequence is a reduced exercise tolerance due to leaky Ca2+-release channels (15). Intriguingly, Waning et al. found that this physiological control mechanism appeared to be dysregulated in cancer-associated TGFβ-induced muscle fatigue (144).
The sarcoplasmic calcium release channel Ryr1 forms a complex with Nox4, as both proteins could be co-precipitated, and Ryr1 present in these complexes was oxidized to a higher extent. Moreover, calstabin levels in these complexes were reduced in muscle from cancer-bearing mice (75, 76). Together, these changes compromised function of the Ryr1, resulting in lower muscle strength. Inhibition of Nox4 by a Nox1/Nox4 inhibitor reverted the oxidation of Ryr1, restored calstabin binding to Ryr1, and normalized strength of extensor digitorum longus (EDL) muscles. Importantly, these molecular alterations were also found in a small cohort of breast cancer patients and in a mouse model of Camurati-Engelmann disease, which is associated with increased bone degradation and elevated circulating TGFβ levels.
Although the identity of numerous circulating humoral factors that induce cachexia is known, the precise mechanisms that link the receptor activation in skeletal muscle membranes to the precise intracellular events leading to ROS activation and protein degradation are still a matter of intensive research.
Using a mouse tumor model, Fukawa et al. recently added another piece to the puzzle of “cachectic signaling” in skeletal muscle (50). A very early event identified by the use of three different tumor cell lines was a rapid induction of β-oxidation of fatty acids. In consequence, oxidative stress increased, indicated by elevated mitochondrial ROS formation and higher glutathione (GSH) levels in myotubes incubated with conditioned media from tumor cells. ROS, in turn, stimulated the activity of the stress kinase p38 MAPK, which, by itself, appears to regulate cachectic pathways.
Of note, Etomoxir, a well-known inhibitor of carnitine palmitoyltransferase I (CPT-I) and β-oxidation, efficiently blocked ROS formation in human myotubes treated with pro-cachectic supernatants of tumor cells. Moreover, systemic as well as local application of Etomoxir also reduced the oxidative stress marker 8-oxoguanidine in vivo in tumor-bearing mice and efficiently reduced the tumor-induced weight loss and p38 MAPK activation. Since cachexia, 8-oxoguanidine, and p38 MAPK activation highly correlated in human skeletal muscle biopsies from patients with gastrointestinal cancer, the axis β-oxidation, mitochondrial ROS, and p38 MAPK appear to represent a common pro-cachectic pathway that could be efficiently targeted by the established drug Etomoxir.
Fibertype-Specific Effects of Cachexia
In contrast to cardiac muscle, which is composed of homogeneous striated muscle cells, skeletal muscles are composed of two different fiber type classes—type I fibers (slow twitch fibers) and type II fibers (fast twitch fibers) (Fig. 7). Type I fibers mainly run on an oxidative profile, whereas type II fibers have to be further distinguished according to their metabolism and force generation. Type II fibers are subclassified into type IIa (oxidative), IId/x (glycolytic), and IIb (fastest glycolytic fibers—not seen in humans) fibers. In adult healthy mice, the type IIb fibers usually account for 70%–80% of total muscle fibers, whereby the exact content varies within the different skeletal muscles, depending on their rather oxidative (e.g., Musculus soleus) or glycolytic (e.g., Musculus plantaris) profile.
FIG. 7.
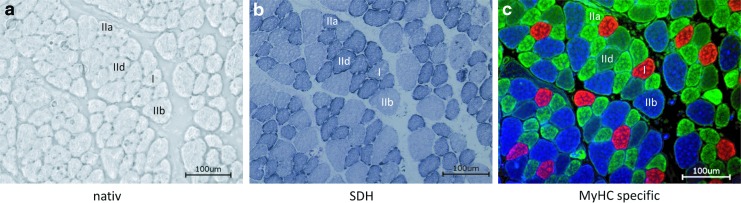
Native and stained cross-sections of murine hindlimb muscle. Serial 8 μm-thick cryosections of (a) native, (b) SDH-stained, and (c) MyHC-specific-stained skeletal muscles (I: MyHC 1 [red]; IIa: MyHC 2a [green]; IId: MyHC 2d/x [unstained/black]; IIb: MyHC 2b [blue]) revealing the fiber-type distribution as well as their oxidative capacity (SDH stain). Bars = 100 μm. MyHC, myosin heavy chain; SDH, succinate dehydrogenase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
During wasting conditions, these fiber types are affected differentially. In cancer, sepsis, and elevated glucocorticoids, type II glycolytic fibers seem to selectively atrophy, suggesting a higher resistance of the oxidative fibers (3, 88, 137). Early work has shown that in patients with chronic heart failure (CHF), skeletal muscle fiber-type composition may shift toward type II fibers. Also, a reduced mitochondrial volume density and cytochrome c oxidase activity (41, 42) was reported. In a mouse model of cardiac cachexia, neither a switch from oxidative to glycolytic fibers nor an atrophy of oxidative fibers was observed, but cross-sectional areas of glycolytic type II fibers were substantially reduced (78).
These fiber type-specific responses to an underlying chronic disease reflect most likely a differential susceptibility of fiber types to physical inactivity as well as disease-associated factors as increased inflammatory cytokines, malnutrition, hypoxia, and oxidative stress (32, 106).
The molecular basis for the higher resistance of oxidative fibers against wasting observed in many pathological states needs to be elucidated. The transcriptional co-activator peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) is a principal regulator of oxidative metabolism and mitochondrial biogenesis. Its transgenic expression in muscle leads to a shift from glycolytic to oxidative type I fibers, characterized by a switch in myosin isoform toward type I, elevated mitochondrial density, and enhanced myoglobin expression (80). Yan and coworkers analyzed skeletal muscle-specific PGC1α expression to determine whether type I fibers would be protected in the context of cardiac cachexia (51).
In transgenic mice with cardiac-specific calsequestrin overexpression, which develop heart failure and cachexia, PGC1α expression antagonized the development of muscle atrophy when compared with calsequestrin-expressing mice. This went along with attenuated FOXO3a levels, suggesting a reduced activation of the ubiquitin-proteasome system and of autophagy, because both systems are under control by FoxO3.
In cachexia-resistant type I fibers, inducible nitric oxide synthase (iNOS) and endothelial NOS (eNOS) were upregulated. Moreover, elevated levels of SOD1, SOD2, and SOD3 contributed to a lower ROS formation by type I fibers than by type II fibers. Further analyses demonstrated a pronounced protection of muscle against wasting by the presence of extracellular SOD (ecSOD, SOD3), since both transgenic mice with muscle-specific overexpression of ecSOD and in situ transfection of muscle by ecSOD expression vectors resulted in muscle protection (101).
Role of Nitric Oxide in Skeletal Muscle Wasting
As mentioned earlier, upregulation of various NOS isoforms occurs in cachexia, raising the question as to what extent NO is involved in modulating cachexia. NO has frequently been termed a double-edged sword (116), because this radical may exert both protective and pathological functions, depending on quantitative effects as well as on the spatial arrangement of NO synthases.
In skeletal muscle, the major NOS isoform is the neuronal (type I) NO synthase (nNOS). nNOS is expressed as different splice variants, giving rise to nNOS isoforms of different size. The main isoform, nNOSμ, is located at the sarcolemma, where it interacts via its N-terminal PDZ domain with α-syntrophin, a component of the dystrophin complex. Sarcolemma-associated nNOS may release NO at high rates due to activity-associated Ca2+ influx. Muscle-derived NO enhances blood flow by dilating blood vessels nearby (105, 136). Most of muscular NO is released by nNOSμ. nNOSβ is another nNOS variant that lacks the PDZ domain and is, therefore, unable to interact with the dystrophin complex. nNOSγ is associated with the Golgi apparatus, where it appears to regulate muscle fatigue (91, 105).
Yet another form of nNOS (mitochondrial NOS [mtNOS]) is claimed to reside inside the mitochondria where it could regulate respiratory chain function, although the existence of an mtNOS is still under debate (140, 151). Thus, in skeletal muscle, there is an extensive compartmentalization of NOS isoforms, and spatially defined actions of different NOS isoforms are clearly documented. Since NO is a diffusible agonist, the question remains as to how specific compartments of NO action can exist within the same cell.
Myoglobin, which is highly expressed in heart and oxidative skeletal muscle, is—when oxygenated—able to efficiently oxidize NO to nitrate. NO can efficiently inhibit mitochondrial respiration, but this function can be blocked efficiently by oxy-myoglobin (48, 55, 148). We hypothesized that myoglobin builds an efficient barrier that separates functional compartments within a muscle cell, allowing the local action of NO, for example, at the sarcolemma and the sarcoplasmic reticulum without a spillover from one compartment to another (12, 54).
To what extent the different actions of NO occur in skeletal muscle under wasting conditions remains to be investigated in detail. iNOS induction occurs in TNFα-induced cachexia in mice (23), in human skeletal muscle biopsies from patients suffering from AIDS and cancer, respectively (109), as well as in cardiac cachexia (5, 149).
Although NOS inhibition appeared to attenuate the extent of muscle wasting in one study of Buck and Choijkier (23), Yu et al. demonstrated that a robust NO-dependent, antioxidative defense may also contribute to the protection in oxidative muscles (149). Specifically, on catabolic stimuli, oxidative muscles have greater NO production and antioxidant gene expression. When the endogenous NO donor, S-Nitrosoglutathione (GSNO), was administered systemically, it mitigated dexamethasone-induced skeletal muscle wasting and attenuated MAFbx/Atrogin-1 messenger RNA (mRNA) expression (101). Moreover, GSNO injection promoted the expression of ecSOD, suggesting that ecSOD is an important player in NO-mediated protection against muscle wasting.
These findings raised the possibility of targeting the NO-dependent antioxidant defense against catabolic muscle wasting. NO could exert its protective effects via canonical cGMP-mediated signaling as well as by direct modification of proteins via nitrosylation, nitrosation, and glutathiolation (56). A more detailed analysis of NO-dependent modifications by proteomic approaches may enable the identification of novel targets in cachexia.
The precise mechanism leading to the NO-dependent modulation of skeletal muscle wasting is not fully understood. A well-defined action of NO is to inhibit the respiratory chain of isolated mitochondria at nanomolar concentrations by nitrosylation of complex IV (33). However, this may result in elevated ROS formation, because a reverse flow of electrons may occur when complex IV is blocked. On the other hand, inhibition of complex I by S-nitrosation is associated with reduced mitochondrial ROS formation. Complex I is considered to represent an “entry point” for electrons, and, therefore, a reduction of ROS formation should be the result of its inhibition. Indeed, at least in the heart, many studies have demonstrated a cardioprotective potential of complex I nitrosation, and the cardioprotection by nitrite was ascribed to this protein modification (31, 110, 125).
Besides its effects on mitochondrial respiration, NO appears to be a major regulator of glucose uptake and mitochondrial biogenesis in skeletal muscle (83), which could explain the protective effects of NO in muscle wasting. Moreover, Lira et al. presented a positive feedback loop involving NO-mediated AMP-dependent protein kinase (AMPK) stimulation, which, in turn, enhances NO formation (82) (Fig. 8).
FIG. 8.
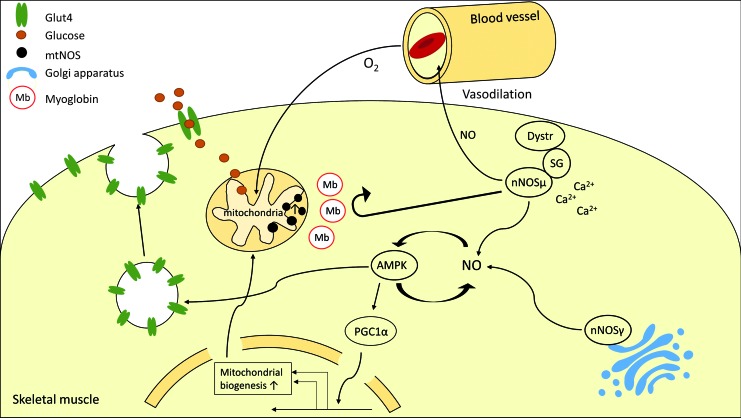
Modulation of skeletal muscle by nitric oxide. nNOSμ associated with the sarcolemma may induce vasodilation, thereby increasing oxygen and nutrient supply by vasodilation. NO enhances AMPK activity and AMPK stimulates NO formation, leading to a feed-forward loop. Activated AMPK, in an NO-dependent manner, promotes translocation of GLUT4 storage vesicles to the sarcolemma, enhancing glucose uptake. Moreover, PGC1α is activated, enhancing mitochondrial biogenesis in skeletal muscle fibers. nNOSγ is associated with the Golgi apparatus. Moreover, a mtNOS has been postulated. The spatial organization of the different NOS compartments may be established by myoglobin, which prevents NO from diffusing between compartments. AMPK, AMP-dependent protein kinase; mtNOS, mitochondrial NOS; nNOS, neuronal NOS; NO, nitric oxide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
AMPK activates PGC1α, which is a master regulator of mitochondrial biogenesis by regulating peroxisome proliferator-activated receptors (PPARs) and nuclear respiratory factor 1 (NRF1)/NRF2 transcriptional activity, resulting in an enhanced oxidative metabolism. Thus, upregulation of eNOS and iNOS could protect muscle from cachexia by promoting an oxidative phenotype of muscle fibers. In particular, iNOS, which is supposed to represent a constitutively active, soluble NOS isoform, could enter the different “nNOS compartments” by diffusion and, hence, drive mitochondrial biogenesis via an AMPK-PGC1α-dependent mechanism.
Besides modulating the action of NO, myoglobin's protective effect in red muscle fibers could result from its ability to directly inactivate ROS. The pseudo-peroxidase activity of myoglobin is associated with oxidation of the central heme-bound Fe2+ to ferric- (Fe3+) or ferryl- (Fe4+) iron species, which may be involved in oxidation of biomolecules such as lipids and amino acids.
Despite its proposed function to enhance oxidative stress, for example, in myocardial infarction (7), studies in isolated hearts of myoglobin knockout mice demonstrated a higher sensitivity to H2O2 and O2−· in the absence of myoglobin. Moreover, myoglobin-deficient hearts displayed a worse recovery from ischemia/reperfusion (47). Thus, in red skeletal muscle, this function of myoglobin may explain, at least in part, why oxidative fibers appear to be more resistant to wasting in the setting of cachexia. Experimental proof of a myoglobin-mediated protection in cachexia awaits the generation of conditional myoglobin knockout mice.
Antioxidative Therapy: A Tool to Treat Skeletal Muscle Wasting?
Current strategies for treatment of skeletal muscle wasting include anti-inflammatory and anabolic strategies. In view of the compelling evidence, that elevated ROS are important elements of the wasting pathology, a preclinical evaluation of ROS-lowering therapy was initiated.
In general, low-molecular-weight compounds with antioxidant activity, including vitamins C and E or inhibitors of ROS-generating enzymes, can be applied. In a rat cancer cachexia model that was induced by an injection of Yoshida AH-130 tumor cells, Springer et al. investigated the effects of various dosages of allopurinol and oxypurinol, inhibitors of XO, which are considered a major source of superoxide production (129). This study was triggered by the observation that uric acid, the end product of the XO reaction, is increased in many patients with heart failure and cancer (39). Interestingly, both XO inhibitors reduced uric acid levels in tumor carrying rats, demonstrating that XO was a major source of uric acid formation and of elevated ROS production in this model.
XO inhibition went along with reduced mortality, skeletal muscle wasting, ROS formation, and attenuated induction of proteasomal genes (129). In line with this pharmacologic approach, overexpression of ecSOD, as mentioned earlier, reduced oxidative stress, expression of atrogenes and attenuated skeletal muscle wasting in a transgenic mouse model of cachexia (101). In contrast to the promising results obtained by directly targeting enzymes involved in ROS formation, an antioxidant mixture containing green tea extracts, ascorbic acid, resveratrol, and a.o. failed to prevent the development of cachexia in a mouse model based on an injection of C26 tumor cells (10). Thus, a more targeted approach instead of using a broad spectrum of antioxidants appears to be a more effective strategy to inhibit ROS formation and ameliorate cachexia.
Although antioxidant therapies appear to be a logic postulate given the involvement of ROS in cachexia, trials to improve other ROS-associated diseases by antioxidants or XO inhibition have failed in many cases or yielded contradictory results. Thus, also in the case of cachexia, the development of effective therapeutic approaches based on reduction of oxidative stress will be a major challenge. For example, XO inhibition by allopurinol represents a relatively specific approach, which appeared to improve endothelial dysfunction in hypertensive patients (24) and positively influenced cardiac function in patients with idiopathic dilative cardiomyopathy (28).
However, the recently published results of the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study demonstrated that despite a reduction of uric acid plasma levels by allopurinol, clinical status, exercise capacity, and left-ventricular ejection fraction were not improved (53). Also, vitamin E-based interventions did not yield positive results in the treatment of heart failure (1, 150).
The limited success of antioxidant therapies despite encouraging results in preclinical models may be, on the one hand, due to the choice of inappropriate antioxidants (130). On the other hand, the approach of just targeting the formation of ROS may be too simple in view of the various sources of ROS formation and mechanisms of inactivation, including mitochondria, Nox enzymes, different forms of SOD, peroxiredoxins, and so on. Under basal conditions, ROS are important parts of intracellular signaling. Thus, extensive inhibition of ROS formation may also disturb basal signal transduction, inducing unwanted side effects.
Depending on the underlying disease (cancer, heart failure, COPD, etc.) leading to cachexia, the comorbidities may even complicate the situation. ROS formation by different sources such as mitochondria, various Nox isoforms, XO, and so on most likely varies depending on the underlying disease and the type of interorgan communication. Thus, targeting may require the application of disease-specific inhibitors. In case of an Nox contribution, this may be a challenging task, since currently inhibitors with sufficient Nox and, even more problematic, sufficient Nox isoform specificity still have to be developed (30, 36, 64).
In view of the limitations of antioxidants and the lack of specific pharmacological tools to inhibit ROS-generating enzymes, the induction of endogenous antioxidant enzymes may represent an alternative therapeutic strategy. Indeed, upregulation of SOD and catalase in humans reduced lipid peroxidation (98), suggesting reduced oxidative stress. However, in a recent paper, Dey et al. investigated the contribution of mitochondrial and cytoplasmic compartments to ROS scavenging in H9c2 cardiac cells (37). It was demonstrated that the formation of NADPH, the electron donor for reduction of oxidized GSH and thioredoxin (TRX) in the cytoplasmic and mitochondrial compartments, may become a limiting factor of the ROS-scavenging capacity of the cell.
Metabolic or mitochondrial dysfunction may lead to a loss of reductive capacity, which is important to regenerate not only endogenous reductants such as TRX and GSH but also exogenous antioxidants such as MitoQ. Thus, to be effective, an antioxidative therapy may critically depend on an intact metabolic flux to sufficiently regenerate NAD(P)H, especially in the case of cachexia, which is frequently associated with mitochondrial dysfunction.
Modulation of Cachexia by Exercise
At present, there are no established pharmacological interventions that successfully treat muscle wasting in sarcopenia and cachexia. Exercise training, however, can attenuate or even reverse the process. It is well accepted that endurance exercise is the most effective (22, 142), whereas mixed results have been obtained regarding the impact of resistance exercise (73, 77). For example, a recent animal study has confirmed the effectiveness of endurance exercise with evidence of preserved muscle function and attenuation of muscle mass loss, possibly due to activation of a mammalian target of rapamycin (mTOR). In this study, resistance exercise does not seem to be beneficial, but induces genes associated with muscle damage or repair, indicative of excessive stress (70).
There are also positive findings in frail elderly humans showing that 3 months of resistance exercise significantly reduced the mRNA levels of TNFα in muscle with concomitant increases of protein levels.
Although it is well known that exercise has direct, positive impacts on muscle under the condition of cachexia, the underlying mechanism remains unclear. Exercise impacts appear to be multi-faceted. For example, endurance exercise prevents the increase in tumor necrosis factor-like weak inducer of apoptosis (TWEAK), blocking subsequent activation of NF-κB and its downstream signaling (102). As mentioned earlier, resistance exercise significantly reduced the mRNA levels of TNFα in muscle along with concomitant increases of protein levels, suggesting that exercise may attenuate muscle wasting by suppressing TNFα-mediated inflammation (59).
A recent animal study by Pigna et al. suggests the importance of autophagy in exercise-mediated protection against muscle wasting in cancer cachexia (107). These authors showed that voluntary running of mice subjected to the C26 cell-induced cancer model prevented loss of muscle mass and function along with improved autophagy flux. The increased autophagy flux may improve muscle homeostasis under the condition of cancer cachexia due to removal of, for example, damaged proteins and mitochondria.
An important mechanism by which endurance exercise provides potent protection against catabolic muscle wasting is by promoting antioxidant defense systems. As mentioned earlier, oxidative muscles, which are locomotive muscles, are more resistant to atrophy than glycolytic muscles under almost all conditions of muscle wasting except for the ones induced by reduced neuromuscular activity (3, 25, 74, 78, 90, 123). Consistently, promotion of the oxidative muscle phenotype by PGC1α overexpression in glycolytic muscles significantly attenuates catabolic muscle wasting induced by CHF (51), which is the most likely mediated by enhanced expression of iNOS and eNOS, production of NO, and expression of antioxidant enzymes, including CuZnSOD, MnSOD, ecSOD, and catalase. All of these changes could contribute to reduced oxidative stress (51).
Further supporting this is the finding that voluntary wheel running in mice (4 weeks of training) induces ecSOD and iNOS expression in skeletal muscle. Consistently, ectopic expression by somatic gene transfer and muscle-specific transgenic overexpression of ecSOD provided potent protection under the condition of glucocorticoid treatment and mitigated all aspects of cardiac cachexia, including body weight loss and exercise intolerance (101). These findings strongly support the role of NO-dependent antioxidant defense (i.e., ecSOD) in protection against catabolic muscle wasting.
Exercise is known to promote secretion of humoral factors from the contracting muscles. For example, IL6, which increases 100-fold in the circulation after prolonged exercise, was associated with muscle damage; however, IL6 also regulates the proliferation of satellite cells and promotes hypertrophic growth (122). Paradoxically, circulating IL6 levels were also associated with suppression of muscle protein synthesis and mTOR signaling in cachectic tumor-bearing mice, an effect that was attenuated by treadmill exercise training (146). Exercise also modulates the effects of myostatin. Myostatin is upregulated under conditions of muscle wasting and prevents myocyte differentiation by inhibiting the Akt/mTOR pathway. Importantly, 6 months of low-intensity endurance training in insulin-resistant adult males led to reduced myostatin (65).
The proof of beneficial effects of exercise on skeletal muscle wasting is not restricted to animal studies. A clinical study explored the impact of exercise training on the expression of antioxidant genes in patients with CHF (81). Skeletal muscle biopsies taken from healthy people and from CHF patients after completion of a 6-month training protocol or continued sedentary lifestyle demonstrated (1) a reduction of SOD, GPX, and catalase mRNA and protein levels in CHF patients compared with healthy control subjects. Moreover, this study also showed that (2) exercise training increased the activity of GPX and Cat in muscle of trained CHF patients. These changes went along with a reduction of lipid peroxides and nitrotyrosine in muscle biopsies. Thus, exercise reduces oxidative stress in skeletal muscle of CHF patients.
More recent data extended the concept of muscle protection by training. The Leipzig Catabolism Study (Leica) demonstrated that after a 4-week training protocol, MuRF1 expression, an indicator of elevated ubiquitin-proteasome-system activity, was significantly reduced in quadriceps muscles of heart failure patients (52). Also, the level of ubiquitinated proteins was lower than in biopsies from sedentary muscle. Concomitantly, IGF-1 expression increased, suggesting that exercise shifted the catabolism-anabolism ratio to a reduced muscle wasting.
Besides the positive effects of exercise training on the expression genes involved in ROS and protein degradation, a downregulation of pro oxidant genes such as Nox could also be part of the beneficial effects of training. At least in the vasculature, exercise training of coronary artery disease (CAD) patients led to reduced expression of Nox4, gp91phox, and p22phox (4).
Overall, exercise activates several different systems that act either independently or synergistically to reduce oxidative stress in muscle. Exercise represents a low-cost, natural, and potently effective strategy that provides protection against muscle wasting.
Abbreviations Used
- ActRIIb
activin receptor IIb
- AIDS
acquired immunodeficiency syndrome
- AMPK
AMP-dependent protein kinase
- AngII
angiotensin II
- AT1R
angiotensin I receptor
- CHF
chronic heart failure
- COPD
chronic obstructive pulmonary disease
- DAG
diacylglycerol
- ecSOD
extracellular superoxide dismutase
- eNOS
endothelial NOS
- FoxO
forkhead box protein O
- GDF
growth differentiation factor
- GPX
glutathione peroxidase
- GSH
glutathione
- GSNO
S-Nitrosoglutathione
- HDAC
histone deacetylases
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- iNOS
inducible NOS
- IRS1
insulin receptor substrate 1
- MAFbx
muscle atrophy F-box
- MnSOD
manganese superoxide dismutase
- mRNA
messenger RNA
- mtNOS
mitochondrial NOS
- mTOR
mammalian target of rapamycin
- MuRF1
Muscle RING Finger 1
- NF-κB
nuclear factor “kappa-light-chain-enhancer” of activated B-cells
- nNOS
neuronal NOS
- NOS
nitric oxide synthase
- Nox
NADPH oxidase
- NRF
nuclear respiratory factor
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3
phosphoinositide 3-kinase
- PKC
proteinkinase C
- PLC
phospholipase C
- ROS
reactive oxygen species
- Ryr1
ryanodine receptor 1
- SOD
superoxide dismutase
- TFEB
transcription factor EB
- TGFβ
tumor growth factor beta
- TNFα
tumor necrosis factor alpha
- TRX
thioredoxin
- XO
xanthine oxidase
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG) via International Research Training Group IRTG1902 to A.G. and partially supported by the National Institutes of Health R01GM109473 to Z.Y.
References
- 1.MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 360: 23–33, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Abrigo J, Rivera JC, Simon F, Cabrera D, and Cabello-Verrugio C. Transforming growth factor type beta (TGF-beta) requires reactive oxygen species to induce skeletal muscle atrophy. Cell Signal 28: 366–376, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, and Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8: 421–432, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, and Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555–562, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Adams V, Yu J, Mobius-Winkler S, Linke A, Weigl C, Hilbrich L, Schuler G, and Hambrecht R. Increased inducible nitric oxide synthase in skeletal muscle biopsies from patients with chronic heart failure. Biochem Mol Med 61: 152–160, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Ago T, Kuroda J, Pain J, Fu C, Li H, and Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alayash AI. Redox biology of blood. Antioxid Redox Signal 6: 941–943, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, and Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Aoyagi T, Terracina KP, Raza A, Matsubara H, and Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 7: 17–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assi M, Derbre F, Lefeuvre-Orfila L, and Rebillard A. Antioxidant supplementation accelerates cachexia development by promoting tumor growth in C26 tumor-bearing mice. Free Radic Biol Med 91: 204–214, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Attisano L. and Wrana JL. Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, and Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Barton ER, Morris L, Musaro A, Rosenthal N, and Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157: 137–148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bechara LR, Moreira JB, Jannig PR, Voltarelli VA, Dourado PM, Vasconcelos AR, Scavone C, Ramires PR, and Brum PC. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int J Cardiol 175: 499–507, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, and Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A 105: 2198–2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, and Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol 45: 466–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandes RP. Triggering mitochondrial radical release: a new function for NADPH oxidases. Hypertension 45: 847–848, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Braun TP, Grossberg AJ, Krasnow SM, Levasseur PR, Szumowski M, Zhu XX, Maxson JE, Knoll JG, Barnes AP, and Marks DL. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J 27: 3572–3582, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitbart A, Auger-Messier M, Molkentin JD, and Heineke J. Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol 300: H1973–H1982, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, and Delafontaine P. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology 142: 1489–1496, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Brum PC, Bacurau AV, Cunha TF, Bechara LR, and Moreira JB. Skeletal myopathy in heart failure: Effects of aerobic exercise training. Exp Physiol 99: 616–620, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Buck M. and Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15: 1753–1765, 1996 [PMC free article] [PubMed] [Google Scholar]
- 24.Butler R, Morris AD, Belch JJ, Hill A, and Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 35: 746–751, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Butori C, Desnuelle C, Hofman P, Paquis V, Durant J, Carles M, Pesce A, and Michiels JF. Muscular involvement in the course of AIDS. Anatomo-clinical study of 17 cases [in French]. Ann Pathol 15: 424–430, 1995 [PubMed] [Google Scholar]
- 26.Cabello-Verrugio C, Acuna MJ, Morales MG, Becerra A, Simon F, and Brandan E. Fibrotic response induced by angiotensin-II requires NAD(P)H oxidase-induced reactive oxygen species (ROS) in skeletal muscle cells. Biochem Biophys Res Commun 410: 665–670, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Cabello-Verrugio C, Cordova G, and Salas JD. Angiotensin II: Role in skeletal muscle atrophy. Curr Protein Pept Sci 13: 560–569, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, and Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 104: 2407–2411, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Carson JA. and Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev 38: 168–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casas AI, Dao VT, Daiber A, Maghzal GJ, Di Lisa F, Kaludercic N, Leach S, Cuadrado A, Jaquet V, Seredenina T, Krause KH, Lopez MG, Stocker R, Ghezzi P, and Schmidt HH. Reactive oxygen-related diseases: Therapeutic targets and emerging clinical indications. Antioxid Redox Signal 23: 1171–1185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, and Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19: 753–759, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, and Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45: 2191–2199, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Cooper CE. and Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J Bioenerg Biomembr 40: 533–539, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano FJ, Doglietto GB, Argiles JM, Baccino FM, and Rossi Fanelli F. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 291: R674–R683, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Cozzoli A, Liantonio A, Conte E, Cannone M, Massari AM, Giustino A, Scaramuzzi A, Pierno S, Mantuano P, Capogrosso RF, Camerino GM, and De Luca A. Angiotensin II modulates mouse skeletal muscle resting conductance to chloride and potassium ions and calcium homeostasis via the AT1 receptor and NADPH oxidase. Am J Physiol Cell Physiol 307: C634–C647, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dao VT, Casas AI, Maghzal GJ, Seredenina T, Kaludercic N, Robledinos-Anton N, Di Lisa F, Stocker R, Ghezzi P, Jaquet V, Cuadrado A, and Schmidt HH. Pharmacology and clinical drug candidates in redox medicine. Antioxid Redox Signal 23: 1113–1129, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dey S, Sidor A, and O'Rourke B. Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. J Biol Chem 291: 11185–11197, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doehner W, Jankowska EA, Springer J, Lainscak M, and Anker SD. Uric acid and xanthine oxidase in heart failure – Emerging data and therapeutic implications. Int J Cardiol 213: 15–19, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Drexler H. Skeletal muscle failure in heart failure. Circulation 85: 1621–1623, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Drexler H, Riede U, Munzel T, Konig H, Funke E, and Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 85: 1751–1759, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Dschietzig TB. Myostatin – From the mighty mouse to cardiovascular disease and cachexia. Clin Chim Acta 433: 216–224, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, Song K, Schmidt S, Bassel-Duby R, Olson EN, and Fielitz J. Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res 117: 424–436, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, and Anker SD. Cachexia: A new definition. Clin Nutr 27: 793–799, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, and Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66: 1142–1174, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Flogel U, Godecke A, Klotz LO, and Schrader J. Role of myoglobin in the antioxidant defense of the heart. FASEB J 18: 1156–1158, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Flogel U, Merx MW, Godecke A, Decking UK, and Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci U S A 98: 735–740, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman LM. The pathophysiology of cardiac cachexia. Curr Opin Support Palliat Care 3: 276–281, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian CN, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama HO, Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan KK, Ho YS, Tan IB, Teh BT, and Shyh-Chang N. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat Med 22: 666–671, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Geng T, Li P, Yin X, and Yan Z. PGC-1alpha promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am J Pathol 178: 1738–1748, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gielen S, Sandri M, Kozarez I, Kratzsch J, Teupser D, Thiery J, Erbs S, Mangner N, Lenk K, Hambrecht R, Schuler G, and Adams V. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation 125: 2716–2727, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tang WH, Dunlap ME, LeWinter MM, Mann DL, Felker GM, O'Connor CM, Goldsmith SR, Ofili EO, Saltzberg MT, Margulies KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, and Hernandez AF. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: The Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study. Circulation 131: 1763–1771, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godecke A. On the impact of NO-globin interactions in the cardiovascular system. Cardiovasc Res 69: 309–317, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, and Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem 278: 21761–21766, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Godecke A, Schrader J, and Reinartz M. Nitric oxide-mediated protein modification in cardiovascular physiology and pathology. Proteomics Clin Appl 2: 811–822, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Gomes-Marcondes MC. and Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett 180: 69–74, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Gorgens SW, Eckardt K, Jensen J, Drevon CA, and Eckel J. Exercise and regulation of adipokine and myokine production. Prog Mol Biol Transl Sci 135: 313–336, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, and Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 15: 475–482, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Guttridge DC, Mayo MW, Madrid LV, Wang CY, and Baldwin AS., Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289: 2363–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Halling JF, Ringholm S, Nielsen MM, Overby P, and Pilegaard H. PGC-1alpha promotes exercise-induced autophagy in mouse skeletal muscle. Physiol Rep 4(3): e12698, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han D, Antunes F, Canali R, Rettori D, and Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem 278: 5557–5563, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K, Seino S, and Takahashi Y. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology 152: 912–921, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, and Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 42: 2023–2029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Z, Wang H, Lee IH, Du J, and Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 119: 3059–3069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Q, Zhou HJ, Zhang H, Huang Y, Hinojosa-Kirschenbaum F, Fan P, Yao L, Belardinelli L, Tellides G, Giordano FJ, Budas GR, and Min W. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation 131: 1082–1097, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ju JS, Jeon SI, Park JY, Lee JY, Lee SC, Cho KJ, and Jeong JM. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci 66: 417–430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kackstein K, Teren A, Matsumoto Y, Mangner N, Mobius-Winkler S, Linke A, Schuler G, Punkt K, and Adams V. Impact of angiotensin II on skeletal muscle metabolism and function in mice: contribution of IGF-1, Sirtuin-1 and PGC-1alpha. Acta Histochem 115: 363–370, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Khamoui AV, Park BS, Kim DH, Yeh MC, Oh SL, Elam ML, Jo E, Arjmandi BH, Salazar G, Grant SC, Contreras RJ, Lee WJ, and Kim JS. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metabolism 65: 685–698, 2016 [DOI] [PubMed] [Google Scholar]
- 71.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, and Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, Hodin RA, and Spiegelman BM. PTH/PTHrP receptor mediates cachexia in models of kidney failure and cancer. Cell Metab 23: 315–323, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, and Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle 5: 199–207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krolick KA. Muscle-derived nitric oxide synthase expression, differences associated with muscle fiber-type, and disease susceptibility in a rat model of myasthenia gravis. Clin Immunol 121: 286–293, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Lehnart SE. Novel targets for treating heart and muscle disease: Stabilizing ryanodine receptors and preventing intracellular calcium leak. Curr Opin Pharmacol 7: 225–232, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, and Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A 103: 7906–7910, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, and Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 61: 1726–1734, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, and Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599–608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li YP, Chen Y, Li AS, and Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol 285: C806–C812, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, and Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 81.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, and Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: Increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, and Criswell DS. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol 588: 3551–3566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lira VA, Soltow QA, Long JH, Betters JL, Sellman JE, and Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab 293: E1062–E1068, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, and Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Masiero E. and Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy 6: 307–309, 2010 [DOI] [PubMed] [Google Scholar]
- 86.McPherron AC, Lawler AM, and Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 87.McPherron AC. and Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A 94: 12457–12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mendell JR. and Engel WK. The fine structure of type II muscle fiber atrophy. Neurology 21: 358–365, 1971 [DOI] [PubMed] [Google Scholar]
- 89.Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL, Blaauw B, DePinho RA, and Sandri M. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6: 6670, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, and Hesselink MK. Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve 31: 339–348, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Moon Y, Balke JE, Madorma D, Siegel MP, Knowels GM, Brouckaert P, Buys E, Marcinek DJ, and Percival J. Nitric oxide regulates skeletal muscle fatigue, fiber type, microtubule organization and mitochondrial ATP synthesis efficiency through cGMP-dependent mechanisms. Antioxid Redox Signal 2016. [Epub ahead of print]; DOI: 10.1089/ars.2016.6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morales MG, Vazquez Y, Acuna MJ, Rivera JC, Simon F, Salas JD, Alvarez Ruf J, Brandan E, and Cabello-Verrugio C. Angiotensin II-induced pro-fibrotic effects require p38MAPK activity and transforming growth factor beta 1 expression in skeletal muscle cells. Int J Biochem Cell Biol 44: 1993–2002, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Morley JE, Thomas DR, and Wilson MM. Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr 83: 735–743, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Moylan JS. and Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35: 411–429, 2007 [DOI] [PubMed] [Google Scholar]
- 95.Muller FL, Liu Y, and Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Nakayama H, Nishida K, and Otsu K. Macromolecular degradation systems and cardiovascular aging. Circ Res 118: 1577–1592, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Nascimbeni AC, Fanin M, Masiero E, Angelini C, and Sandri M. Impaired autophagy contributes to muscle atrophy in glycogen storage disease type II patients. Autophagy 8: 1697–1700, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nelson SK, Bose SK, Grunwald GK, Myhill P, and McCord JM. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic Biol Med 40: 341–347, 2006 [DOI] [PubMed] [Google Scholar]
- 99.Nichenko AS, Southern WM, Atuan M, Luan J, Peissig KB, Foltz SJ, Beedle AM, Warren GL, and Call JA. Mitochondrial maintenance via autophagy contributes to functional skeletal muscle regeneration and remodeling. Am J Physiol Cell Physiol 1: C190–C200, 2016 [DOI] [PubMed] [Google Scholar]
- 100.Nisimoto Y, Diebold BA, Cosentino-Gomes D, and Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okutsu M, Call JA, Lira VA, Zhang M, Donet JA, French BA, Martin KS, Peirce-Cottler SM, Rembold CM, Annex BH, and Yan Z. Extracellular superoxide dismutase ameliorates skeletal muscle abnormalities, cachexia, and exercise intolerance in mice with congestive heart failure. Circ Heart Fail 7: 519–530, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Padrao AI, Figueira AC, Faustino-Rocha AI, Gama A, Loureiro MM, Neuparth MJ, Moreira-Goncalves D, Vitorino R, Amado F, Santos LL, Oliveira PA, Duarte JA, and Ferreira R. Long-term exercise training prevents mammary tumorigenesis-induced muscle wasting in rats through the regulation of TWEAK signalling. Acta Physiol (Oxf) 2016. [Epub ahead of print]; DOI: 10.1111/apha.12721 [DOI] [PubMed] [Google Scholar]
- 103.Penna F, Baccino FM, and Costelli P. Coming back: autophagy in cachexia. Curr Opin Clin Nutr Metab Care 17: 241–246, 2014 [DOI] [PubMed] [Google Scholar]
- 104.Penna F, Costamagna D, Pin F, Camperi A, Fanzani A, Chiarpotto EM, Cavallini G, Bonelli G, Baccino FM, and Costelli P. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol 182: 1367–1378, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Percival JM, Anderson KN, Huang P, Adams ME, and Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest 120: 816–826, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piepoli MF. and Crisafulli A. Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp Physiol 99: 609–615, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Pigna E, Berardi E, Aulino P, Rizzuto E, Zampieri S, Carraro U, Kern H, Merigliano S, Gruppo M, Mericskay M, Li Z, Rocchi M, Barone R, Macaluso F, Di Felice V, Adamo S, Coletti D, and Moresi V. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep 6: 26991, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raben N, Hill V, Shea L, Takikita S, Baum R, Mizushima N, Ralston E, and Plotz P. Suppression of autophagy in skeletal muscle uncovers the accumulation of ubiquitinated proteins and their potential role in muscle damage in Pompe disease. Hum Mol Genet 17: 3897–3908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramamoorthy S, Donohue M, and Buck M. Decreased Jun-D and myogenin expression in muscle wasting of human cachexia. Am J Physiol Endocrinol Metab 297: E392–E401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, and Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114: 1601–1610, 2014 [DOI] [PubMed] [Google Scholar]
- 111.Rom O. and Reznick AZ. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic Biol Med 98: 218–230, 2016 [DOI] [PubMed] [Google Scholar]
- 112.Rosner MH, Ronco C, and Okusa MD. The role of inflammation in the cardio-renal syndrome: a focus on cytokines and inflammatory mediators. Semin Nephrol 32: 70–78, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Russell ST, Eley H, and Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal 19: 1797–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Ryden M, Arvidsson E, Blomqvist L, Perbeck L, Dicker A, and Arner P. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem Biophys Res Commun 318: 168–175, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Sanders PM, Russell ST, and Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 93: 425–434, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schmidt HH. and Walter U. NO at work. Cell 78: 919–925, 1994 [DOI] [PubMed] [Google Scholar]
- 117.Schmidt K, von Haehling S, Doehner W, Palus S, Anker SD, and Springer J. IGF-1 treatment reduces weight loss and improves outcome in a rat model of cancer cachexia. J Cachexia Sarcopenia Muscle 2: 105–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmierer B. and Hill CS. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8: 970–982, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Schulz E, Wenzel P, Munzel T, and Daiber A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid Redox Signal 20: 308–324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Semprun-Prieto LC, Sukhanov S, Yoshida T, Rezk BM, Gonzalez-Villalobos RA, Vaughn C, Michael Tabony A, and Delafontaine P. Angiotensin II induced catabolic effect and muscle atrophy are redox dependent. Biochem Biophys Res Commun 409: 217–221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. Nox4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, and Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Shah AJ, Sahgal V, Quintanilla AP, Subramani V, Singh H, and Hughes R. Muscle in chronic uremia—A histochemical and morphometric study of human quadriceps muscle biopsies. Clin Neuropathol 2: 83–89, 1983 [PubMed] [Google Scholar]
- 124.Shintani F, Nakaki T, Kanba S, Kato R, and Asai M. Role of interleukin-1 in stress responses. A putative neurotransmitter. Mol Neurobiol 10: 47–71, 1995 [DOI] [PubMed] [Google Scholar]
- 125.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, and Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med 204: 2089–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shringarpure R, Grune T, Mehlhase J, and Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem 278: 311–318, 2003 [DOI] [PubMed] [Google Scholar]
- 127.Song YH, Godard M, Li Y, Richmond SR, Rosenthal N, and Delafontaine P. Insulin-like growth factor I-mediated skeletal muscle hypertrophy is characterized by increased mTOR-p70S6K signaling without increased Akt phosphorylation. J Investig Med 53: 135–142, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, and Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115: 451–458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Springer J, Tschirner A, Hartman K, Palus S, Wirth EK, Ruis SB, Moller N, von Haehling S, Argiles JM, Kohrle J, Adams V, Anker SD, and Doehner W. Inhibition of xanthine oxidase reduces wasting and improves outcome in a rat model of cancer cachexia. Int J Cancer 131: 2187–2196, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol 101: 14D–19D, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Stewart MD, Ritterhoff T, Klevit RE, and Brzovic PS. E2 enzymes: More than just middle men. Cell Res 26: 423–440, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S, and Delafontaine P. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 342: 143–147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sullivan-Gunn MJ, Campbell-O'Sullivan SP, Tisdale MJ, and Lewandowski PA. Decreased NADPH oxidase expression and antioxidant activity in cachectic skeletal muscle. J Cachexia Sarcopenia Muscle 2: 181–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, and Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci U S A 108: 16098–16103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tabony AM, Yoshida T, Galvez S, Higashi Y, Sukhanov S, Chandrasekar B, Mitch WE, and Delafontaine P. Angiotensin II upregulates protein phosphatase 2Calpha and inhibits AMP-activated protein kinase signaling and energy balance leading to skeletal muscle wasting. Hypertension 58: 643–649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, and Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal Nitric oxide synthase. Circ Res 92: 554–560, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Tiao G, Lieberman M, Fischer JE, and Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol 272: R849–R856, 1997 [DOI] [PubMed] [Google Scholar]
- 138.Torti FM, Dieckmann B, Beutler B, Cerami A, and Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science 229: 867–869, 1985 [DOI] [PubMed] [Google Scholar]
- 139.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, and Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21: 489–495, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Venkatakrishnan P, Nakayasu ES, Almeida IC, and Miller RT. Absence of nitric-oxide synthase in sequentially purified rat liver mitochondria. J Biol Chem 284: 19843–19855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.von Haehling S, Lainscak M, Springer J, and Anker SD. Cardiac cachexia: A systematic overview. Pharmacol Ther 121: 227–252, 2009 [DOI] [PubMed] [Google Scholar]
- 142.von Haehling S, Steinbeck L, Doehner W, Springer J, and Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol 45: 2257–2265, 2013 [DOI] [PubMed] [Google Scholar]
- 143.Waning DL. and Guise TA. Cancer-associated muscle weakness: What's bone got to do with it? Bonekey Rep 4: 691, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, Chiechi A, Wright LE, Umanskaya A, Niewolna M, Trivedi T, Charkhzarrin S, Khatiwada P, Wronska A, Haynes A, Benassi MS, Witzmann FA, Zhen G, Wang X, Cao X, Roodman GD, Marks AR, and Guise TA. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med 21: 1262–1271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, and Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146, 2006 [DOI] [PubMed] [Google Scholar]
- 146.White JP, Puppa MJ, Gao S, Sato S, Welle SL, and Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab 304: E1042–E1052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wing SS. and Goldberg AL. Glucocorticoids activate the ATP-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am J Physiol 264: E668–E676, 1993 [DOI] [PubMed] [Google Scholar]
- 148.Wunderlich C, Flogel U, Godecke A, Heger J, and Schrader J. Acute inhibition of myoglobin impairs contractility and energy state of iNOS-overexpressing hearts. Circ Res 92: 1352–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 149.Yu Z, Li P, Zhang M, Hannink M, Stamler JS, and Yan Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS One 3: e2086, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yusuf S, Dagenais G, Pogue J, Bosch J, and Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 154–160, 2000 [DOI] [PubMed] [Google Scholar]
- 151.Zaobornyj T. and Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol 303: H1283–H1293, 2012 [DOI] [PubMed] [Google Scholar]