Abstract
We recently demonstrated that the electrical perceptual threshold (EPT) examination reveals spared sensory function at lower spinal segments compared with the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) examination in humans with chronic incomplete cervical spinal cord injury (SCI). Here, we investigated whether discrepancies in sensory function detected by both sensory examinations change over time after SCI. Forty-five participants with acute (<1 year), chronic (≥1–10 years), and extended-chronic (>10 years) incomplete cervical SCI and 30 control subjects were tested on dermatomes C2–T4 bilaterally. EPT values were higher in subjects with acute (2.5 ± 0.8 mA), chronic (2.2 ± 0.7 mA), or extended-chronic (2.8 ± 1.1 mA) SCI compared with controls (1.0 ± 0.1 mA). The EPT examination detected sensory impairments in spinal segments above (2.3 ± 0.9) and below (4.2 ± 2.6) the level detected by the ISNCSCI sensory examination in participants with acute and chronic SCI, respectively. Notably, both examinations detected similar levels of spared sensory function in the extended-chronic phase of SCI (0.8 ± 0.5). A negative correlation was found between differences in EPT and ISNCSCI sensory levels and time post-injury. These observations indicate that discrepancies between EPT and ISNCSCI sensory scores are time-dependent, with the EPT revealing impaired sensory function above, below, or at the same spinal segment as the ISNCSCI examination. We propose that the EPT is a sensitive tool to assess changes in sensory function over time after incomplete cervical SCI.
Key words: : American Spinal Injury Association Impairment Scale (AIS), electrical perceptual threshold, light touch, pinprick, tetraplegia
Introduction
Transmission in sensory pathways is typically altered in humans with incomplete spinal cord injury (SCI), resulting in impairments that might interfere with the control of residual motor function.1 The current gold standard examination to assess sensory function after SCI is the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), which is used to determine the level and severity of injury.2 Although the ISNCSCI examination is extensively used in the clinic, accumulating evidence supports the view that there is a need for quantitative sensitive measurements of sensory function that complement its outcomes. It has been proposed that the electrical perceptual threshold (EPT) examination, which measures the sensory threshold or minimally detectable electrical stimulus intensity applied to the skin,3 is a sensitive tool to assess sensory function that can complement the ISNCSCI examination after human SCI.4–9
The ISNCSCI and EPT examinations have both been used to assess persons immediately and at well-defined periods after SCI. Using the ISNCSCI, it was found that most sensory and motor recovery occurs within the first months after cervical SCI,10–12 but some sensorimotor recovery was also detected 1–5 years post-SCI.13 Interestingly, the EPT detected subclinical changes in sensory function that were not identified by the ISNCSCI examination as early as the first month after SCI8 and also in persons with a median time of 6.5 years post-SCI.9 Although understanding the nature and extent of recovery in sensory function in humans with SCI is critical for proper development of clinical studies, rehabilitation therapies, and clinical trials, the extent to which the EPT and ISNCSCI sensory scores change over time after incomplete SCI still remains unknown.
Some lines of evidence support the view that discrepancies between the EPT and ISNCSCI sensory examinations may vary according to the time after SCI. For example, a study comparing the pinprick and light touch sensory sections of the ISNCSCI examination with the EPT in humans with incomplete SCI revealed that the number of participants with a sensory level detected by EPT above the level assessed by the ISNCSCI examination was larger ∼1 month compared with later months after injury.8
It was recently shown in a group of SCI participants that the EPT examination detected spared sensory function below the ISNCSCI sensory examination in the majority of persons with chronic cervical SCI between 1–10 years post-injury.9 It has also been proposed that the EPT examination provides a comprehensive assessment of sensory function because EPTs correlate with somatosensory evoked potentials (SSEPs),14 and by which have a strong prognostic value after SCI.15 Further, the number of discrepancies reported between light touch and pinprick sensory scores in persons with SCI became larger in those with incomplete SCI.16 Thus, we expected that discrepancies between the ISNCSCI and EPT sensory examinations in persons with incomplete cervical SCI will vary over time, with the EPT examination detecting sensory levels above and below the ISNCSCI examination in the acute and chronic phase of SCI, respectively.
Methods
Subjects
Forty-five participants with SCI (age = 50.3 ± 13.6 years, 3 female; Table 1) and 30 age-comparable controls (mean age = 46.0 ± 20.1 years, 15 female; p = 0.5) were enrolled in the study. All subjects gave informed consent to experimental procedures, which were approved by the local ethics committee at the University of Miami. We included participants with cervical SCI (C1–C8) and, as determined by the American Spinal Injury Association Impairment Scale (AIS).
Table 1.
Spinal Cord Injury Participants
| ACUTE (<1 year) | EPT sensory level | ISNCSCI sensory light touch | ISNCSCI sensory pinprick | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI participants | Age (years) | Sex | Injury (months) | Etiology | Medications | Spasm score | Left | Right | ISNCSCI | AIS | Left | Right | Left | Right |
| 1 | 43 | M | 1.2 | T | GBP | 4 | C3 | C2 | C5 | D | T4 | C5 | C6 | C5 |
| 2 | 58 | M | 1.4 | T | none | 0 | C4 | C7 | C6 | D | C6 | C7 | C6 | C7 |
| 3 | 36 | M | 1.5 | NT | GBP | 4 | C2 | C4 | C5 | C | T1 | C5 | C5 | C5 |
| 4 | 25 | M | 1.5 | T | BAC | 4 | C5 | C4 | C5 | B | C5 | C5 | C5 | C5 |
| 5 | 61 | M | 3 | T | GBP | 4 | C3 | C3 | C4 | D | C4 | T4 | C4 | C6 |
| 6 | 52 | M | 5 | T | BAC, GBP | 3 | C1 | C2 | C4 | B | C5 | C7 | C4 | C6 |
| 7 | 35 | M | 6 | T | BAC, TIZ | 4 | C5 | C8 | C8 | C | T2 | T2 | C8 | C8 |
| CHRONIC (≥1 to 10 years) | EPT sensory level | ISNCSCI sensory light touch | ISNCSCI sensory pinprick | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI participants | Age (years) | Sex | Injury (years) | Etiology | Medications | Spasm score | Left | Right | ISNCSCI | AIS | Left | Right | Left | Right |
| 8 | 61 | M | 1.2 | T | GBP, TIZ | 4 | C8 | C8 | C2 | D | C3 | C2 | C5 | C4 |
| 9 | 49 | M | 1.6 | T | BAC, GBP | 4 | C4 | C4 | C3 | B | C4 | C3 | C4 | C3 |
| 10 | 55 | M | 2 | T | none | 4 | C5 | C5 | C3 | C | C5 | C5 | C4 | C4 |
| 11 | 61 | M | 2.5 | T | none | 1 | C4 | C2 | C3 | D | C3 | C3 | C4 | C5 |
| 12 | 54 | M | 2.5 | T | BAC, TIZ | 3 | C6 | C5 | C2 | D | C3 | C2 | C2 | C3 |
| 13 | 59 | M | 2.7 | T | none | 1 | T2 | T3 | C3 | D | T1 | C4 | C7 | C3 |
| 14 | 40 | M | 3.2 | T | BAC | 4 | C8 | C8 | C6 | C | C7 | C7 | C6 | C6 |
| 15 | 58 | M | 3.6 | T | BAC | 1 | T3 | T2 | C4 | D | C5 | C6 | C4 | C4 |
| 16 | 64 | M | 4.3 | NT | BAC | 3 | C5 | C4 | C2 | B | T4 | C2 | C7 | C7 |
| 17 | 53 | M | 4.6 | T | none | 1 | T4 | T2 | C4 | D | T4 | T1 | T1 | C4 |
| 18 | 54 | M | 4.6 | T | BAC, GBP | 2 | C8 | T2 | C7 | C | C7 | T3 | T3 | C7 |
| 19 | 68 | M | 5.8 | T | none | 3 | T2 | T2 | C2 | D | C2 | C3 | C2 | C2 |
| 20 | 23 | F | 6 | T | none | 2 | T4 | T4 | C4 | B | C6 | C4 | C6 | C4 |
| 21 | 63 | M | 6.2 | T | TIZ | 3 | C1 | C4 | C1 | C | C2 | C1 | C4 | C4 |
| 22 | 64 | M | 6.5 | NT | BAC | 0 | T2 | T4 | C5 | D | C6 | T4 | C5 | C5 |
| 23 | 27 | M | 6.5 | T | BAC | 1 | T1 | T1 | C6 | B | C7 | C6 | C6 | C7 |
| 24 | 56 | M | 6.6 | T | BAC | 4 | C5 | C4 | C3 | C | C5 | C3 | C5 | C3 |
| 25 | 61 | M | 7.3 | T | BAC, GBP | 4 | C4 | C4 | C2 | D | C2 | C3 | C3 | C3 |
| 26 | 28 | M | 7.9 | T | BAC | 4 | C8 | C7 | C4 | C | C5 | C4 | C4 | C4 |
| 27 | 44 | M | 8 | T | BAC | 4 | C4 | C3 | C2 | B | T4 | C2 | C3 | C2 |
| 28 | 65 | M | 8.8 | T | none | 0 | C6 | C8 | C2 | D | C6 | C3 | C2 | C6 |
| 29 | 31 | M | 9 | T | BAC | 4 | C7 | C7 | C6 | B | C6 | C7 | C6 | C7 |
| 30 | 35 | M | 9.3 | T | none | 1 | C6 | C6 | C5 | D | T4 | C5 | T4 | C5 |
| 31 | 54 | M | 9.6 | T | none | 1 | C5 | C4 | C2 | B | C3 | C2 | C2 | C2 |
| 32 | 80 | M | 9.8 | T | none | 1 | C4 | C4 | C3 | D | T4 | T4 | C3 | C3 |
| EXT. CHRONIC (>10 years) | EPT sensory level | ISNCSCI sensory light touch | ISNCSCI sensory pinprick | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCI participants | Age (years) | Sex | Injury (years) | Etiology | Medications | Spasm score | Left | Right | ISNCSCI | AIS | Left | Right | Left | Right |
| 33 | 50 | M | 12 | T | BAC | 1 | T4 | T4 | T4 | D | T4 | T4 | T4 | T4 |
| 33 | 56 | M | 12.9 | T | BAC | 1 | C6 | C7 | C7 | D | C7 | T2 | C7 | T1 |
| 34 | 49 | M | 13.1 | T | none | 1 | C4 | C3 | C4 | D | C5 | C5 | C4 | C4 |
| 35 | 51 | M | 14.5 | T | none | 2 | C4 | C5 | C4 | B | C4 | C4 | C4 | C4 |
| 36 | 32 | M | 14.8 | T | none | 4 | C7 | C7 | C7 | C | C8 | C8 | C7 | C7 |
| 37 | 39 | M | 15 | T | BAC, TIZ | 4 | C1 | C2 | C1 | C | C1 | C1 | C1 | C1 |
| 38 | 62 | F | 16.7 | T | BAC, GBP | 2 | C5 | C6 | C6 | C | T3 | T4 | T1 | C6 |
| 40 | 39 | M | 18.9 | T | none | 1 | T2 | T4 | T2 | D | T2 | T2 | T2 | T2 |
| 39 | 40 | M | 19 | T | BAC | 4 | C6 | C7 | C6 | B | C6 | C6 | C6 | C6 |
| 40 | 47 | F | 20 | T | none | 2 | C4 | C4 | C3 | B | C4 | C3 | C4 | C4 |
| 41 | 73 | M | 20.9 | T | none | 4 | C2 | C3 | C2 | C | C3 | C3 | C2 | C2 |
| 44 | 37 | M | 21.5 | T | none | 2 | T4 | T4 | T3 | C | T4 | T4 | T3 | T4 |
| 42 | 63 | M | 24.1 | T | BAC | 0 | C4 | C4 | C4 | B | C4 | C4 | C4 | C4 |
| 46 | 44 | M | 26 | T | none | 5 | C5 | C4 | C5 | C | C5 | C5 | C5 | C5 |
| 44 | 49 | M | 32 | T | none | 2 | C5 | C5 | C5 | C | C7 | C7 | C6 | C5 |
| 45 | 69 | M | 34 | T | none | 4 | C4 | C4 | C5 | C | C7 | T4 | C7 | C5 |
EPT, electrical perceptual threshold; ISNCSCI, International Standards for Neurological Classification of Spinal Cord Injury; GBP, gabapentin; BAC, baclofen; TIZ, tizanidine.
Persons with SCI underwent a clinical examination by an experienced spinal cord physician before enrollment in the study and were not included if they had a history of severe musculoskeletal injury, peripheral nerve injury, brain injury, or any other severe medical condition. SCI subjects were assigned to three different groups referred as: acute (<1 year post-injury, mean = 2.8 ± 1.9 months; n = 7), chronic (≥1–10 years post-injury, mean = 5.5 ± 2.7 years, n = 25), and extended-chronic (>10 years post-injury, mean = 19.7 ± 6.6 years, n = 13).
The group with acute SCI was defined according to previous literature indicating that neurological scores improved progressively and tended to stabilize by around 1 year post-injury.17 In this group, four subjects were tested as inpatients at Jackson Memorial Hospital and the other three at The Miami Project to Cure Paralysis. The chronic SCI group was defined based on our previous results highlighting that differences in sensory levels detected by the EPT and ISNCSCI sensory examination followed a similar pattern in persons with SCI up to 10 years post-injury.9 Because in our previous work, two of the individuals with a SCI for more than 10 years showed different responses, the group with extended-chronic SCI included participants with an injury for more than 10 years. Individuals in the chronic and extended-chronic group were tested at The Miami Project to Cure Paralysis. Evidence showed that although differences exist across age in EPT values in control subjects, old females, young females, and young males do not show such differences.9
Our control subjects ranged from 21–77 years of age, which was within the age range of SCI subjects included in each SCI group. No differences were found in age between SCI groups and control subjects (acute SCI = 44.3 ± 13.3, ranged from 25 to 61 years, p = 0.6; chronic SCI = 52.3 ± 1 4.4 ranged from 23–80 years, p = 0.3; extended-chronic SCI = 50.0 ± 11.9, ranged from 32–73 years, p = 0.6). Because previous results also indicated differences in EPTs between older males compared with younger males and older females, we used the corresponding age-matched and gender group to compare our EPT values in persons with SCI and the control group.9
EPT
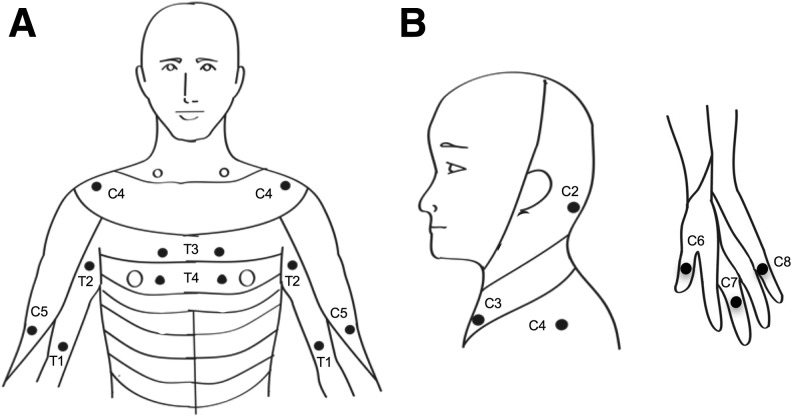
The EPT examination was performed using constant current square wave electrical pulses (0.5 ms pulse width duration, 3 Hz stimulation frequency, DS7A, Digitimer Ltd.). Stimuli were delivered to the skin over the ISNCSCI sensory key points in 22 dermatomes between C2 and T4 (Fig. 1) on both sides of the body by using disposable adhesive electrodes. The cathode was positioned over the ISNCSCI sensory key point, and the anode was placed on the ipsilateral arm to the applied stimulus. The stimulus intensity was manually increased in increments of 0.1 mA up to 10 mA. Each subject was given a familiarization trial to recognize the electrical pulses. Subjects were asked to report verbally when the first sensation was felt.
FIG. 1.
Experimental setup. A, B. Sensory key points by spinal dermatomes reproduced from the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). ISNCSCI key points in dermatomes from C2–T4 were tested in the study bilaterally in participants with acute (<1 year), chronic (≥1–10 years), and extended-chronic (>10 years) incomplete cervical spinal cord injury.
The procedure was repeated three times on each dermatome. The intensity was increased in steps by 0.1 mA, and the time when the stimulus was first perceived was recorded. Then, the intensity was increased two steps above that, and decreased in steps of 0.1 mA until the stimulus was not perceived anymore. The EPT (mA) was calculated as the mean of the intensities when sensation was not perceived in each trial (lowest descending stimulus intensity). The perceived stimulus was described as a light “tapping” or gentle “pulsing” sensation and was not reported as painful by any of the subjects. Subjects were blinded to the amplitude of the stimulus current.
Cumulative distribution functions (CDFs) of EPT values were created for control subjects and SCI participants in dermatomes located above and below the ISNCSCI and EPT sensory level. This function describes the probability that a real-valued random variable x with a given probability distribution will be found to have a value less than or equal to x.
ISNCSCI
SCI subjects participated in a neurological assessment from an ISNCSCI-certified assessor, following the standards by the ISNCSCI guidelines.2 All ISNCSCI sensory examinations were completed by the same examiner. The ISNCSCI examination involved sensory but not motor assessment. The sensory examination included the pinprick and light touch components from dermatomes C2–T4 on both sides of the body (Table 1). The ISNCSCI sensory level was defined as the most caudal intact dermatome for both pinprick and light touch sensation. Note that some sensation could be detected below the ISNCSCI sensory level in some of the dermatomes (Table 1).
Data analysis
In control subjects, mean EPT values and values 2 standard deviation (SD) above the mean were calculated for each dermatome. In SCI participants, EPTs were analyzed in two ways. As in controls, mean EPT values and values 2 SD above the mean for each dermatome were calculated. An EPT was considered “abnormal” when it was >2 SD of the mean value of an age and sex comparable control group. We also assessed significant deviations of EPT values in SCI participants from the mean results in age and sex comparable controls using Z-scores.9 A Z-score represents the distance between the raw score and the population mean in units of the SD. A negative Z-score is found when the raw score is below the mean and positive when it is above.
A Kruskal-Wallis one-way analysis of variance (ANOVA) was performed to determine the effect of GROUP (acute SCI, chronic SCI, extended-chronic SCI, controls) on EPT values. The same test was conducted to examine the effect of DERMATOME (acute above, acute below, chronic above, chronic below, extended-chronic above, extended-chronic below, controls) on EPT values and GROUP on age. Bonferroni post hoc analysis was used to test for significant comparisons. Paired t tests were performed to test the difference between groups and sides as needed.
Z-scores for the SCI population were computed for each dermatome by using the following formula: (SCI EPT value – EPT mean value for control subjects)/(SD for control subjects). A Pearson correlation analysis was used to examine the relationship between differences in sensory level detected by the EPT and ISNCSCI sensory level and time post-injury. Significance was set at p < 0.05. Group data are presented as the mean ± SD in the text.
Results
EPT
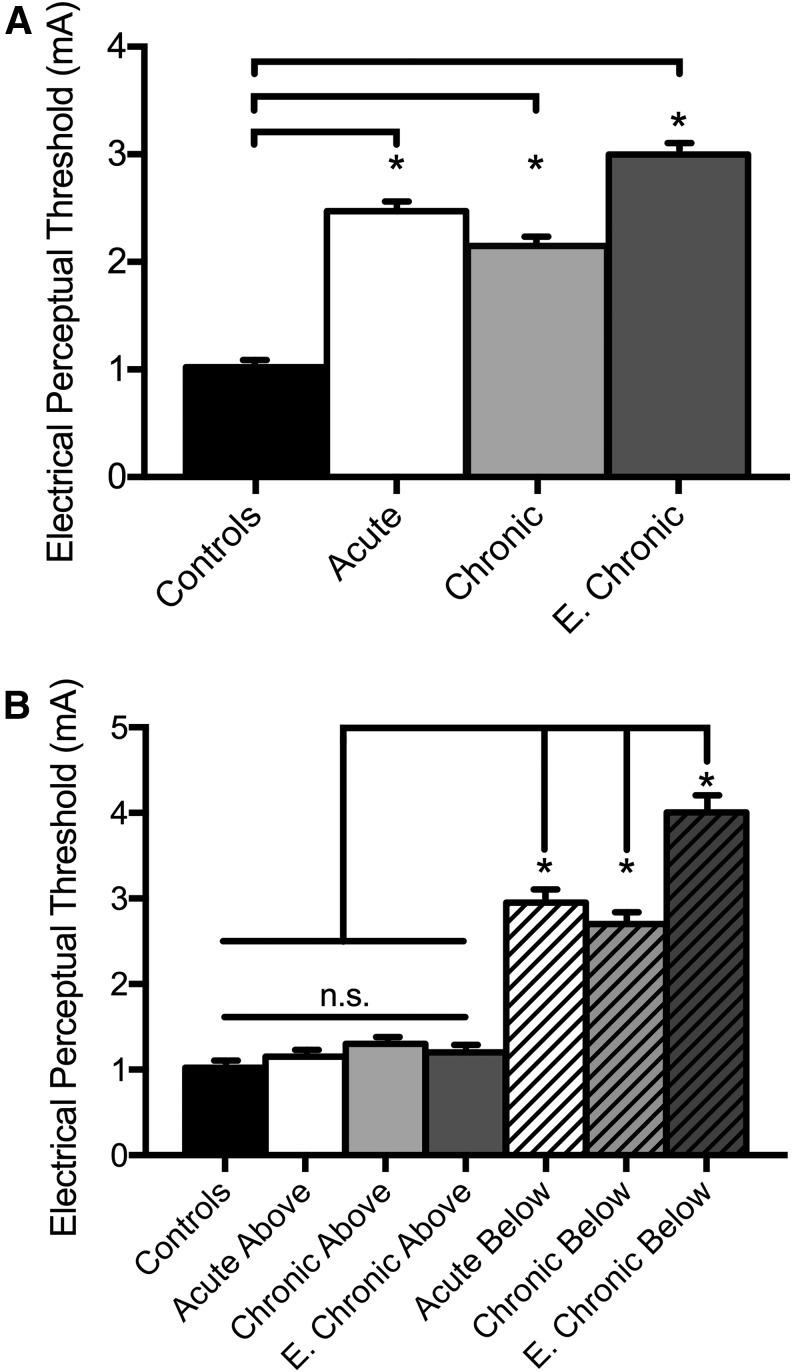
Our ANOVA test showed an effect of GROUP (H = 44.0, p < 0.001) on EPT values. Post hoc testing showed that mean EPT values across all dermatomes were higher in subjects with acute (2.5 ± 0.8 mA, p < 0.05), chronic (2.2 ± 0.7 mA, p < 0.05), and extended-chronic (3.0 ± 1.1 mA, p < 0.05) SCI compared with control subjects (1.0 ± 0.1 mA; Fig. 2A). To further understand the origin of the increases in mean EPT values in SCI participants compared with control subjects, we separated the intensities needed in dermatomes located above and below the sensory level detected by the EPT examination in each SCI participant (Fig. 2B).
FIG. 2.
Electrical perceptual threshold (EPT) values. (A) The abscissa shows the group tested [Controls, back bar; Acute SCI, white bar; Chronic SCI, light gray bar; E. Chronic SCI (extended-chronic SCI), dark gray bar]. The ordinate shows the stimulus intensity (mA) used when the EPT was detected. Note that mean EPT values were higher in all SCI groups compared with control subjects. (B) The abscissa shows the group tested when we separated the dermatomes located above [Acute SCI, white bar; Chronic SCI, light gray bar; E. Chronic SCI (extended-chronic SCI), dark gray bar] and below [Acute SCI, white bar with lines; Chronic SCI, light gray bar with lines; E. Chronic SCI (extended-chronic SCI), dark gray bar with lines] the sensory level detected by the EPT examination in each SCI participant. The ordinate shows the stimulus intensity (mA) used when the EPT was detected. Note that in all SCI groups, mean EPT values in dermatomes above the sensory level detected by the EPT examination were similar to control subjects but were higher in dermatomes located below the sensory level detected by the EPT examination. Error bars indicate standard errors; *p < 0.05.
Here, we found an effect of DERMATOME (H = 82.3, p < 0.001) on EPT values. We found in all SCI groups that mean EPT values in dermatomes located above the sensory level were similar to control subjects (controls = 1.0 ± 0.1 mA, acute above = 1.2 ± 0.1 mA, chronic above = 1.3 ± 0.3 mA, extended-chronic above = 1.3 ± 0.3 mA, p > 0.05), suggesting that the EPT examination can accurately assess sensory function in spinal segments not affected by the injury regardless of the time post-injury. Notably, EPT values in dermatomes located below the sensory level detectedby the EPT examination were higher after SCI compared with controls (controls = 1.0 ± 0.1 mA, acute below = 3.0 ± 0.2 mA, chronic below = 2.7 ± 0.1 mA, extended-chronic below = 4.0 ± 0.4 mA, p < 0.05). These results suggest that the EPT examination is sensitive to detect differences in threshold over time after SCI and that differences in mean EPT values between SCI and control subjects originated in dermatomes located below the sensory level detected by the EPT examination.
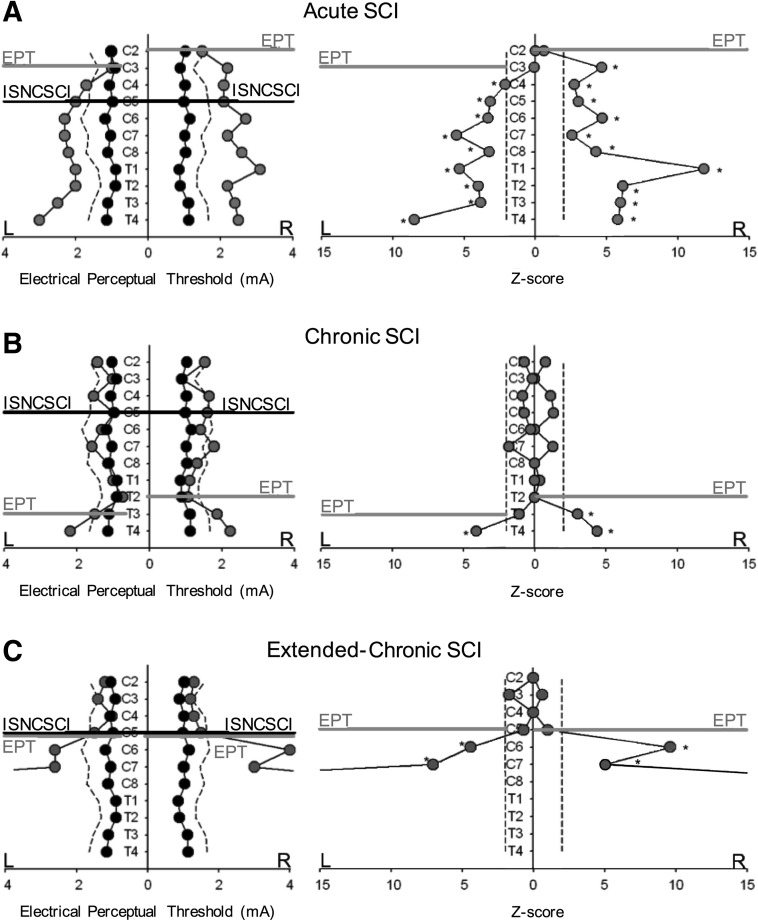
Figure 3 illustrates EPT values and their subsequent Z-score analysis in representative SCI participants from each group. Note that in the participant with acute SCI (Table 1, subject #1; Fig. 3A), the EPT examination showed bilateral sensory asymmetric deviations of EPT values from the mean of control subjects from dermatomes C3 on the left and C2 on the right side. The level of sensory impairment detected by the EPT was located two (left side) and three (right side) spinal segments above the ISNCSCI sensory level as shown by the difference between the black and gray lines. In agreement, the Z-score analysis revealed a significant deviation of EPT values from the mean of control subjects below C3 and C2 on the left and right side, respectively.
FIG. 3.
Electrical perceptual threshold (EPT) vs. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) in representative spinal cord injury (SCI) participants. EPT values and Z-scores are shown in representative SCI participants (gray circles, left and right graphs, respectively) compared with control subjects (black circles, left graphs). Note that a Z-score represents the distance between the raw score and the population mean in units of the standard deviation. A positive Z-score indicates that the raw score is above the mean. Dashed lines in each graph represent values 2 standard deviation from the mean of sex and age-matched controls. ISNCSCI sensory level is shown by a horizontal black line, and the EPT level is shown by a horizontal gray line. In the SCI participant with acute SCI (A, gray circles), the EPT and Z-scores showed a bilateral asymmetric sensory impairment located two (left side) and three (right side) spinal segments above the ISNCSCI sensory level as shown by the difference between the black and gray lines. In the participant with chronic SCI (B, gray circles), the EPT and Z-scores showed a bilateral asymmetric sensory impairment below dermatome T3 on the left side and T2 on the right side. In the participant with extended-chronic SCI (C, gray circles), the EPT and the Z-score analysis revealed significant bilateral symmetric deviations of EPT values from the mean of control subjects from C5. Thus, EPT and ISNCSCI sensory levels were located at the same spinal segment.
In the participant with chronic SCI (Table 1, subject #15; Fig. 3B), the EPT results showed a bilateral asymmetric sensory impairment below dermatome T3 on the left side and below dermatome T2 on the right side. Thus, the sensory level detected by the EPT was located 6 and 5 segments below the ISNCSCI sensory level on the left and right side, respectively. Similarly, the Z-score analysis revealed significant bilateral deviations of EPT values from the mean of control subjects below T3 and T2 on the left and right size, respectively.
In the participant with extended-chronic SCI (Table 1, subject #44; Fig. 3C), the EPT and Z-scores revealed significant bilateral symmetric deviations of EPT values from the mean of control subjects below C5. Notably, EPT and ISNCSCI sensory levels were located at the same spinal segment.
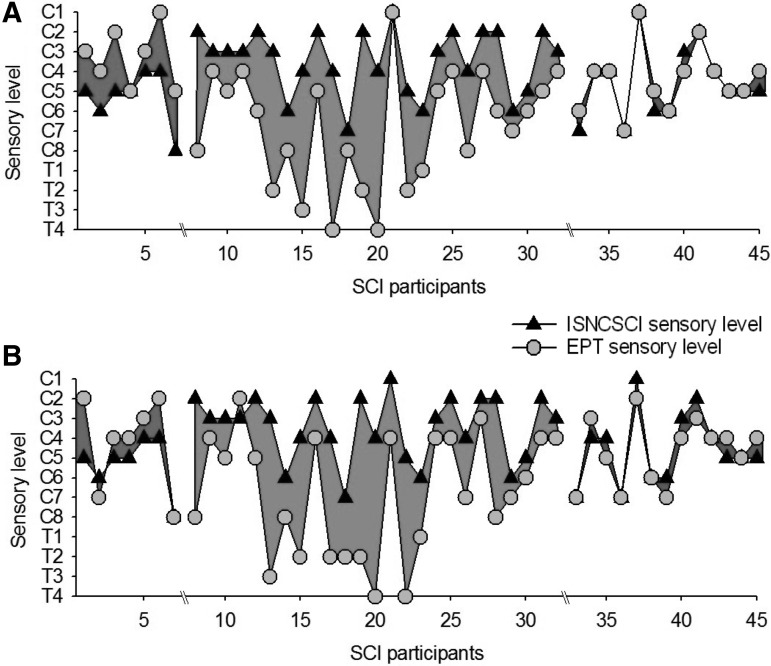
Figure 4 illustrates the EPT and ISNCSCI sensory level in all SCI subjects tested. In the group with acute SCI, note that all subjects showed EPT levels higher than the ISNCSCI sensory level in at least one side of the body (6/7 left side, 5/7 right side). On average, the EPT examination detected impairments in sensory function 2.3 ± 0.9 spinal segments above the level detected by the ISNCSCI examination (range −3 to 1; a negative number indicates that the sensory level detected by the EPT was lower than the ISNCSCI sensory examination).
FIG. 4.
Electrical perceptual threshold (EPT) vs. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) in all spinal cord injury (SCI) participants. In both graphs, the abscissa shows all SCI participants tested (n = 45), and the ordinate shows the sensory level detected by the EPT (gray circles) and ISNCSCI (black triangles) examinations in the left (A) and right (B) side of the body in participants with acute (shaded area to the left, <1 year), chronic (shaded area in the middle, ≥10 years), and extended-chronic (shaded area to the right, >10 years) SCI. Note that regardless of the side tested, the sensory level detected by the EPT was above or below the sensory level detected by the ISNCSCI examination in the majority of SCI participants with acute and chronic SCI, respectively. Very small differences were observed between results from both sensory examinations in persons with extended-chronic SCI.
In contrast, in the group with chronic SCI, the majority of participants showed raised EPTs several segments below the ISNCSCI sensory level, regardless of the side tested (24/25 left side, 25/25 right side). The EPT examination indicated a sensory level 4.2 ± 2.6 spinal segments below that detected by the ISNCSCI examination (range −1 to 8; a negative number indicates that the sensory level detected by the EPT was higher than the ISNCSCI sensory examination).
Note that here we defined EPT level as the last segment where EPT values were similar to control subjects, but comparable results were found if EPT levels were defined as the first segment where EPT values deviated from the mean of controls (5.1 ± 2.7 spinal segments below that detected by ISNCSCI examination).9 Also, note that these discrepancies are because of differences with both subtests of the ISNCSCI sensory examination. Interestingly, in the group with extended-chronic SCI, the majority of subjects showed similar sensory levels when tested with both examinations. On average, the EPT examination detected a sensory level 0.8 ± 0.5 segments below the level detected by the ISNCSCI examination (range −1 to 1; a negative number indicates that the sensory level detected by the EPT was lower than the ISNCSCI sensory examination).
Note that a negative correlation was found for differences between EPT and ISNCSCI sensory levels and the time post-SCI (r = −0.45, p < 0.001). Thus, participants with longer times with SCI showed a smaller difference between EPT and ISNCSCI sensory level.
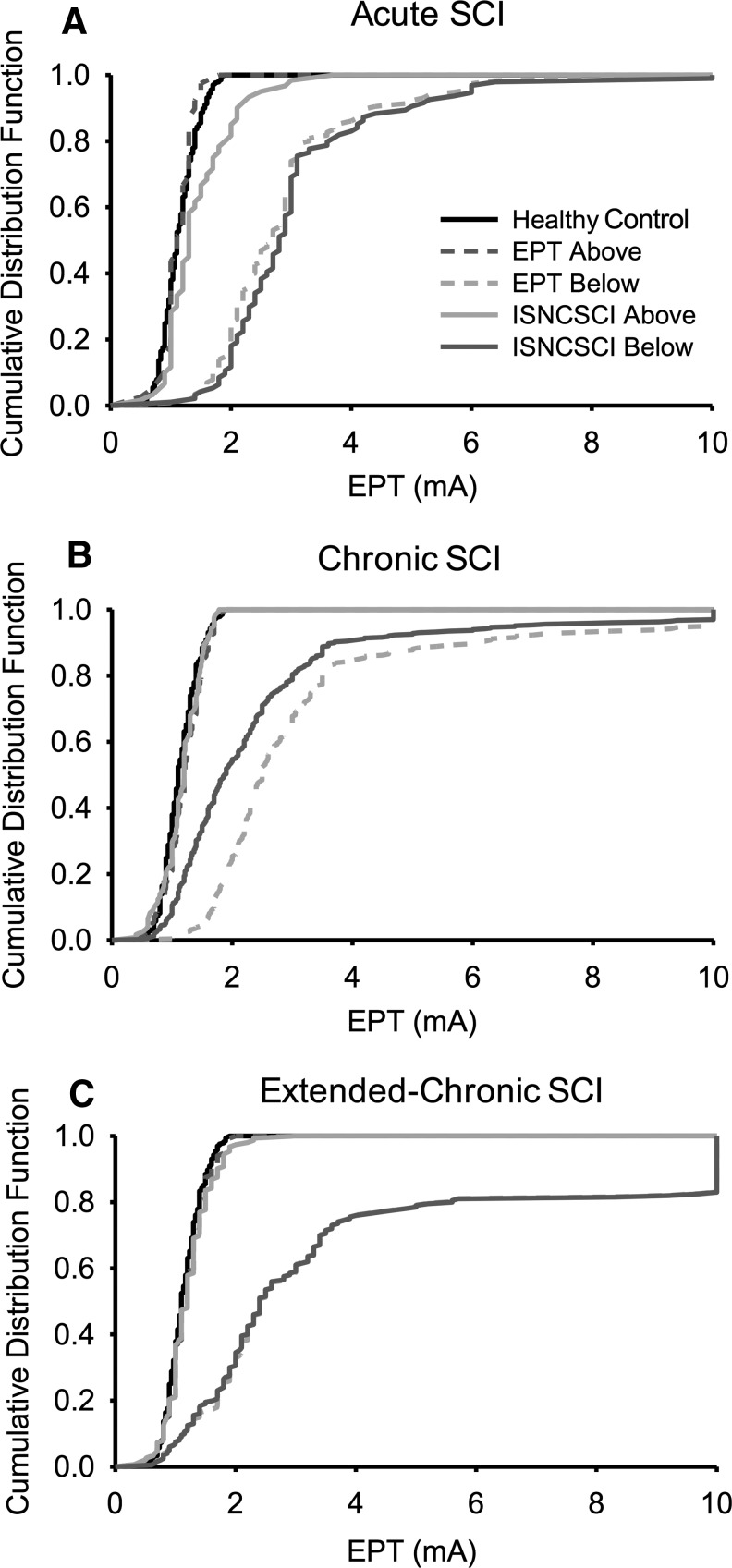
To further analyze differences across participants, we computed CDFs of EPT values in each group (Fig. 5). Note that in SCI subjects, EPT values of all dermatomes were divided into those located above and below the sensory level detected by the EPT and ISNCSCI sensory examination. Figure 5A shows that in control subjects, EPT values were distributed almost symmetrically (center peak at ∼1.1 mA, range 0.7–1.8 mA).
FIG. 5.
Cumulative distribution function (CDF). CDF plots were constructed in participants with acute (A), chronic (B), and extended-chronic (C) spinal cord injury (SCI) and compared with control subjects. In all graphs, the abscissa shows the stimulus intensity (mA) used during testing, and the ordinate shows the number of times that intensities were repeated regardless of the dermatome tested (expressed as a % when the sensory level was detected by the International Standards for Neurological Classification of Spinal Cord Injury [ISNCSCI] and electrical perceptual threshold [EPT] examinations). A–C, the black solid line shows EPT values from control subjects while the dark gray dashed and light gray solid lines show values for dermatomes above the EPT and ISNCSCI sensory level, respectively. The light gray dashed and dark gray solid lines show values below the EPT and ISNCSCI sensory level, respectively.
In subjects with acute SCI, CDFs in dermatomes above the sensory level detected by the EPT were similar to controls but shifted to the right when the sensory level was detected by the ISNCSCI examination, consistent with the view that some dermatomes above the ISNCSCI level required higher intensities during testing.
In the group with chronic SCI, dermatomes above EPT and ISNCSI sensory level showed a similar CDF as controls. Dermatomes below the EPT level showed a more spread asymmetric distribution (center peak at ∼1.8 mA, range from 0.7–10 mA) compared with when the ISNCSI sensory level was used (center peak at ∼2.4 mA, range from 1.0–10 mA), consistent with the view that the EPT detected impaired dermatomes below the ISNCSI sensory examination.
In the extended-chronic SCI group, CDFs were similar to controls in dermatomes above the EPT and ISNCSI sensory level and more spread and with higher values than acute and chronic SCI groups in dermatomes below sensory level detected by both examinations (center peak at ∼2.4 mA, range from 0.7–10 mA). Note that >20% of the EPT values are located above the maximum intensity.
Discussion
Our results indicate that the EPT examination revealed impaired sensory function ∼2 spinal segments above the sensory level detected by the ISNCSCI examination in participants with acute SCI. In contrast, the EPT revealed spared sensory function ∼4 spinal segments below the ISNCSCI sensory examination in participants with chronic SCI. Notably, sensory scores from both examinations were similar to each other in the extended-chronic SCI group. A negative correlation was found between differences in EPT and ISNCSCI sensory levels and the time post-SCI. Altogether, our results indicate for the first time that discrepancies between EPT and ISNCSCI sensory scores are time-dependent, with the EPT revealing impaired sensory function above, below, or at the same spinal segment as the ISNCSCI examination depending on the time after SCI.
EPT vs. ISNCSCI: Acute SCI
Our results in participants with acute SCI agree with previous findings showing that the EPT examination revealed changes in sensory function that were not detected by the ISNCSCI sensory examination within the first few months after SCI.8 This is also consistent with studies reporting that when the EPT detected discrepancies above those detected by the ISNCSCI examination, these difference were usually one or two spinal segments,4,8 as we found.
An important question is why the EPT examination detected sensory impairments above and not below the ISNCSCI examination in the acute phase of SCI. Intriguingly, sensory impairments examined by other sensory tests have also been reported to be a few spinal segments above the level reported by the ISNCSCI examination after SCI.18,19 Because most sensory and motor recovery occurs within the first months after cervical SCI,8,10–12 one might expect that our results in the acute phase will be influenced by this factor. This does not seem to be the case because recovery might indicate changes below and not above the ISNCSCI sensory examination.
It is possible that physiological factors taking place in the first few months post-SCI contributed to our results. For example, Wallerian degeneration in the spinal cord, identified by magnetic resonance imaging, takes place within the first few months post-injury.20 Wallerian degeneration can be observed rostral to the injury in the dorsal columns as early as a few days after the injury, a region that contains the axons that mediate sensory function measured by the EPT and ISNCSCI tests.14
Evidence also showed that other physiological events in the spinal cord are different before and after ∼1 year post-injury. Phagocytic cell invasion in the spinal cord reaches a plateau ∼1 year post-injury, and astrocyte alterations begin ∼1 year post-injury.20 These processes could contribute to differences in the functional state of the spinal cord within the first few months post-injury and thus to the ability to detect sensory function by both sensory examinations.
A higher degree of accuracy might be needed to detect changes in one or two spinal segments.4,8 Thus, another possibility is that the lack of sensitivity of the nominal scale used by the ISNCSCI sensory examination affected our findings. This is consistent with results showing that examiners tend to overestimate the level of injury in the acute phase of SCI8 and that the reliability of some aspects of the ISNCSCI sensory examination is lower compared with the motor examination in the acute phase of SCI.21 Also, our previous results showed that discrepancies between ISNCSCI sensory levels detected by consecutive ISNCSCI sensory examinations was ∼1.6 dermatomes, which is within the range of differences that we found in the group with acute SCI.9
EPT vs. ISNCSCI: Chronic SCI
In participants with chronic SCI, consistent with our previous results,9 we found that the EPT examination detected sensory deficits ∼4 spinal segments below the level indicated by the ISNCSCI sensory examination. It is possible that physiological changes taking place in the chronic phase of SCI contributed to the differences found between sensory examinations in this phase of SCI.
After the first months after SCI, a gradual increase in all reflexes, and different degrees and symptoms of spasticity,22 which involve pathological changes in transmission in large afferent fibers,1 develop in more than 60% of individuals. The posterior part of the cord contains the posterior-column-medial lemniscus pathway, which mediates well-localized fine touch and conscious proprioception. Importantly, the posterior spinal cord is involved in conducting sensory information assessed by the EPT,14 and pathological sensory responses mediated by the posterior spinal cord likely reflect pathology in large afferent fibers.14,15
Because in the EPT examination a mixed population of fibers in the periphery are activated, it is possible that in the chronic phase of SCI, where transmission is generally increased in excitatory reflex pathways and decreased in inhibitory pathways,1 the EPT activates afferents a few spinal segments below the ISNCSCI examination. It is also possible that sensorimotor recovery during the chronic phase of SCI might have contributed to our results.
This must be interpreted with caution, however, because the time course of the mechanisms contributing to recovery and the definition of recovery after SCI still remain unclear.
So far, evidence has shown that the EPT examination has good validity in examining sensory function after SCI,4,25 good intra- and inter-rater reliability in SCI26 and control27,28 subjects, and good sensitivity to detect changes in sensory function over time after SCI.5 Similarly, studies showed that the ISNCSCI sensory examination shows adequate intra- and inter-rater reliability, good validity in assessing sensory function after SCI, and can detect changes in a longitudinal manner after SCI (for review, see29).
When both examinations are compared, however, some differences have been detected. For example, evidence showed that test-retest rater reliability, measured by calculating intra-class correlation coefficients, is lower on sensory levels assessed by the ISNCSCI compared with the EPT examination when assessing participants with chronic incomplete SCI.9 Thus, our findings support the view that the EPT is a sensitive tool to assess recovery of sensory function that can reveal changes not detected by the ISNCSCI sensory examination after chronic human SCI.
EPT vs. ISNCSCI: Extended-chronic SCI
Unexpectedly, considering the results in the 1–10 years chronic SCI group, in the extended-chronic SCI group sensory scores detected by both sensory examinations were similar. We found that persons with SCI for >10 years were those who showed smaller differences between the sensory level detected by the EPT and ISNCSCI. This is consistent with our previous results in two persons with SCI for >10 years showing similar EPT and ISNCSCI sensory scores.9 This is also supported by the negative relationship found between differences in EPT and ISNCSCI sensory level and time post-SCI.
It is unclear whether these results are related to changes in the EPT and/or the ISNCSCI examination because our tests were conducted over time in different subjects. Because these individuals had a more chronic injury, it is also less likely that modifications in spinal cord processes related to the injury affected our findings.
Other changes, however, have been reported over time in humans after SCI. Tactile afferent units innervating the surface of the hand have higher indentation force thresholds and larger receptive fields in humans with SCI for several years compared with controls.30 Thus, an increase in skin stiffness might have affected the ability of individuals to detect the sensory stimulus by either examination.
This is supported by the higher mean EPT values found in participants with extended-chronic compared with chronic SCI. It is unlikely that this was related to the lack of stimulus intensity, because mean EPT values in this group were ∼2.8 mA, and the intensity was increased up to 10 mA without being able to detect a response. It is less likely that this factor affected the group with chronic SCI because mean EPT values were similar in participants with chronic compared with acute SCI. For EPTs, higher intensities needed to stimulate the skin in the extended-chronic phase of SCI might be reflected in the close values to the ISNCSCI examination.
We cannot exclude the possibility that this prolonged period after injury is a better period to compare the outcomes from these sensory examinations because physiological processes affecting the spinal cord in the acute and chronic phases of SCI are possibly more stable. Regardless of the factor affecting our outcomes, our results indicate that discrepancies between EPT and ISNCSCI sensory scores change over time in humans with incomplete cervical SCI.
Functional significance
Assessment tools that provide more accurate characterization of the nature and extent of recovery in sensory function after SCI are critical for the development of rehabilitation approaches, clinical studies, and research outcomes of clinical trials.31,32 For example, if the ISNCSCI sensory examination revealed that a sensory level for an individual is C8 but in fact this participant has impaired sensory function a few segments above and/or below, the ISNCSCI result limits the focus of therapeutic approaches to muscles that receive a different level of innervation and prevents better quantification of progression and/or evolution of the injury.
Our results showing that in some participants light touch and/or pinprick tests revealed a 1 and/or 2 score on one side in dermatomes located below the sensory level detected by the ISNCSCI examination highlights the lack of sensitivity of the ISNCSCI examination, which is likely influenced by the quantification procedures and by the restrictions imposed by the ISNCSCI examination to define normality. Although there is a push in the field to report more than a single sensory neurological level, note that still a “normal” sensory level corresponds to the most caudal segment at which both sides of the body show a score of 2. This immediately excludes the possibility of detecting asymmetries and does not consider possible variations between light touch and pin prick scores.
Conclusion
Our results extend previous findings by showing that the EPT examination is an accurate tool to detect sensory changes that were not detected by the ISNCSCI sensory examination over time after incomplete cervical SCI and highlight the need to examine transmission in other sensory modalities in a time-dependent manner after SCI.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.D'Amico J.M., Condliffe E.G., Martins K.J., Bennett D.J., and Gorassini M.A. (2014). Recovery of neuronal and network excitability after spinal cord injury and implications for spasticity. Front. Integr. Neurosci. 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davey N.J., Nowicky A.V., and Zaman R. (2001). Somatopy of perceptual threshold to cutaneous electrical stimulation in man. Exp. Physiol. 86, 127–130 [DOI] [PubMed] [Google Scholar]
- 4.Savic G., Bergstrom E.M., Frankel H.L., Jamous M.A., Ellaway P.H., and Davey N.J. (2006). Perceptual threshold to cutaneous electrical stimulation in patients with spinal cord injury. Spinal Cord 44, 560–566 [DOI] [PubMed] [Google Scholar]
- 5.Savic G., Frankel H.L., Jamous M.A., Jones P.W., and King N.K. (2011). Sensitivity to change of the cutaneous electrical perceptual threshold test in longitudinal monitoring of spinal cord injury. Spinal Cord 49, 439–444 [DOI] [PubMed] [Google Scholar]
- 6.Kramer J.L., Moss A.J., Taylor P., and Curt A. (2008). Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J. Neurotrauma 25, 1019–1026 [DOI] [PubMed] [Google Scholar]
- 7.King N.K., Savic G., Frankel H., Jamous A., and Ellaway P.H. (2009). Reliability of cutaneous electrical perceptual threshold in the assessment of sensory perception in patients with spinal cord injury. J. Neurotrauma 26, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 8.Lauschke J.L., Leong G.W., Rutkowski S.B., and Waite P.M. (2011). Changes in electrical perceptual threshold in the first 6 months following spinal cord injury. J. Spinal Cord Med. 34, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macklin R.A., Brooke V.J., Calabro F.J., Ellaway P.H., and Perez M.A. (2016). Discrepancies between clinical assessments of sensory function and electrical perceptual thresholds after incomplete chronic cervical spinal cord injury. Spinal Cord 54, 16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters R.L., Adkins R.H., Yakura J.S., and Sie I. (1994). Motor and sensory recovery following incomplete tetraplegia. Arch. Phys. Med. Rehabil. 75, 306–311 [DOI] [PubMed] [Google Scholar]
- 11.Curt A., Keck M.E./, and Dietz V. (1998). Functional outcome following spinal cord injury: significance of motor-evoked potentials and ASIA scores. Arch. Phys. Med. Rehabil. 79, 81–86.1 [DOI] [PubMed] [Google Scholar]
- 12.Steeves J.D., Kramer J.K., Fawcett J.W., Cragg J., Lammertse D.P., Blight A.R., Marino R.J., Ditunno J.F., Jr., Coleman W.P., Geisler F.H., Guest J., Jones L., Burns S., Schubert M., van Hedel H.J., and Curt A.; EMSCI Study Group. (2011). Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49, 257–265 [DOI] [PubMed] [Google Scholar]
- 13.Kirshblum S., Millis S., McKinley W., and Tulsky D. (2004). Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817 [DOI] [PubMed] [Google Scholar]
- 14.Kramer J.L., Moss A.J., Taylor P., and Curt A. (2008). Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J. Neurotrauma 25, 1019–1026 [DOI] [PubMed] [Google Scholar]
- 15.Curt A., and Dietz V. (1999). Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord 37, 157–165 [DOI] [PubMed] [Google Scholar]
- 16.Vasquez N., Gall A., Ellaway P.H., and Craggs M.D. (2013). Light touch and pin prick disparity in the International Standard for Neurological Classification of Spinal Cord Injury (ISNCSCI) Spinal Cord 51, 375–378 [DOI] [PubMed] [Google Scholar]
- 16.Maffiuletti N.A., Herrero A.J., Jubeau M., Impellizzeri F.M., and Bizzini M. (2008). Differences in electrical stimulation thresholds between men and women. Ann. Neurol. 63, 507–512 [DOI] [PubMed] [Google Scholar]
- 17.Smith H.C., Savic G., Frankel H.L., Ellaway P.H., Maskill D.W., Jamous M.A., and Davey N.J. (2000). Corticospinal function studied over time following incomplete spinal cord injury. Spinal Cord 38, 292–300 [DOI] [PubMed] [Google Scholar]
- 18.Hayes K.C., Wolfe D.L., Hsieh J.T., Potter P.J., Krassioukov A., and Durham C.E. (2002). Clinical and electrophysiologic correlates of quantitative sensory testing in patients with incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 83, 1612–1619 [DOI] [PubMed] [Google Scholar]
- 19.Nicotra A., and Ellaway P.H. (2006). Thermal perception thresholds: assessing the level of human spinal cord injury. Spinal Cord 44, 617–624 [DOI] [PubMed] [Google Scholar]
- 20.Becerra J.L., Puckett W.R., Hiester E.D., Quencer R.M., Marcillo A.E., Post M.J., and Bunge R.P. (1995). MR-pathologic comparisons of wallerian degeneration in spinal cord injury. AJNR Am. J. Neuroradiol. 16, 125–133 [PMC free article] [PubMed] [Google Scholar]
- 21.Marino RJ., Jones L., Kirshblum S., Tal J., Dasgupta A. (2008) Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med 31, 166–170 [DOI] [PMC free article] [PubMed]
- 22.Adams M.M., and Hicks A.L. (2005). Spasticity after spinal cord injury. Spinal Cord 43, 577–586 [DOI] [PubMed] [Google Scholar]
- 23.Cortes M., Elder J., Rykman A., Murray L., Avedissian M., Stampas A., Thickbroom G.W., Pascual-Leone A., Krebs H.I., Valls-Sole J., and Edwards D.J. (2013). Improved motor performance in chronic spinal cord injury following upper-limb robotic training. NeuroRehabilitation 33, 57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunday K.L., and Perez M.A. (2012). Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr. Biol. 22, 2355–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer J.L., Moss A.J., Taylor P., and Curt A. (2008). Assessment of posterior spinal cord function with electrical perception threshold in spinal cord injury. J. Neurotrauma 25, 1019–1026 [DOI] [PubMed] [Google Scholar]
- 26.King N.K., Savic G., Frankel H., Jamous A., and Ellaway P.H. (2009). Reliability of cutaneous electrical perceptual threshold in the assessment of sensory perception in patients with spinal cord injury. J. Neurotrauma 26, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 27.Davey N.J., Nowicky A.V., and Zaman R. (2001). Somatopy of perceptual threshold to cutaneous electrical stimulation in man. Exp. Physiol. 86, 127–130 [DOI] [PubMed] [Google Scholar]
- 28.Leong G.W., Gorrie C.A., Ng K., Rutkowski S., and Waite P.M. (2009). Electrical perceptual threshold testing: a validation study. J. Spinal Cord Med. 32, 140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furlan J.C., Fehlings M.G., Tator C.H., and Davis A.M. (2008). Motor and sensory assessment of patients in clinical trials for pharmacological therapy of acute spinal cord injury: psychometric properties of the ASIA Standards. J. Neurotrauma 25, 1273–1301 [DOI] [PubMed] [Google Scholar]
- 30.Thomas C.K, and Westling G. (1995). Tactile unit properties after human cervical spinal cord injury. Brain 118, 1547–1556 [DOI] [PubMed] [Google Scholar]
- 31.Steeves J.D.Bench to bedside: challenges of clinical translation. (2015). Prog. Brain Res. 218, 227–239 [DOI] [PubMed] [Google Scholar]
- 32.Geisler F.H., Coleman W.P., Grieco G., and Poonian D.; Sygen Study Group. (2001). Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 26, Suppl 24, S68–S86 [DOI] [PubMed] [Google Scholar]