Abstract
Musca domestica L. were collected from cattle diagnosed with bovine ringworm to evaluate the potential of the house fly to disseminate Trichophyton verrucosum E. Bodin, a fungal dermatophyte that is the causative agent for ringworm in cattle. Fungal isolates were cultured from 45 individual flies on supplemented Sabouraud dextrose agar, and isolates were identified using morphological and microscopic approaches. Each isolate was identified further by PCR amplification of the ribosomal DNA locus with fungal-specific primers and subsequent amplicon sequencing. Trichophyton verrucosum was not identified using these approaches. However, 35 different fungal species representing 17 genera were cultured from collected flies, including several species that are allergenic and pathogenic to humans and animals. Several species within the fungal orders Hypocreales, Microascales, Onygenales, Saccharomycetales, Xylaniales, and Agaricales were observed for the first time on house flies. The most frequent fungus recovered was Cladosporium cladosporoides Fresen, which is known to be a ubiquitous, airborne allergen to humans.
Keywords: Musca domestica, bovine ringworm, Cladosporium, dermatophyte
The house fly, Musca domestica L., is an important medical and veterinary insect pest, as it breeds in septic environments and occupies habitats that overlap with humans and animals (Malik et al. 2007). House flies can harbor pathogenic bacteria, excreting viable isolates in their vomitus and feces (Joyner et al. 2013, Nayduch et al. 2013), and can disseminate them mechanically to various hosts (Levine and Levine 1991, Ahmad et al. 2007, Wang et al. 2011). Additionally, house flies can disseminate common fungi implicated as incidental pathogens, including Aspergillus spp. and Penicillium spp. (Sales et al. 2002, Zarrin et al. 2007, Davari et al. 2012, Srivoramas et al. 2012, Phoku et al. 2014, Yousef 2014, Kassiri et al. 2015, Phoku et al. 2016).
Dermatophytic fungi belong to the family Arthrodermataceae and to principally two genera: Trichophyton and Microsporum, anamorphs of the genus Arthroderma (Graser et al. 1999). Microsporum equinum and Microsporum canis are the causative agents of ringworm in horses and dogs, respectively; Trichophyton verrucosum E. Bodin causes ringworm in cattle and occasionally in humans (English 1972), while other Trichophyton spp., e.g., T. rubrum Castell, T. tonsurans Malmsten, and T. interdigitale Priestley, cause various fungal diseases in humans. The highly contagious, infective propagules of bovine ringworm are arthroconidia that are typically spread by direct contact with infected animals or fomites such as fence posts or halters, as well as pastures, where the fungus can persist in the soil (Ajello 1974). Bovine ringworm manifests as round, hairless patches on the hide, and lesions may become purulent as a result of the animal scratching the infected area against fences or tree trunks.
House flies have been implicated as a mechanical vector of T. mentagrophytes, as they can transmit this rodent ringworm to guinea pigs (Koch and Rieth 1958). In addition, Koch noted the suspicious prevalence of house flies associated with a ringworm outbreak among penned cattle in Germany, although transmission was not demonstrated (Koch 1964).
In June 2014, a bovine ringworm outbreak occurred at the experimental facilities of the USDA-ARS Cattle Fever Tick Research laboratory located in Hidalgo County in south Texas. The outbreak coincided with a noticeable, dramatic increase in the house fly population attributed to increased suitable developmental habitat created by hay trampled into mud and feces by a small herd of penned cattle; one animal was noted as having ringworm on arrival in May. By June, over half of the animals in the small herd were infected, notably on the head around the eyes (Fig. 1). The fungus then spread to two of ten cattle in a nearby pasture ∼20 m away and did not involve direct or indirect (halters, stanchions) contact, which is the typical route of transmission. Cases of bovine keratoconjunctivitis occurred simultaneously. However, while face flies, Musca autumnalis (L.), are known to vector the causative bacterial agent, Moraxella bovis (Arends et al. 1984), that fly species does not occur in the area where this study was conducted. House flies and stable flies, Stomoxys calcitrans (L.), also have been implicated in its dissemination (Brown et al. 1998). Ringworm can appear on any part of the body, but the house flies observed on these animals preferred to aggregate on the face, likely attracted to lachrymal fluids. The coincidental occurrence of both ringworm and keratoconjunctivitis, and an increase in the house fly population, as well as the predominance of lesions and flies on the faces of the animals, led us to hypothesize that house flies could be mechanically vectoring the infectious stages of the T. verrucosum fungus within the local herd.
Fig. 1.

Angus calf infected with bovine ringworm at the study site (Edinburg, TX).
Materials and Methods
Insect Collection and Sample Preparation
Flies were collected on two separate occasions (05 June and 17 June 2014) from Angus cattle diagnosed with bovine ringworm. Cattle were stanchioned to ease fly collection using aerial nets to gather flies swarming around the faces of those infected. Flies were collected from one calf per collection date, with a total of two calves sampled. The flies that were collected were enumerated and individually stored (1 fly per 10 ml sterile vial) at −20 °C until processed. Two easily distinguished species were collected: house flies and horn flies, Haematobia irritans (L.), the latter of which were discarded. Although the face fly does not occur in south Texas, all specimens were examined under a dissecting microscope for diagnostic characteristics that differentiate M. autumnalis from M. domestica (Gojmerac 1977). Individual flies were macerated in a sterile glass borosilicate tissue homogenizer with 250 µl of 0.85% saline solution following Zarrin et al. (2007), and the homogenate was immediately plated. Flies were not surface-sterilized, thus the homogenate represented microorganisms present on the exoskeleton and in the gut.
Colony Culture Methods
Sabouraud dextrose agar (Beckton, Dickinson and Company, Sparks, MD) supplemented with chloramphenicol (50 µg/ml; Alfa Aesar, Haverhill, MA), cycloheximide (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO), thiamine (125 µg/ml; Sigma-Aldrich), and myo-inositol (0.5 mg/ml; Sigma-Aldrich) [SDAsupp] was used to selectively isolate and culture fungi from fly homogenates. The fly homogenate (50 µl) was spread on each of four SDAsupp plates using sterile, disposable Lazy-L-Spreaders (Genesee Scientific, San Diego, CA), and plates were incubated at 27 °C, for 7 d to promote fungal colony growth. Each individual colony on a plate was assigned a number and scored as a colony-forming unit (cfu) from the corresponding fly. A T. verrucosum isolate (Sigma-Aldrich) was prepared and plated as above, to confirm suitability of growth conditions.
Fungal Isolate Identification
Colony morphology, microscopic examination for representative fungal structures, and PCR amplification of fungal colony DNA were collectively used to identify each morphologically distinct fungal isolate. The T. verrucosum isolate described previously, was used as a positive control for all techniques. Fungal structures were observed by spreading a single isolate onto a microscope slide containing a drop of lactophenol cotton blue (Hardy Diagnostics, Santa Maria, CA). A coverslip was placed over the preparation and subsequently sealed with Permount (Fisher Scientific, Waltham, MA). Preparations were visualized at 400 and 1000× magnification using a compound microscope, and digital photomicrographs of the cellular tissues were taken. PCR amplification and sequence verification of the positive control were completed. Fungal genomic DNA was extracted from individual colonies, as in Murray et al. (2005). Approximately 20 mg of hyphae were disrupted with 0.5 mm glass beads in 100 mM sodium chloride, 10 mM Tris-HCl, 1 mM EDTA [STE] buffer (500 mg beads/ml STE). The suspension was mixed vigorously for 5 min using a Vortex Genie-2 with the TurboMix attachment (Scientific Industries, Ocala, FL), and cellular debris and glass beads were removed by centrifugation. The resulting supernatant (1 μl) was used as template for PCR amplification with universal primers designed to amplify regions at the rRNA locus, including the internal transcribed spacer (ITS)-1, 5.8S, ITS-2, and partial regions of 18S and 28S rRNA (White et al. 1990). The primer sequences used were ITS-1: 5′ –TCCGTAGGTGAACCTGCGG – 3′ and ITS-4: 5′ – TCCTCCGCTTATTGATATGC – 3′. Each reaction consisted of 20 mM Tris HCl, pH 8.4, 40 mM KCl, 1.75 mM MgCl2, 0.2 mM dNTP mix, 0.35 μM each of ITS-1 and ITS-4 primers, and 0.5 U Platinum Taq polymerase (Invitrogen, Carlsbad, CA). Products were amplified with an MJ Research PTC-200 thermocycler using the following cycling conditions: 94 °C, 3 min; 35 cycles of: 94 °C, 1 min, 60 °C, 1 min, and 72 °C, 1 min; and a final extension, 72 °C, 5 min. Amplicons were treated with ExoSAP-IT (exonuclease I and shrimp alkaline phosphatase; USB Corporation, Cleveland, OH) at 37 °C, 15 min, and the enzyme inactivated at 80 °C, 15 min. The treated sample subsequently was used in cycle sequencing with BigDye, version 3.1 chemistry (Life Technologies, Foster City, CA) and either the ITS-1 or the ITS-4 primer, and the reactions were analyzed on an ABI3130xl Genetic Analyzer (Life Technologies). Resulting forward and reverse sequence data were manually aligned, and these individual contigs were compared with publicly available databases using the blast-n algorithm at the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov), as well as MycoBank at the International Mycological Association Fungal Database (http://www.mycobank.org).
Results and Discussion
A combination of morphological evaluation, microscopic observations, and molecular techniques were utilized to identify fungal spores recovered from 45 house flies. Fungi were recovered from 36 of the 45 flies. Absence of culturable fungi from nine of the flies may be a result of fly age, with newly or recently emerged adults having limited exposure to the environment resulting in no fungal isolate recovery. Among those positive flies, most were coinfected with multiple fungi.
These isolates comprised 35 species in 17 fungal genera (Table 1). The majority, 26 fungal species, were recorded for the first time from house flies. A total of 2,217 cfus were cultured from collected flies. The fungi most frequently cultured were Cladosporium spp. (85%), Penicillium spp. (3.4%), and Aspergillus spp. (2.8%), genera which are typically saprophytic and commonly found in soil. A number of the species identified are known allergens, or are associated with animal and human mycoses (Table 1). In this study, the observed preference of house flies for the eye region of bovine hosts, along with the typical niches occupied by house flies, may have predisposed them to these fungal isolates. Indeed, Cladosporium spp., Penicillium spp., and Aspergillus spp. dominate the fungal communities isolated from conjunctival cultures of healthy animals, which appear to be due to environmental exposure (Samuelson et al. 1984, Sgorbini et al. 2010).
Table 1.
Ascomycete species recovered from 45 individual house flies collected from two bovine hosts with active ringworm infections
| Order | Fungi (previous record)a | No. of fliesb | CFUc | Freq. | Importanced |
|---|---|---|---|---|---|
| Agaricales | Asterophora parasitica Buillard | 1 | 1 | 0.05 | |
| Capnodiales | Cladosporium sp. (5, 7) | 10 | 69 | 3.11 | |
| Cladosporium cladosporoides Fresen (4) | 25 | 1681 | 75.82 | Airborne at Texas cattle feedlots (Wilson et al. 2002); Flies from horse stables in Germany (Gestmann et al. 2012) | |
| Cladosporium oxysporum Berkeley & Curtis | 3 | 53 | 2.39 | Human cutaneous phaeohyphomycosis infections (Gugnani et al. 2006) | |
| Cladosporium perangustum Bensch et al. | 4 | 47 | 2.12 | ||
| Cladosporium sphaerospermum Penzig | 1 | 15 | 0.68 | Human respiratory tract infections (Yew et al. 2016) | |
| Cladosporium tenuissimum Cooke | 2 | 38 | 1.71 | ||
| Eurotiales | Aspergillus sp. (1, 3, 7) | 2 | 37 | 1.67 | |
| Aspergillus deflectus Fennel & Raper | 1 | 2 | 0.09 | Canine mycosis (Robinson et al. 2000) | |
| Aspergillus ochraceopetaliformis Batista et al | 1 | 4 | 0.18 | Onychomycosis (Brasch et al. 2009) | |
| Aspergillus subramanianii Visagie et al | 1 | 1 | 0.05 | ||
| Aspergillus sydowii Bainier & Sartory | 1 | 12 | 0.54 | Onychomycosis (Nouripour-Sisakht et al. 2015) | |
| Aspergillus versicolor Vuillemin | 2 | 7 | 0.32 | Canine aspergillosis (Zhang et al. 2012); Allergen of swine production facilities (Sabino et al. 2012) | |
| Penicillium sp. (1, 2, 5, 6, 7) | 2 | 10 | 0.45 | ||
| Penicillium citrinum Thorn (3) | 4 | 1 | 0.05 | Allergen in cattle production facilities (Abd-Elall et al. 2009) | |
| Penicillium griseofulvum Diercykx | 2 | 65 | 2.93 | ||
| Hypocreales | Acremonium sp. | 1 | 1 | 0.05 | |
| Acremonium brachypenium Gams | 2 | 17 | 0.77 | Triatomine-associated (Moraes et al. 2001) | |
| Acremonium persicinum (Gams) | 1 | 2 | 0.09 | Opportunistic human pathogen, lung (Perdomo et al. 2011) | |
| Acremonium potronii Vuillemin | 1 | 2 | 0.09 | Human keratitis (Forster et al. 1975) | |
| Nectria mauritiicola Hennings | 1 | 2 | 0.09 | ||
| Stillbella fimetaria Persoon | 1 | 1 | 0.05 | Colonizes dung of herbivores (Lehr et al. 2006) | |
| Microascales | Scopulariopsis chartarum Smith | 1 | 1 | 0.05 | |
| Onygenales | Gymnascella aurantiaca Peck | 2 | 18 | 0.81 | |
| Pleosporales | Alternaria sp. (1, 2, 6) | 2 | 4 | 0.18 | |
| Alternaria alternata Fries (4) | 1 | 1 | 0.05 | ||
| Epicoccum nigrum Link (3) | 1 | 58 | 2.62 | Fungal sinusitis in the southeastern US (Noble et al. 1997) | |
| Leptosphaerulina sp. | 1 | 1 | 0.05 | ||
| Phaeosphaeria sp. | 1 | 1 | 0.05 | ||
| Saccharomycetales | Candida panamensis Suh et al. | 1 | 1 | 0.05 | Beetle-associated (Suh et al. 2006) |
| Kluyveromyces marxianus Hansen | 3 | 21 | 0.95 | Cow and goat milk (Delavenne et al. 2011) | |
| Pichia jadinii Sartory et al. | 1 | 28 | 1.26 | ||
| Pichia kudriavzevii Boidin et al. | 1 | 1 | 0.05 | Bovine mastitis, Dairy cattle rumenal fluid (Hayashi et al. 2013, Sirisan et al. 2013) | |
| Xylaniales | Hansfordia sinuosae Li & Cheng | 1 | 14 | 0.63 |
Fungi previously reported in the literature are noted. 1Davari et al. (2012), 2Kassiri et al. (2015), 3Phoku et al. (2016), 4Sales et al. (2002), 5Srivoramas et al. (2012), 6Yousef (2014), 7Zarrin et al. (2007).
Total number of flies from which particular fungal isolates were recovered.
Number of colony-forming units (CFU).
References to importance of fungal isolates as disease agents or allergens.
Cladosporium cladosporoides Fresen (Fig. 2A), representing 76% of the fungal isolates in this study, is a widely distributed species within one of three species complexes of the genus Cladosporium (Bensch et al. 2015). Cladosporium cladosporoides was previously isolated from M. domestica collected at a city dump in Brazil (Sales et al. 2002) and from flies in horse stables in Germany (Gestmann et al. 2012). The fungus produces emodin (2-methyl-4,5,7-trihydroxyanthraquinone), a diarrheagenic mycotoxin (Ogórek et al. 2012), and is a common allergen. Cladosporium spores are ubiquitous in air samples collected both indoors and outdoors (Khan and Wilson 2003), including those associated with Texas cattle feedlots (Wilson et al. 2002). Three additional species within the C. cladosporoides complex were recovered including C. oxysporum (2.4%) that can cause human cutaneous phaeohyphomycosis infections (Gugnani et al. 2006), C. perangustum (2.1%), and C. tenuisimum (1.7%) that is typically isolated from plant substrates but was reported from human clinical samples, i.e., the respiratory tract (Bensch et al. 2015, Sandoval-Denis et al. 2015). Cladosporiumsphaerospermum (0.7%), a representative from a second Cladosporium species complex, was isolated from a single fly. It is associated with allergic diseases of the upper respiratory tract in humans (Yew et al. 2016).
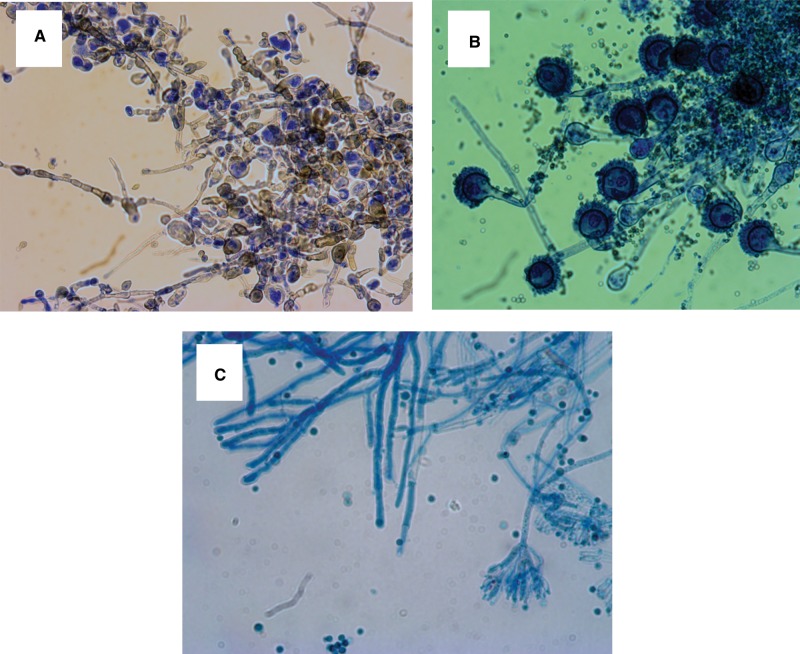
Fig. 2.
Representative images of fungal isolates cultured from house flies at the study site. Specimens were visualized using a compound microscope. (A) Cladosporium cladosporoides Fresen hyphae and conidia stained with lactophenol blue, 400× magnification. (B) Aspergillus versicolor Vuillemin hyphae and conidia stained with lactophenol blue, 400× magnification. (C) Penicillium griseofulvin Diercykx hyphae, conidia and conidiophores, 1,000× magnification.
Aspergillus spp. were recovered in the current study (63 cfus), more than half of which were only identifiable to the genus level based on sequence similarity searches. While Aspergillus spp. are commonly found on M. domestica (Zarrin et al. 2007, Davari et al. 2012, Phoku et al. 2016), five species isolated here represent the first records: A. deflectus Fennel & Raper, a dermatophyte in dogs that causes otitis externa and paronchyia (Robinson et al. 2000); A. ochraceopetaliformis Batista & Maia and A. sydowii Bainier & Sartory, causative agents of onychomycosis (Brasch et al. 2009, Nouripour-Sisakht et al. 2015); and A. versicolor Vuillemin (Fig. 2B), a prevalent allergen in swine production facilities, that can produce mycotoxins in damp, indoor environments (Engelhart et al. 2002, Sabino et al. 2012).
Thirteen additional fungal isolates were identified from single fly specimens and are the first records from house flies (Table 1), providing an interesting prospective use of these insects as environmental indicators of fungal flora in a cattle setting. For example, Pichia jadinii Sartory was recovered possibly due to its use as an additive in livestock feed (Ignatova et al. 2002), while Stillbella fimetaria Lindau is a coprophilic fungus likely encountered in cattle dung (Lehr et al. 2006).
Trichophyton verrucosum was not recovered from the house fly specimens collected during the course of this study.Richard (1963) was unable to demonstrate T. equinum transmission by stable flies, and Pascoe and Connole (1974) noted a M. gypseum outbreak on horses that was coincident with an increase in stable fly populations. However, they, were not able to directly attribute a role for flies in fungal dissemination. Previous efforts to isolate pathogenic dermatophytes from flies collected at both urban and rural settings resulted in identification of T. mentagrophytes and T. terrestre from house flies (Gip and Svensson 1968, Pinetti et al. 1974), but attempts to isolate T. verrucosum on flies from ringworm-infected hosts have been unsuccessful (Koch 1964). Results from these and the current study suggest that T. verrucosum arthroconidia, the asexual spores that are the infective elements in a skin infection (Markey et al. 2013), are not acquired and disseminated by house flies. Gymnothecial ascospores produced by some pathogenic fungi cling to arthropods due to the interwoven nature of the spore perideal wall, while arthroconidia have a different structure and may adhere to arthropods via electrostatic interactions that are likely transient and weaker (Greif and Currah 2007). While adherence of the arthroconidia to human skin is strongly time dependent (Zurita and Hay 1987), it is unclear whether this is the same for cuticular surfaces. Arthroconidia are most prevalent on the infected hairs of cattle hosts (Ajello 1974), and it is probable that the host is most contagious prior to hair loss. By the time our samples were taken, the denuded lesions were obvious because of hair loss. It may be that our fly collections, although sampled from cattle with ringworm lesions, were less than optimal in terms of contagion. Indeed, canine ringworm (M. canis) was detected on the surface of experimentally inoculated house flies up to 5 d after introduction (Cafarchia et al. 2009), indicating that collections at timepoints after the peak of the inoculum may be suboptimal for culturing the fungus.
An intriguing alternative reason for our inability to recover T. verrucosum could be a result of competitive interference among fungi in our laboratory cultures (Shearer 1995). Penicillium spp. (Fig. 2C) represented 3.4% of cfus recovered in the current study, and they produce the antifungal compound, griseofulvin, which has been used to treat dermatophytoses caused by Microsporum spp. and Trichophyton spp. (El Nakeeb et al. 1965). For example, a macroconidia of Trichophyton was observed and photographed (Fig. 3), but it did not grow and develop into a colony and therefore could not be sequenced. Based on microscopy, this macroconidia appeared to be the common Trichophyton ajelloi, a geophilic fungus. In this instance, chemical antibiosis in our laboratory cultures may have impacted recovery. Further, bacterial–fungal community interactions may impact prevalence and recovery of fungal isolates. It is unclear how bacterial diversity may influence Trichophyton spp., in particular, and evaluating microbiomes of the whole fly and the bovine host skin is desirable to assess this relationship.
Fig. 3.

Macroconidia of Trichopyton ajelloi stained with lactophenol blue and visualized using a compound microscope, 1,000× magnification.
Whether or not house flies are vectors of dermatophytic fungi, our results show that they are carriers of a diverse variety of spores some of which are potential pathogens depending on the mode of infection. The most abundant fungal species cultured from these flies are known to be ubiquitous in distribution, or their presence was consistent with a rural or pastoral setting. Thus, we expect that surveys of fungal spores on houseflies in different environmental contexts would find species not reported here. Although our study failed to implicate house flies as vectors of ringworm conidia, given the circumstances, we do not consider our result to be definitive on the matter.
Acknowledgments
We would like to express gratitude to the following individuals that assisted with elements of the project: Greta Buckmeier for DNA sequencing, Jason Tidwell for technical support with PCR, Michael Moses for administrative assistance, Joni Ortiz for assistance with specimen preparation, Dr. Daniel Murray for intellectual support and review of the manuscript., Dr. Shelly Mitchell D.V.M. for diagnosing ringworm. The senior author extends a special thanks to her Masters thesis committee members Chris Zito, Charles Morgan, Irene Reed, and Kristin Martin from the University of Saint Joseph for their support during the project. This research was funded by Texas A&M Agrilife and USDA-ARS, National Program 104, Project #3094-32000-038-00D.
References Cited
- Abd-Elall A. M., Mohamed M. E., Awadallah M. A.. 2009. Potential airborne microbial hazards for workers on dairy and beef cattle farms in Egypt. Vet Ital. 45: 275–285. [PubMed] [Google Scholar]
- Ahmad A., Nagaraja T. G., Zurek L.. 2007. Transmission of Escherichia coli O157:H7 to cattle by house flies. Prev. Vet. Med. 80: 74–81. [DOI] [PubMed] [Google Scholar]
- Ajello L. 1974. Natural history of the dermatophytes and related fungi. Mycopathol. Mycol. Appl. 53: 93–110. [DOI] [PubMed] [Google Scholar]
- Arends J. J., Wright R. E., Barto P. B., Lusby K. S.. 1984. Transmission of Moraxella bovis from blood agar cultures to Hereford cattle by face flies (Diptera: Muscidae). J. Econ. Entomol. 77: 394–398. [DOI] [PubMed] [Google Scholar]
- Bensch K., Groenewald J. Z., Braun U., Dijksterhuis J., de Jesus Yanez-Morales M., Crous P. W.. 2015. Common but different: The expanding realm of Cladosporium. Stud. Mycol. 82: 23–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch J., Varga J., Jensen J. M., Egberts F., Tintelnot K.. 2009. Nail infection by Aspergillus ochraceopetaliformis. Med. Mycol. 47: 658–662. [DOI] [PubMed] [Google Scholar]
- Brown M. H., Brightman A. H., Fenwick B. W., Rider M. A.. 1998. Infectious bovine keratoconjunctivitis: A review. J. Vet. Intern. Med. 12: 259–266. [DOI] [PubMed] [Google Scholar]
- Cafarchia C., Lia R. P., Romito D., Otranto D.. 2009. Competence of the housefly, Musca domestica, as a vector of Microsporum canis under experimental conditions. Med. Vet. Entomol. 23: 21–25. [DOI] [PubMed] [Google Scholar]
- Davari B., Khodavaisy S., Ala F.. 2012. Isolation of fungi from housefly (Musca domestica L.) at Slaughter House and Hospital in Sanandaj, Iran. J. Prev. Med. Hyg. 53: 172–174. [PubMed] [Google Scholar]
- Delavenne E., Mounier J., Asmani K., Jany J. L., Barbier G., Le Blay G.. 2011. Fungal diversity in cow, goat and ewe milk. Int. J. Food Microbiol. 151: 247–251. [DOI] [PubMed] [Google Scholar]
- El Nakeeb M. A., McLellan W. L. Jr., Lampen J. O.. 1965. Antibiotic action of griseofulvin on dermatophytes. J. Bacteriol. 89: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart S., Loock A., Skutlarek D., Sagunski H., Lommel A., Farber H., Exner M.. 2002. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 68: 3886–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English M. P. 1972. The epidemiology of animal ringworm in man. Br. J. Dermatol. 86: 78–87. [Google Scholar]
- Forster R. K., Rebell G., Stiles W.. 1975. Recurrent keratitis due to Acremonium potronii. Am. J. Ophthalmol. 79: 126–128. [DOI] [PubMed] [Google Scholar]
- Gestmann F., Förster M., Mehlhorn H., Sievert K., Messler S., Neuhausen N., Petersdorf S., Pfeffer K.. 2012. Flies as vectors of microorganisms potentially inducing severe diseases in humans and animals, pp. 195–226. InMehlhorn H. (ed.), Arthropods as vectors of emerging diseases. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- Gip L., Svensson S. A.. 1968. Can flies cause the spread of dermatophytosis? Acta Dermato Venereol. 48: 26–29. [PubMed] [Google Scholar]
- Gojmerac W. L. 1977. Identifying and controlling flies on dairy, beef, other livestock, and pets. University of Wisconsin Cooperative Extensive Publication A2118.
- Graser Y., El Fari M., Vilgalys R., Kuijpers A. F., De Hoog G. S., Presber W., Tietz H.. 1999. Phylogeny and taxonomy of the family Arthrodermataceae (dermatophytes) using sequence analysis of the ribosomal ITS region. Med. Mycol. 37: 105–114. [PubMed] [Google Scholar]
- Greif M. D., Currah R. S.. 2007. Patterns in the occurrence of saprophytic fungi carried by arthropods caught in traps baited with rotted wood and dung. Mycologia 99: 7–19. [DOI] [PubMed] [Google Scholar]
- Gugnani H. C., Ramesh V., Sood N., Guarro J., Moin Ul H., Paliwal-Joshi A., Singh B.. 2006. Cutaneous phaeohyphomycosis caused by Cladosporium oxysporum and its treatment with potassium iodide. Med. Mycol. 44: 285–288. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Sugita T., Hata E., Katsuda K., Zhang E., Kiku Y., Sugawara K., Ozawa T., Matsubara T., Ando T., et al. 2013. Molecular-based identification of yeasts isolated from bovine clinical mastitis in Japan. J. Vet. Med. Sci. 75: 387–390. [DOI] [PubMed] [Google Scholar]
- Ignatova E. A., Nagornaia S. S., Sudenko V. I., Podgorskii V. S.. 2002. [On the identity of Candida utilis and Pichia jadinii yeast species]. Mikrobiol. Z. 64: 20–26. [PubMed] [Google Scholar]
- Joyner C., Mills M. K., Nayduch D.. 2013. Pseudomonas aeruginosa in Musca domestica L.: temporospatial examination of bacteria population dynamics and house fly antimicrobial responses. PLoS ONE 8: e79224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassiri H., Zarrin M., Veys-Behbahani R., Faramarzi S., Kasiri A.. 2015. Isolation and identification of pathogenic filamentous fungi and yeasts from adult house fly (Diptera: Muscidae) captured from the hospital environments in Ahvaz City, southwestern Iran. J. Med. Entomol. 52: 1351–1356. [DOI] [PubMed] [Google Scholar]
- Khan N. N., Wilson B. L.. 2003. An environmental assessment of mold concentrations and potential mycotoxin exposures in the greater Southeast Texas area. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 38: 2759–2772. [DOI] [PubMed] [Google Scholar]
- Koch H., Rieth H.. 1958. Endemische trichophytie bei meerschweinchen. Arch. Klin Exp. Dermatol. 205: 577–585. [PubMed] [Google Scholar]
- Koch H. A. 1964. Fliegen als überträger von dermatophyten. Hautarzt 15: 365–366. [PubMed] [Google Scholar]
- Lehr N. A., Meffert A., Antelo L., Sterner O., Anke H., Weber R. W.. 2006. Antiamoebins, myrocin B and the basis of antifungal antibiosis in the coprophilous fungus Stilbella erythrocephala (syn. S. fimetaria). FEMS Microbiol. Ecol. 55: 105–112. [DOI] [PubMed] [Google Scholar]
- Levine O. S., Levine M. M.. 1991. Houseflies (Musca domestica) as mechanical vectors of shigellosis. Rev. Infect. Dis. 13: 688–696. [DOI] [PubMed] [Google Scholar]
- Malik A., Singh N., Satya S.. 2007. House fly (Musca domestica): A review of control strategies for a challenging pest. J. Environ. Sci. Health B. 42: 453–469. [DOI] [PubMed] [Google Scholar]
- Markey B., Leonard F., Archambault M., Cullinare A., Maguire D.. 2013. Clinical veterinary microbiology, 2nd edn.Mosby-Elsevier. [Google Scholar]
- Moraes A. M., Junqueira A. C., Costa G. L., Celano V., Oliveira P. C., Coura J. R.. 2001. Fungal flora of the digestive tract of 5 species of triatomines vectors of Trypanosoma cruzi, Chagas 1909. Mycopathologia 151: 41–48. [DOI] [PubMed] [Google Scholar]
- Murray K. D., Aronstein K. A., Jones W. A.. 2005. A molecular diagnostic method for selected Ascosphaera species using PCR amplification of internal transcribed spacer regions of rDNA. J. Apic. Res. 44: 61–64. [Google Scholar]
- Nayduch D., Cho H., Joyner C.. 2013. Staphylococcus aureus in the house fly: Temporospatial fate of bacteria and expression of the antimicrobial peptide defensin. J. Med. Entomol. 50: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J. A., Crow S. A., Ahearn D. G., Kuhn F. A.. 1997. Allergic fungal sinusitis in the southeastern USA: Involvement of a new agent Epicoccum nigrum Ehrenb. ex Schlecht. 1824. J. Med. Vet. Mycol. 35: 405–409. [PubMed] [Google Scholar]
- Nouripour-Sisakht S., Mirhendi H., Shidfar M. R., Ahmadi B., Rezaei-Matehkolaei A., Geramishoar M., Zarei F., Jalalizand N.. 2015. Aspergillus species as emerging causative agents of onychomycosis. J. Mycol. Med. 25: 101–107. [DOI] [PubMed] [Google Scholar]
- Ogórek R., Lejman A., Pusz W., Miłuch A., Miodyńska P.. 2012. Characteristics and taxonomy of Cladosporium fungi. Mikol Lek. 19: 80–85. [Google Scholar]
- Pascoe R. R., Connole M. D.. 1974. Dermatomycosis due to Microsporum gypseum in horses. Aust. Vet. J. 50: 380–383. [DOI] [PubMed] [Google Scholar]
- Perdomo H., Sutton D. A., Garcia D., Fothergill A. W., Cano J., Gene J., Summerbell R. C., Rinaldi M. G., Guarro J.. 2011. Spectrum of clinically relevant Acremonium species in the United States. J. Clin. Microbiol. 49: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoku J. Z., Barnard T. G., Potgieter N., Dutton M. F.. 2014. Fungi in housefly (Musca domestica L.) as a disease risk indicator-A case study in South Africa. Acta Trop. 140: 158–165. [DOI] [PubMed] [Google Scholar]
- Phoku J. Z., Barnard T. G., Potgieter N., Dutton M. F.. 2016. Fungal dissemination by housefly (Musca domestica L.) and contamination of food commodities in rural areas of South Africa. Int. J. Food Microbiol. 217: 177–181. [DOI] [PubMed] [Google Scholar]
- Pinetti P., Lostia A., Tarantino F.. 1974. The role played by flies in the transmission of the human and animal dermatophytic infection. Mycopathol. Mycol. Appl. 54: 131–134. [DOI] [PubMed] [Google Scholar]
- Richard J. L. 1963. Studies on the possible transmission of Trichophyton equinum by the common stable fly. Proc. Iowa Acad. Sci. 70: 114–120. [Google Scholar]
- Robinson W. F., Connole M. D., King T. J., Pitt J. I., Moss S. M.. 2000. Systemic mycosis due to Aspergillus deflectus in a dog. Aust. Vet. J. 78: 600–602. [DOI] [PubMed] [Google Scholar]
- Sabino R., Faisca V. M., Carolino E., Verissimo C., Viegas C.. 2012. Occupational exposure to Aspergillus by swine and poultry farm workers in Portugal. J. Toxicol. Environ. Health a. 75: 1381–1391. [DOI] [PubMed] [Google Scholar]
- Sales M.S.N., Costa G. L., Bittencourt V. R.. 2002. Isolation of fungi in Musca domestica Linnaeus, 1758 (Diptera: Muscidae) captured at two natural breeding grounds in the municipality of Seropédica, Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 97: 1107–1110. [DOI] [PubMed] [Google Scholar]
- Samuelson D. A., Andresen T. L., Gwin R. M.. 1984. Conjunctival fungal flora in horses, cattle, dogs, and cats. J. Am. Vet. Med. Assoc. 184: 1240–1242. [PubMed] [Google Scholar]
- Sandoval-Denis M., Sutton D. A., Martin-Vicente A., Cano-Lira J. F., Wiederhold N., Guarro J., Gené J.. 2015. Cladosporium species recovered from clinical samples in the United States. J. Clin. Microbiol. 53: 2990–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgorbini M., Barsotti G., Nardoni S., Brombin M., Sbrana A., Mancianti F., Corazza M.. 2010. Seasonal prevalence of fungi in the conjunctival fornix of healthy cows during a 2-year study. Vet. Ophthalmol. 13: 227–234. [DOI] [PubMed] [Google Scholar]
- Shearer C. A. 1995. Fungal competition. Can. J. Bot. 73: 1259–1264. [Google Scholar]
- Sirisan V., Pattarajinda V., Vichitphan K., Leesing R.. 2013. Isolation, identification and growth determination of lactic acid-utilizing yeasts from the ruminal fluid of dairy cattle. Lett. Appl. Microbiol. 57: 102–107. [DOI] [PubMed] [Google Scholar]
- Srivoramas T., Chaiwong T., Sanford M. R.. 2012. Isolation of fungi from adult house fly, Musca domestica, and the blow fly, Chrysomya megacephala, in Ubon Ratchathani province, Northeastern Thailand. Int. J. Parasitol. Res. 4: 53–56. [Google Scholar]
- Suh S. O., Nguyen N. H., Blackwell M.. 2006. A yeast clade near Candida kruisii uncovered: Nine novel Candida species associated with basidioma-feeding beetles. Mycol. Res. 110: 1379–1394. [DOI] [PubMed] [Google Scholar]
- Wang Y. C., Chang Y. C., Chuang H. L., Chiu C. C., Yeh K. S., Chang C. C., Hsuan S. L., Lin W. H., Chen T. H.. 2011. Transmission of Salmonella between swine farms by the housefly (Musca domestica). J. Food Prot. 74: 1012–1016. [DOI] [PubMed] [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J.. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322. InInnis M., Gelfand D. H., Shinsky J. J., White T. J. (eds.), PCR Protocols: A guide to methods and applications. Academic Press, Inc, London [Google Scholar]
- Wilson S. C., Morrow-Tesch J., Straus D. C., Cooley J. D., Wong W. C., Mitlohner F. M., McGlone J. J.. 2002. Airborne microbial flora in a cattle feedlot. Appl. Environ. Microbiol. 68: 3238–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew S. M., Chan C. L., Ngeow Y. F., Toh Y. F., Na S. L., Lee K. W., Hoh C. C., Yee W. Y., Ng K. P., Kuan C. S.. 2016. Insight into different environmental niches adaptation and allergenicity from the Cladosporium sphaerospermum genome, a common human allergy-eliciting Dothideomycetes. Sci. Rep. 6: 27008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef A. F. 2014. Isolation of fungi from house fly (Musca domestica) at slaughter house in public places in Riyadh. Egypt Acad. J. Biol. Sci. 7: 151–155. [Google Scholar]
- Zarrin M., Vazirianzadeh B., Solary S. S., Mahmoudabadi A. Z., Rahdar M.. 2007. Isolation of fungi from house fly (Musca domestica) in Ahwaz, Iran. Pak. J. Med. Sci. 23: 917–919. [Google Scholar]
- Zhang S., Corapi W., Quist E., Griffin S., Zhang M.. 2012. Aspergillus versicolor, a new causative agent of canine disseminated aspergillosis. J. Clin. Microbiol. 50: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita J., Hay R. J.. 1987. Adherence of dermatophyte microconidia and arthroconidia to human keratinocytes in vitro. J. Invest Dermatol. 89: 529–534. [DOI] [PubMed] [Google Scholar]