Abstract
Cardiovascular disease accounts for almost half of all deaths worldwide, and has now surpassed infectious disease as the leading cause of death and disability in developing countries. At present, therapies such as LDL-lowering statins and anti-hypertensive drugs have begun to bend the morality curve for coronary artery disease (CAD); yet, as we come to appreciate the more complex pathophysiological processes in the vessel wall, there is an opportunity to fine-tune therapies to more directly target mechanisms that drive CAD. MicroRNAs have been identified that control vascular cell homeostasis,1–3 lipoprotein metabolism4,5–9 and inflammatory cell function.10 Despite the importance of these miRNAs in driving atherosclerosis and vascular dysfunction, therapeutic modulation of miRNAs in a cell- and context-specific manner has been a challenge. In this review, we will summarize the emergence of microRNA-based therapies as an approach to treat CAD by specifically targeting the pathways leading to the disease. We will focus on the latest development of nanoparticles as a means to specifically target the vessel wall, and what the future of these nanomedicines may hold for the treatment of CAD.
MicroRNAs and CAD
MicroRNAs (miRNAs) belong to a class of non-coding RNA that is highly conserved across species. miRNAs are short and single stranded, between 20–22 nucleotides (nt) in their mature form, and form duplexes with complementary sequences in the 3′ untranslated region (UTR) of mRNAs. The specificity of the miRNA:mRNA interaction is derived from the seed region of the miRNA, a 5–7nt region that is either partially or totally complementary to its target mRNA sequence. Target gene repression is achieved through either mRNA degradation or translational inhibition, the former occurring only when the miRNA:mRNA duplex is perfectly complementary. Despite being only 5–7nt in length, miRNA seed sequences result in remarkable specificity, and even a single nucleotide base change can completely abolish a miRNA:mRNA interaction. Conversely, multiple genes within a given pathway can contain targeting sequences for the same miRNA, allowing a single miRNA to repress an entire gene set simultaneously. This coordinate genetic regulation makes miRNAs uniquely powerful as functional modulators that can be targeted therapeutically.
During atherogenesis, excess circulating lipids activate endothelial cells (EC) to produce pro-inflammatory factors that promote the recruitment of monocytes into the subendothelial space. As monocyte-derived macrophages engulf accumulated lipids, transforming into inflammatory foam cells, they secrete factors that activate smooth muscle cells (SMCs), induce further monocyte recruitment, and promote macrophage proliferation. At each of these stages, miRNAs have been shown to both promote and protect from atherogenesis (Figure 1). The details outlining the mechanisms of miRNA function in CAD have been reviewed extensively elsewhere, therefore we will instead highlight miRNAs whose therapeutic targeting in the vessel wall shows promise but may benefit from nanoparticle-based targeted delivery to avoid the accompanying risks of systemic or non-vascular manipulation.
Figure 1.
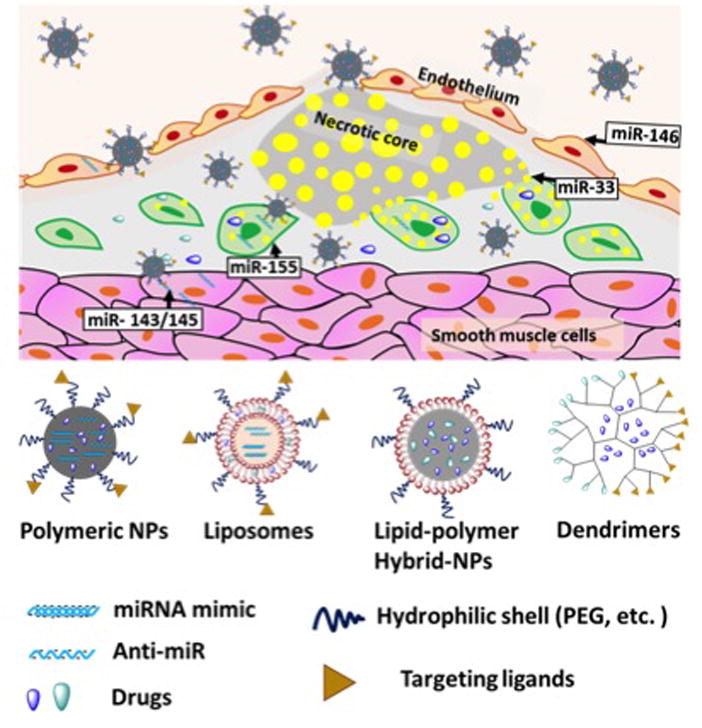
MicroRNAs in the atherosclerotic plaque that would benefit from nanoparticle (NP) delivery of RNA therapeutics (top). Schematic representation of different NP platforms (bottom).
miR-33
Discovered simultaneously by a number of groups, miR-33 was identified in a feedback loop with its host gene SREBP-2 to attenuate cholesterol efflux from cells during times of sterol depletion.5, 7, 11 Since its initial characterization as an ABCA1-targeting miRNA, multiple other lipid and energy metabolism genes have been identified as miR-33 targets.12–14 Blocking miR-33 using either genetic or anti-sense approaches reduces lesion burden in mouse models of progressing and regressing atherosclerosis.13, 15–18 Some of these benefits may be derived from the high-density lipoprotein (HDL)-raising effects of miR-33 antagonism, but in many cases miR-33 inhibition protected from atherosclerosis in the absence of increases in HDL. However, longer-term studies in mice of miR-33 blockade raised concerns over the development of fatty liver and/or elevated circulating triglycerides,19–21 though this did not occur in all mouse studies nor was it observed in non-human primates.22, 23 Although this may be related to variations in biodistribution of different chemically modified anti-sense oligonucleotides (ASO), as well as differences in total versus partial knockdown, there is nonetheless concern that what may be good for the atherosclerotic plaque may not be good for the liver.
miR-21
miR-21 was identified as being elevated in a number of vascular diseases, suggesting that its modulation may offer some protection against disease.24 In SMCs, miR-21 serves to block the expression of its target genes PTEN, SPRY1, PDCD4 and TPM1 and promotes SMC proliferation.25, 26 As such, blocking miR-21 using ASOs or by genetic deletion reduces SMC proliferation and neointimal formation in injured vessels,27, 28 and local delivery of anti-miR21 via coated stents prevents neointimal hyperplasia.2 miR-21 is also a potent regulator of cell death in other cell types and tissues, and antagonism of miR-21 induces apoptosis in renal cell carcinoma cells.29 This may pose some risk for renal injury if miR-21 is inhibited systemically. Indeed, Wang et al noted that while systemic anti-miR21 reduced neoinitmal formation in stented arteries, it also caused significant kidney damage.2 This provides an example where tissue-specific delivery of miR-21 inhibitors could offer improved therapeutic profile than systemic delivery.
miR-143/miR-145
Early studies of miRNA function in atherosclerosis identified the miRNAs miR-143 and miR-145 as critical regulators of SMC function.30 Transcribed as a cluster, miR-143/145 are master regulators of SMC differentiation via targeting pluripotency factors KLF4, myocardin and ELK-1.31 In the vessel wall, release of miR-143/145 from mouse and human vascular ECs into the extracellular space enables the transfer of miR143/145 to SMCs, where they induce the repression of ELK1 and KLF4 and protect from atherosclerosis.32 However, other studies in mice show that the absence of miR-143/145 reduces the development of atherosclerosis likely via de-repression of macrophage ABCA1 and enhanced cholesterol efflux.33 These studies highlight the cell-specific nature of miR-143/145 function and its potential opposing roles. miR-143/145 are also potent tumor suppressors, and play a significant role in the development of carcinomas potentially via activation of the RAS pathway.34 As such, therapies aimed at delivering and/or inhibiting miR143/145 in the vessel wall must take into account its diverse and potentially beneficial roles in other tissues.
Inflammatory miRNAs
A number of miRNAs have been identified that regulate the immune response in the vessel wall during inflammation and/or atherosclerosis.35 Thus far, the most well characterized are miR-155, miR-146 and miR-33. miR-155 has been described as a multifaceted immune regulatory miRNA that functions in monocytes, T cells and B cells. In macrophages, miR-155 promotes inflammation, and inhibition leads to improved cardiac responses to hypertrophy.36 However, the role of miR-155 in atherosclerosis is stage dependent,37 promoting atherosclerosis at early stages10 but protecting from it at later stages.38 Indeed, a recent study demonstrated the opposing roles for miR-155 in ECs vs monocytes, and suggest that the balance of expression of this miRNA has the potential to dramatically alter the inflammatory response in the artery wall.39, 40 Similarly, miR-146a is a potent anti-inflammatory miRNA that serves to limit NFkB activation in a feedback loop, and in the atherosclerotic plaque, delivery of miR-146a mimics limits the development of lesions in mice.41 However, as miR-146a acts to limit inflammation in response to bacterial infection and other stimuli, the utility of miR-146a replacement therapy for a chronic disease like atherosclerosis would be limited.
These miRNAs provide examples of attractive therapeutic targets to treat CAD via over-expression or inhibition strategies, yet are hampered by their cell-, tissue- and context-specific roles. Below we will describe some of the challenges associated with miRNA-targeting therapies, and provide some insight into how nanoparticles (NP) are emerging as a critical tool to increase the therapeutic promise of these potent modulators of CAD.
The challenges of miRNA-targeting therapies
Although both miRNA mimics and anti-miRs have great therapeutic potential, each of these miRNA modulators have unique challenges that must be addressed for successful development of miRNA therapies. In order to be recognized correctly in the cytosol by the RNA-induced silencing complex (RISC), the cellular machinery responsible for the miRNA- and siRNA-mediated gene repression, synthetic miRNA mimics must be double-stranded. This results in the potential of immune reactivity in response to dsRNA, which triggers an anti-viral response. Moreover, miRNA mimics have very short half-life in the circulation, and any chemical modification to improve their stability might interfere with their processing into RISC and decreased effector function. On the other hand, microRNA inhibitors (anti-miRs) need only bind to a single-stranded mature miRNA to block their function, and thus generally do not activate the immune system. As a result, anti-miRs can be chemically modified to improve their stability and affinity for the targeted miRNA. These chemical modifications are focused on the sugar, the base or the linkage between nucleotides of miRNA. Since the nuclease action involves 2′-OH in the ribose ring, substitution of this 2′–OH group with 2′-O-methyl, 2′-O-methoxyethyl or 2′-fluoro groups has been explored to increase the stability.42 Other modifications include changing phosphodiester linkage to phosphorothioate bonds, by replacing non-bridging oxygen atom with a sulfur atom, and locking the ribose rings via methylene bridges connecting the 2′-O and the 4′-C atoms, constraining the ribose to C3′-endo conformation (locked nucleic acid, LNA).43 Currently, LNA based miRNA modulators show promising results in different tumor mouse models.44 While these modifications improve resistance to nuclease degradation, they also increased possible cytotoxicity and side effects due to the sustained retention of anti-miRs in the body. Despite the advantages of chemical modifications of miRNA modulators, cellular uptake, biodistribution, off-target effects, and toxicity still remain major limitations for miRNA therapies. Due to the phosphate groups on the back bone, both miRNA mimics and anti-miRs are negatively charged, making it difficult for them to leave the circulation and enter the cells.45, 46 To circumvent these challenges, both viral and non-viral nucleotide delivery platforms have been explored as a delivery vehicles for miRNA therapeutics. However, these approaches have their limitations and hence non-viral delivery strategies have been highly sought-after. Nevertheless, choosing the appropriate delivery platform to improve the stability in the circulation, augment the spatio-temporal delivery of the miRNAs, and successful endosomal escape appears to be most important factors influencing miRNA therapeutics.
Can nanoparticles offer a solution for therapeutic miRNA modulation?
NP-based therapeutic platforms: composition, synthesis and biophysicochemical properties
To date, numerous engineered NP platforms have been developed for wide variety of disease applications and the following have made a significant advances towards the clinic: liposomes, polymeric NPs, dendrimers, metal-based, and carbon-based materials (Figure 1). Liposomes are vesicle-like structures consisting of a single or multiple bilayered membranes composed of natural or synthetic lipids. They were one of the first nanostructured platforms discovered to have potential as drug delivery vehicles.47 Typically, liposomes are spherical in shape comprised of an aqueous core and a lipid bilayer shell, and their size can vary from 20 nm to several micrometers.48 Liposomes are extensively used in a variety of biomedical and pharmaceutical applications mainly due to their biocompatibility, biodegradability and their ability to encapsulate both hydrophobic and hydrophilic payloads.48 In order to improve the half-life in the circulation and decrease phagocytic clearance, polyethylene glycol (PEG) was introduced on the surface of liposomes by using PEG-modified lipids as one of the starting components. Additionally, the surface of liposomes can be decorated with targeted ligands for site-specific drug delivery. Doxil was the first FDA-approved liposomal formulation entered into clinic for the treatment of AIDS related Kaposi’s syndrome over 20 years ago.49
Polymeric NPs along with liposomes are widely used in nanomedicinal research and consist of biodegradable, biocompatible polymeric materials that are either hydrophobic, hydrophilic or amphiphilic in nature. Most of the polymeric NPs reported in the literature are based on polylactide, polyglycolide, polycaprolactone, and polylactide-co-glycolide. Polymeric NPs are very versatile in nature with tunable sizes, shapes and drug release properties and as such, are used as drug delivery vehicles for wide range of therapeutics50 such as hydrophobic and hydrophilic small molecules, proteins, nucleotides, and peptides, and comprise the majority of nanomedicines currently in clinical trials.51, 52 Polymeric NP-based nanomedicines are being tested for use in cancer,53, 54 cardiovascular diseases,55 diabetes,56 bone-healing therapies57 and vaccinations. They can also be used as so-called theranostics by conjugating contrast agents to the surface of polymeric NPs for visualization by MRI or other non-invasive imaging modality.58
Dendrimers named after the Greek word ‘dendron’ for tree, are chemically synthesized macromolecules with well-defined tree-like architectures. Typically dendrimers consist of a central core with two or more reactive functional groups that are covalently attached to repeating chemical moieties (generations). They can either encapsulate or covalently conjugate a variety of therapeutic and imaging agents in their dendric core or on the surface for various biomedical applications.59 Lipoprotein modified NPs are HDL-based natural nanoparticles. While the HDL is known for its role in cholesterol transport, it can also act as carriers for systemic delivery of drugs and imaging agents.60 Inorganic Metal-oxide NPs such as mesoporous silica NPs, iron oxide and gold NPs, are physicochemically and biochemically stable NP platforms with high surface area, and less drug leakage. These NP platforms are extensively used in theranostics and multimodality imaging applications.61, 62 Carbon based materials such as fullerenes, carbon nanotubes and graphene sheets63, 64 are widely used for various clinical applications including the delivery of nucleic acids for the purpose of gene therapy.65
The physicochemical properties (size, shape, surface charge, rigidity) of the NP platforms can influence their uptake by different organs, tissues and cells. Therefore substantial efforts have been made in the optimization of these properties through the effective design of NPs.66 Based on the application as well as physiochemical properties of the payload, several preparation methods were developed for different NP platforms which vary in the method of emulsification, solvent evaporation, and film hydration.67 A detailed discussion of these primary properties of NPs, the optimization of which is essential for the development of efficient therapeutic NPs, have been reported elsewhere.66 Although delivery to the atherosclerotic plaque has yet to be perfected, the flexibility in NP design will likely allow for fine-tuning NP properties to achieve the more desirable and on-target effects.
Lessons learned from siRNA therapies for cardiovascular disease applications
Given that miRNA and siRNA share a number of structural similarities, the development of siRNA delivery methods has the potential to be extrapolated to miRNAs. In mammalian systems, miRNAs are encoded in the genome and are processed in the nucleus by the cell of origin, whereas siRNAs primarily occur following exogenous delivery.68 In order to functionally bind its target mRNA(s), both classes of small RNA need to be single-stranded, yet require efficient and physiological loading into the RISC complex. While siRNAs lead to degradation of target mRNA, miRNAs lead to either degradation or translational inhibition of target mRNA. In both cases, an ideal delivery platform would be one that acheives a high load of RNA per NP, but efficiently releases these RNAs from the platform where they can successfully escape from the endosomes and distribute evenly in the cytosol.50 In recent years, different siRNA nanodelivery approaches have been used to target cardiovascular diseases. Systemic delivery of siRNA nanovehicles has been explored for cardiovascular diseases applications. But in most cases, even though respective gene knockdown achieved, these NPs accumulated to a greater extent in organs such as liver and spleen than the target tissue (heart or plaques).69 Some of the initial strategies were focused on local injection of nanoplatforms into target sites and were moderately successful. Local delivery of the siRNA-containing polyethyleneimine polymer-based NPs,70, 71 or pH sensitive polyketal-based NPs71 into the myocardium resulted in appropriate target gene knockdown and validated the delivery platforms for siRNA, however, more studies are needed with systemic administration of these NPs to evaluate their potential for broad applicability.
Taking advantage of the pattern of NP organ accumulation observed with systemic delivery, different approaches were developed to indirectly target the cardiovascular system. In one example, intravenous administration of cationic liposomal NPs containing siRNA against C-C chemokine receptor 2 (CCR2) accumulated more in the spleen and bone marrow, and were up taken by monocytes. These NPs effectively silenced the CCR2 mRNA in inflammatory monocyte subsets and inhibited their migration, leading to decreased inflammatory monocytes in atherosclerotic plaques, reduced infarct size after coronary artery occlusion, prolonged normoglycemia in diabetic mice after pancreatic islet transplantation.72 Also recently, polyethylenimine (PEI)-based polymeric endothelial-avid NPs containing multiple different siRNA that can effectively silence multiple targets was reported. Here NPs were loaded with five siRNAs targeting five endothelial cellular adhesion molecules (ICAM1, ICAM2, VCAM1, E-selectin and P-selectin), and simultaneously reduced their expression in ApoE−/− mice with accelerated atherosclerosis after MI.73 These latest studies show tremendous potential for the development of vascular- and inflammatory-cell specific NPs that can target more than one gene at once, and provides excitement for the possibility that multiple miRNAs may be targeted simultaneously using similar technology.
Other NP applications in cardiovascular diseases
There are a growing number of examples of drugs that could have enormous benefit in the atherosclerotic plaque but have untoward effects on other organs, namely the liver. Therefore methods of targeting only the cardiovascular system without unwanted systemic effects have been highly sought after. Over the last decade, the progress in cancer nanotechnology74 improved our understanding of nano-bio interactions,75 and made inroads in developing nanomedicine-based CVD therapies and diagnostics.76, 77 A variety of NP platforms containing drugs, proteins, and biologics have been tested pre-clinically and provide some encouraging results that NPs may soon be used for cardiovascular-specific applications.
Liver X receptors (LXRs) decrease inflammation by modulating the expression of key inflammatory genes, making LXRs and their ligands attractive candidates for therapeutic intervention in cardiovascular diseases. However, LXR activation in the liver promotes SREBP1-driven lipogenesis which results in hepatosteatosis, making systemic delivery of LXR agonists an impossibility for the treatment of atherosclerosis. In this context potential LXR-based nanotherapies for the management of inflammation and atherosclerosis were explored by delivery of LXR agonists specifically to sites of inflammation. The synthetic LXR agonist GW3965 containing NPs (LXR-NPs) were significantly more effective than the naked drug alone in inducing LXR target genes while down regulating proinflammatory mediators.78 Interestingly, in mice with existing atherosclerotic plaques, systemic administration of LXR-NPs resulted in significant reduction in atherosclerotic lesion area without increasing total cholesterol or triglycerides in the liver and plasma.79 In other strategies, NP platforms were used in modulating the polarity of monocytes and macrophages toward a less inflammatory phenotype to prevent plaque destabilization and markers of rupture. Here polymeric NPs were loaded with peroxisome proliferator–activated receptor-γ agonist pioglitazone (pio-NPs) and delivered to circulating monocytes. In atherosclerotic mice studies, pio-NPs reduced markers of plaque rupture in mouse brachiocephalic arteries by decreasing circulating inflammatory monocytes and suppressing the proteinase activity of plaque macrophages.80
More recently, the importance of the family of proresolving mediators as potent supressors of inflammation has been revealed, and as such, therapies aimed to delivery these specialized proresolution mediators (SPMs) could have significant potential to suppress inflammation and promote anti-inflammatory mechanisms. Spatiotemporal delivery of SPMs to atherosclerotic plaques using NP platforms improved the bioavailability of the mediators and enhanced the therapeutic efficacy. In a recent proof-of-concept study, proresolving mediator Ac2-26-containing polymeric NP platforms showed enhanced resolution to a much greater extent than free Ac2-26 peptide in acute inflammation settings. These NP were decorated with collagen IV (Col IV)–binding peptide on the surface of the NPs for the targeted delivery to sites of injury.52 When evaluated for their therapeutic effect on chronic, non-resolving inflammatory conditions, Col IV–Ac2-26 NPs showed promise in mice with advanced atherosclerotic plaques.81 Col IV–Ac2-26 NPs homed to atherosclerotic lesions and stabilized vulnerable lesions by reducing oxidative stress and collagenase activity in a myeloid-FPR2/ALX–dependent manner. These findings suggest a new modality to combat inflammation in atherosclerosis without compromising the host defense system by targeted delivery of proresolving mediators. In a separate study, Col-IV targeted NPs containing anti-inflammatory cytokine interleukin 10 (IL-10) showed similar potential in the treatment of atherosclerosis, and further augmented the strategy of tempo-spatial delivery of proresolving mediators67. These examples of NP-delivered therapeutics provide support for the potential of plaque-specific delivery of therapeutics with less than desirable whole-body effects.
Future perspectives
miRNAs have established themselves as key regulators of vascular disease, and have generated tremendous excitement for their potential to be therapeutically manipulated. And while the identification of unique miRNAs continues to grow, and gene knock-out and anti-sense models have provided some insight into the role of specific miRNAs in disease, our understanding of the cell- and tissue-specific role of miRNAs is lagging. Similarly, the presence or change in expression of a particular miRNA does not always lead to changes in its target gene expression, as not all miRNAs bound to Ago2 are functionally active82. On the other hand, the translation of NP-delivered miRNA therapies will require several improvements in NP platforms to maximize tissue-specific delivery, improve miRNA stability and customize the pharmacokinetics/pharmacodynamics and biodistribution profiles. Major advances in the development of NPs for cardiovascular applications have been made in the area of diagnostic imaging,76,83 and theranostics84,85 and developing miRNA and NPs tagged with imaging agents and studying them in animal models will improve our understanding of factors that drive plaque NP accumulation and the fate of miRNAs and vehicles once inside the plaque. In contrast to small molecules and peptides, which may readily pass through cellular compartments, miRNAs are relatively unstable and must avoid degradation during transit through the endosomal pathway to load into the RISC complex. These unique features of RNA-based therapies make the design of NP platforms a challenge. However the understanding of miRNAs and advances in nanomedicine technology are both moving at a rapid pace, and merging of the two concepts will help in the translation of both of these powerful therapeutic tools.
Acknowledgments
Sources of funding
KJR is supported by the Canadian Institutes for Health Research (MOP130365) and the National Institutes of Health (R01HL119047).
References
- 1.Vengrenyuk Y, Nishi H, Long X, Ouimet M, Savji N, Martinez FO, Cassella CP, Moore KJ, Ramsey SA, Miano JM, Fisher EA. Cholesterol loading reprograms the microrna-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol. 2015;35:535–546. doi: 10.1161/ATVBAHA.114.304029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Deuse T, Stubbendorff M, Chernogubova E, Erben RG, Eken SM, Jin H, Li Y, Busch A, Heeger CH, Behnisch B, Reichenspurner H, Robbins RC, Spin JM, Tsao PS, Schrepfer S, Maegdefessel L. Local microrna modulation using a novel anti-mir-21-eluting stent effectively prevents experimental in-stent restenosis. Arterioscler Thromb Vasc Biol. 2015;35:1945–1953. doi: 10.1161/ATVBAHA.115.305597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao WJ, Rosenblat JD, Roth NC, Kuliszewski MA, Matkar PN, Rudenko D, Liao C, Lee PJ, Leong-Poi H. Therapeutic angiogenesis by ultrasound-mediated microrna-126-3p delivery. Arterioscler Thromb Vasc Biol. 2015;35:2401–2411. doi: 10.1161/ATVBAHA.115.306506. [DOI] [PubMed] [Google Scholar]
- 4.Canfran-Duque A, Lin CS, Goedeke L, Suarez Y, Fernandez-Hernando C. Micro-rnas and high-density lipoprotein metabolism. Arterioscler Thromb Vasc Biol. 2016;36:1076–1084. doi: 10.1161/ATVBAHA.116.307028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of mir-33 from an srebp2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marquart TJ, Allen RM, Ory DS, Baldan A. Mir-33 links srebp-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107:12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. Mir-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarling EJ, Ahn H, de Aguiar Vallim TQ. The nuclear receptor fxr uncouples the actions of mir-33 from srebp-2. Arterioscler Thromb Vasc Biol. 2015;35:787–795. doi: 10.1161/ATVBAHA.114.304179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meiler S, Baumer Y, Toulmin E, Seng K, Boisvert WA. Microrna 302a is a novel modulator of cholesterol homeostasis and atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:323–331. doi: 10.1161/ATVBAHA.114.304878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du F, Yu F, Wang Y, Hui Y, Carnevale K, Fu M, Lu H, Fan D. Microrna-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34:759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. Microrna-33 and the srebp host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen RM, Marquart TJ, Albert CJ, Suchy FJ, Wang DQ, Ananthanarayanan M, Ford DA, Baldan A. Mir-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol Med. 2012;4:882–895. doi: 10.1002/emmm.201201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper ME, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-mir33 in atherosclerosis. Circ Res. 2015;117:266–278. doi: 10.1161/CIRCRESAHA.117.305624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez CM, Goedeke L, Rotllan N, Yoon JH, Cirera-Salinas D, Mattison JA, Suarez Y, de Cabo R, Gorospe M, Fernandez-Hernando C. Microrna 33 regulates glucose metabolism. Mol Cell Biol. 2013;33:2891–2902. doi: 10.1128/MCB.00016-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie T, Baba O, Kuwabara Y, Chujo Y, Watanabe S, Kinoshita M, Horiguchi M, Nakamura T, Chonabayashi K, Hishizawa M, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. Microrna-33 deficiency reduces the progression of atherosclerotic plaque in apoe−/− mice. J Am Heart Assoc. 2012;1:e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouimet M, Ediriweera HN, Gundra UM, Sheedy FJ, Ramkhelawon B, Hutchison SB, Rinehold K, van Solingen C, Fullerton MD, Cecchini K, Rayner KJ, Steinberg GR, Zamore PD, Fisher EA, Loke P, Moore KJ. Microrna-33-dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J Clin Invest. 2015;125:4334–4348. doi: 10.1172/JCI81676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of mir-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. Journal of Clinical Investigation. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic silencing of microrna-33 inhibits the progression of atherosclerosis in ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2013 doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen RM, Marquart TJ, Jesse JJ, Baldan A. Control of very-low density lipoprotein secretion by n-ethylmaleimide-sensitive factor and mir-33. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goedeke L, Salerno A, Ramirez CM, Guo L, Allen RM, Yin X, Langley SR, Esau C, Wanschel A, Fisher EA, Suarez Y, Baldan A, Mayr M, Fernandez-Hernando C. Long-term therapeutic silencing of mir-33 increases circulating triglyceride levels and hepatic lipid accumulation in mice. EMBO Mol Med. 2014;6:1133–1141. doi: 10.15252/emmm.201404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie T, Nishino T, Baba O, Kuwabara Y, Nakao T, Nishiga M, Usami S, Izuhara M, Sowa N, Yahagi N, Shimano H, Matsumura S, Inoue K, Marusawa H, Nakamura T, Hasegawa K, Kume N, Yokode M, Kita T, Kimura T, Ono K. Microrna-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nature communications. 2013;4:2883. doi: 10.1038/ncomms3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karunakaran D, Richards L, Geoffrion M, Barrette D, Gotfrit RJ, Harper ME, Rayner KJ. Therapeutic inhibition of mir-33 promotes fatty acid oxidation but does not ameliorate metabolic dysfunction in diet-induced obesity. Arterioscler Thromb Vasc Biol. 2015;35:2536–2543. doi: 10.1161/ATVBAHA.115.306404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ. Inhibition of mir-33a/b in non-human primates raises plasma hdl and lowers vldl triglycerides. Nature. 2011;478:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krichevsky AM, Gabriely G. Mir-21: A small multi-faceted rna. Journal of cellular and molecular medicine. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, Tsao PS. Microrna-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4:122ra122. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, Liu Y, Cheang TY, Huang XL, Wang SM. Microrna-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 27.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. Microrna expression signature and antisense-mediated depletion reveal an essential role of microrna in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 28.McDonald RA, White KM, Wu J, Cooley BC, Robertson KE, Halliday CA, McClure JD, Francis S, Lu R, Kennedy S, George SJ, Wan S, van Rooij E, Baker AH. Mirna-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. Eur Heart J. 2013;34:1636–1643. doi: 10.1093/eurheartj/eht105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang A, Liu Y, Shen Y, Xu Y, Li X. Mir-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. Urology. 2011;78:474e413–479. doi: 10.1016/j.urology.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of mir-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell death and differentiation. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. Mir-145 and mir-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through mirnas. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 33.Sala F, Aranda JF, Rotllan N, Ramirez CM, Aryal B, Elia L, Condorelli G, Catapano AL, Fernandez-Hernando C, Norata GD. Mir-143/145 deficiency attenuates the progression of atherosclerosis in ldlr−/−mice. Thrombosis and haemostasis. 2014;112:796–802. doi: 10.1160/TH13-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kent OA, McCall MN, Cornish TC, Halushka MK. Lessons from mir-143/145: The importance of cell-type localization of mirnas. Nucleic acids research. 2014;42:7528–7538. doi: 10.1093/nar/gku461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karunakaran D, Rayner KJ. Macrophage mirnas in atherosclerosis. Biochimica et biophysica acta. 2016 doi: 10.1016/j.bbalip.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schurmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, de Windt LJ, Lutgens E, Wouters K, de Winther MP, Zacchigna S, Giacca M, van Bilsen M, Papageorgiou AP, Schroen B. Macrophage microrna-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 37.Wei Y, Zhu M, Corbalan-Campos J, Heyll K, Weber C, Schober A. Regulation of csf1r and bcl6 in macrophages mediates the stage-specific effects of microrna-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 38.Donners MMPC, Wolfs IMJ, Stöger LJ, van der Vorst EPC, Pöttgens CCH, Heymans S, Schroen B, Gijbels MJJ, de Winther MPJ. Hematopoietic mir155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PloS one. 2012;7:e35877–e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pankratz FBX, Zeiser R, Leonhard F, Kreuzaler S, Hilgendorf I, Smolka C, Helbing T, Hoefer I, Esser JS, Kustermann M, Moser M, Bode C, Grundmann S. Microrna-155 exerts cell-specific anti-angiogenic but pro-arteriogenic effects during adaptive neovascularization. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.014579. In press. [DOI] [PubMed] [Google Scholar]
- 40.Rayner KJ. Microrna-155 in the heart: The right time at the right place in the right cell. Circulation. 2015;131:1533–1535. doi: 10.1161/CIRCULATIONAHA.115.016327. [DOI] [PubMed] [Google Scholar]
- 41.Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein e enhances microrna-146a in monocytes and macrophages to suppress nuclear factor-kappab-driven inflammation and atherosclerosis. Circ Res. 2015;117:e1–e11. doi: 10.1161/CIRCRESAHA.117.305844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis S, Lollo B, Freier S, Esau C. Improved targeting of mirna with antisense oligonucleotides. Nucleic acids research. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gandellini P, Profumo V, Folini M, Zaffaroni N. Micrornas as new therapeutic targets and tools in cancer. Expert opinion on therapeutic targets. 2011;15:265–279. doi: 10.1517/14728222.2011.550878. [DOI] [PubMed] [Google Scholar]
- 44.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S. Silencing of microrna families by seed-targeting tiny lnas. Nature genetics. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broderick JA, Zamore PD. Microrna therapeutics. Gene therapy. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of mir-10b inhibits metastasis in a mouse mammary tumor model. Nature biotechnology. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bangham AD. Liposomes: The babraham connection. Chemistry and physics of lipids. 1993;64:275–285. doi: 10.1016/0009-3084(93)90071-a. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature reviews. Drug discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 49.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clinical pharmacokinetics. 2003;42:419–436. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 50.Gadde S. Multi-drug delivery nanocarriers for combination therapy. MedChemComm. 2015;6:1916–1929. [Google Scholar]
- 51.Pridgen EM, Langer R, Farokhzad OC. Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomedicine (Lond) 2007;2:669–680. doi: 10.2217/17435889.2.5.669. [DOI] [PubMed] [Google Scholar]
- 52.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MA, Zheng YR, Gadde S, Pfirschke C, Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, Kolishetti N, Pittet M, Lippard SJ, Farokhzad OC, Weissleder R. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic pt(iv) pro-drug. Nature communications. 2015;6:8692. doi: 10.1038/ncomms9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valencia PM, Pridgen EM, Perea B, Gadde S, Sweeney C, Kantoff PW, Bander NH, Lippard SJ, Langer R, Karnik R, Farokhzad OC. Synergistic cytotoxicity of irinotecan and cisplatin in dual-drug targeted polymeric nanoparticles. Nanomedicine (Lond) 2013;8:687–698. doi: 10.2217/nnm.12.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y-J, Larsson M, Huang W-T, Chiou S-H, Nicholls SJ, Chao J-I, Liu D-M. The use of polymer-based nanoparticles and nanostructured materials in treatment and diagnosis of cardiovascular diseases: Recent advances and emerging designs. Progress in Polymer Science. 2016;57:153–178. [Google Scholar]
- 56.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: Challenges and opportunities. Nature reviews. Drug discovery. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Progress in Polymer Science. 2010;35:403–440. [Google Scholar]
- 58.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chemical Society reviews. 2012;41:2656–2672. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 59.Caminade A-M, Turrin C-O. Dendrimers for drug delivery. Journal of Materials Chemistry B. 2014;2:4055–4066. doi: 10.1039/c4tb00171k. [DOI] [PubMed] [Google Scholar]
- 60.Thaxton CS, Rink JS, Naha PC, Cormode DP. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Advanced drug delivery reviews. doi: 10.1016/j.addr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, Li Y, Shi J. A ph-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32:7711–7720. doi: 10.1016/j.biomaterials.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 62.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: Insights into atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao W, Shim G, Lee S, Choe YS, Oh YK. Safety and tumor tissue accumulation of pegylated graphene oxide nanosheets for co-delivery of anticancer drug and photosensitizer. Biomaterials. 2013;34:3402–3410. doi: 10.1016/j.biomaterials.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. Journal of controlled release : official journal of the Controlled Release Society. 2014;173:75–88. doi: 10.1016/j.jconrel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 65.Bitounis D, Ali-Boucetta H, Hong BH, Min DH, Kostarelos K. Prospects and challenges of graphene in biomedical applications. Adv Mater. 2013;25:2258–2268. doi: 10.1002/adma.201203700. [DOI] [PubMed] [Google Scholar]
- 66.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chemical Society reviews. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamaly N, Fredman G, Fojas JJ, Subramanian M, Choi WI, Zepeda K, Vilos C, Yu M, Gadde S, Wu J, Milton J, Carvalho Leitao R, Rosa Fernandes L, Hasan M, Gao H, Nguyen V, Harris J, Tabas I, Farokhzad OC. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS nano. 2016;10:5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carthew RW, Sontheimer EJ. Origins and mechanisms of mirnas and sirnas. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel sirna-lipoplex technology for rna interference in the mouse vascular endothelium. Gene therapy. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 70.Kim D, Hong J, Moon H-H, Nam HY, Mok H, Jeong JH, Kim SW, Choi D, Kim SH. Anti-apoptotic cardioprotective effects of shp-1 gene silencing against ischemia–reperfusion injury: Use of deoxycholic acid-modified low molecular weight polyethyleneimine as a cardiac sirna-carrier. Journal of Controlled Release. 2013;168:125–134. doi: 10.1016/j.jconrel.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 71.Somasuntharam I, Boopathy AV, Khan RS, Martinez MD, Brown ME, Murthy N, Davis ME. Delivery of nox2-nadph oxidase sirna with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials. 2013;34:7790–7798. doi: 10.1016/j.biomaterials.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic sirna silencing in inflammatory monocytes in mice. Nature biotechnology. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sager HB, Dutta P, Dahlman JE, Hulsmans M, Courties G, Sun Y, Heidt T, Vinegoni C, Borodovsky A, Fitzgerald K, Wojtkiewicz GR, Iwamoto Y, Tricot B, Khan OF, Kauffman KJ, Xing Y, Shaw TE, Libby P, Langer R, Weissleder R, Swirski FK, Anderson DG, Nahrendorf M. Rnai targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Science translational medicine. 2016;8:342ra380. doi: 10.1126/scitranslmed.aaf1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced drug delivery reviews. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller MA, Gadde S, Pfirschke C, Engblom C, Sprachman MM, Kohler RH, Yang KS, Laughney AM, Wojtkiewicz G, Kamaly N, Bhonagiri S, Pittet MJ, Farokhzad OC, Weissleder R. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Science translational medicine. 2015;7:314ra183. doi: 10.1126/scitranslmed.aac6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:750–757. doi: 10.1161/ATVBAHA.110.221499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mulder WJ, Fayad ZA. Nanomedicine captures cardiovascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:801–802. doi: 10.1161/ATVBAHA.108.165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gadde S, Even-Or O, Kamaly N, Hasija A, Gagnon PG, Adusumilli KH, Erakovic A, Pal AK, Zhang XQ, Kolishetti N, Shi J, Fisher EA, Farokhzad OC. Development of therapeutic polymeric nanoparticles for the resolution of inflammation. Adv Healthc Mater. 2014;3:1448–1456. doi: 10.1002/adhm.201300688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, Farokhzad OC, Fisher EA. Nanoparticles containing a liver x receptor agonist inhibit inflammation and atherosclerosis. Adv Healthc Mater. 2015;4:228–236. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakashiro S, Matoba T, Umezu R, Koga J, Tokutome M, Katsuki S, Nakano K, Sunagawa K, Egashira K. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in apoe−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:491–500. doi: 10.1161/ATVBAHA.115.307057. [DOI] [PubMed] [Google Scholar]
- 81.Fredman G, Kamaly N, Spolitu S, Milton J, Ghorpade D, Chiasson R, Kuriakose G, Perretti M, Farokhzad O, Tabas I. Targeted nanoparticles containing the proresolving peptide ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Science translational medicine. 2015;7:275ra220. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.La Rocca G, Olejniczak SH, Gonzalez AJ, Briskin D, Vidigal JA, Spraggon L, DeMatteo RG, Radler MR, Lindsten T, Ventura A, Tuschl T, Leslie CS, Thompson CB. In vivo, argonaute-bound micrornas exist predominantly in a reservoir of low molecular weight complexes not associated with mrna. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:767–772. doi: 10.1073/pnas.1424217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segers FM, den Adel B, Bot I, van der Graaf LM, van der Veer EP, Gonzalez W, Raynal I, de Winther M, Wodzig WK, Poelmann RE, van Berkel TJ, van der Weerd L, Biessen EA. Scavenger receptor-ai-targeted iron oxide nanoparticles for in vivo mri detection of atherosclerotic lesions. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1812–1819. doi: 10.1161/ATVBAHA.112.300707. [DOI] [PubMed] [Google Scholar]
- 84.Cyrus T, Zhang H, Allen JS, Williams TA, Hu G, Caruthers SD, Wickline SA, Lanza GM. Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:820–826. doi: 10.1161/ATVBAHA.107.156281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Godin B, Sakamoto JH, Serda RE, Grattoni A, Bouamrani A, Ferrari M. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol Sci. 2010;31:199–205. doi: 10.1016/j.tips.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


