Abstract
Telomeres are essential in maintaining chromosome integrity and in controlling cellular replication. Attrition of telomere length in peripheral blood mononuclear cells (PBMCs) with age is well documented from cross-sectional studies. But the actual in vivo changes in telomere lengths and its relationship with the contributing factors within the individuals with age have not been fully addressed. In the present paper, we report a longitudinal analysis of telomere length in the PBMCs, lymphocytes and monocytes of 216 human subjects aged from 20–90 years assessed at 0-, 5- and 12-year follow-up. For the 5- and 12-year follow-up, telomere length in the PBMCs decreased in 34 % and 46 %, exhibited no detectable change in 56 % and 47 % and increased in 10 % and 7 % of the subjects respectively. The rate of telomere change was distinct for T-cells, B-cells and monocytes for any given subject. Telomerase activity declined with age in the resting T-cells and B-cells and the activated T-cells. Finally, a significant portion of telomere attrition in T-cells with age was explained by a decline in the telomerase activity, decreased naïve cells and the change in physiological conditions such as elevated blood glucose and interleukin (IL)-6 levels. These findings show that changes in the telomere length of the PBMCs with age in vivo occur at different rates in different individuals and cell types and reveal that changes in the telomere length in the T-cells with age is influenced by the telomerase activity, naïve T-cell percentage and changes in health conditions.
Keywords: aging, glucose, interleukin 6 (IL-6), lymphocyte, telomerase, telomere length
INTRODUCTION
Telomeres, the termini of linear chromosomes, consist of a tandem repeat of the DNA sequence (TTAGGG)n and binding proteins and function to protect the integrity of chromosomes [1-3]. Studies of human primary cells in culture show loss of a small portion of telomere DNA repeats, ranging from 15–70 bp per cell division [4-6]. With the advance in age, the telomeres of blood leucocytes or lymphocytes shorten by 15–50 bp/year, as analysed primarily by cross-sectional analysis [7-9]. Telomere length attrition in a cell accumulates over the course of history of cell divisions and critically shortened or ‘uncapped’ telomeres cause the cell to exit the active cell cycle (senescence) or undergo apoptosis [4,5,10]. The intact function of telomeres is a key determinant of the finite proliferative capacity of primary cells in culture known as the ‘Hayflick limit’. Epidemiological studies reveal that short telomeres correlates with changes in physiological conditions, such as inflammation, [11] and various chronic illnesses, such as cardiovascular diseases [12] and diabetes [13]. However, whether the telomere attrition causes an age-related decline of immune function in humans remains to be determined.
Telomerase is an enzyme that synthesizes telomeres and compensates for telomere loss that occurs with cell division [14,15]. Telomerase activity is highly expressed in haematopoietic stem cells [16,17] during T-cell development in the thymus [18] and during B-cell differentiation in the germinal centre [19-21], but is reduced to low or undetectable levels in the resting lymphocytes [18,22]. Upon activation, telomerase activity is rapidly up-regulated in the lymphocytes (both T-cells and B-cells) and partially compensates for the loss of telomere length in the actively dividing T-cells [18,23]. The importance of telomerase is further demonstrated by its ability to extend the replicative lifespan of the T-cells when the ectopic expression of the telomerase reverse transcriptase (TERT) is introduced in T-cells [24,25], as well as by those genetic defects of telomerase components that show reduced cellular proliferation that leads to the loss of functions of proliferating tissues and organs such as bone marrow and skin [26,27].
It is known that absolute numbers and relative percentages of human-lymphocyte subsets and monocytes in the blood change with age [28]. In cross-sectional analyses, the proportion of naïve lymphocytes in the blood is progressively lower with increased age, whereas the proportion of memory lymphocytes, particularly, CD28− T-cells, is progressively higher with older ages [29]. Whether such changes of lymphocytes and monocytes occur at a constant rate or are non-linear over the lifespan has not been determined. More importantly, it is unknown how changes in the lymphocyte subset composition, possibly in conjunction with age-related changes in telomerase activity, may account for the observed average telomere length shortening in leucocytes/lymphocytes with older ages.
To address these questions, we conducted a longitudinal analysis of telomere length in peripheral blood mononuclear cells (PBMCs), T-cells, B-cells and monocytes to study parallel compositional changes of lymphocyte subsets in 216 participants from the Baltimore Longitudinal Study of Aging (BLSA) (http://www.blsa.nih.gov) assessed at baseline and at 5-year follow-up. For 158 of these participants, we also report data for a 12-year follow-up. In a subset of this sample, we explored longitudinal changes in the telomerase activity in resting and activated T-cells and B-cells.
MATERIALS AND METHODS
Study design and participants
We performed a longitudinal study with PBMCs, lymphocytes (T-cells and B-cells) and monocytes isolated from PBMCs of BLSA participants at first visit and 5-year (n = 216) or 12-year (n = 158) follow-up under an Institutional Review Board (IRB)-approved protocol (protocol number 03-AG-0325). Demographic characterization of these participants is summarized in Supplementary Table S1. At each visit, 50 ml of blood was drawn from the participants under fasting condition. PBMCs were isolated from blood and cryopreserved in liquid nitrogen. Two cryopreserved PBMCs with an average of 5 years apart or 2–3 DNA samples 12 years apart were used in the experiments. For the 5-year follow-up, PB-MCs from both time-points were thawed and counted on the day of the experiment. The recovery of frozen PBMC was 77 ± 0.3% (mean ± S.E.M.). B-cells, monocytes and T-cells were sequentially isolated from the thawed PBMC by magnetic-bead conjugated antibodies against CD19, CD14 and CD2 (Life Technologies). Isolated T-cells and B-cells were allowed to recover in an incubator for 3–4 h before being stimulated with anti-CD3 plus interleukin (IL)-2 (20 unit/ml; Hoffmann-La Roche) and pokeweed mitogen (PWM; 20 μg/ml; Sigma-Aldrich) for 72 h respectively, for telomerase measurement.
Analysis of PBMC composition by flow cytometry
Antibodies used for flow cytometry analysis included: CD2–Tri-Colour (TC); CD4–phycoerythrin (PE) and CD4–allophycocyanin (APC); CD28–FITC; CD8–TC; CD19–APC; CD45RA–APC from Life Technologies; CD14–PE; CD27–PE and IgM–FITC from BD Biosciences. Freshly thawed PBMCs of each visit were stained with 3–4 antibodies: T-cells (CD2, CD4, CD8, CD45RA and CD28); B-cells (CD19, IgM and CD27); monocytes (CD14). The data were collected on a BD FAC-SCalibur or BD FACSCanto II and analysed by Cell-Quest (BD Biosciences).
Telomere length measurement
The procedures for telomere length measurement by the terminal restriction fragment (TRF) [30] and by the quantitative PCR (qPCR)-based method [31] were as described previously. The TRF method was used for all 5-year follow-up samples and the qPCR method was used for the 12-year follow-up samples. The mean TRF was calculated from 2–40 kb. DNA from Jurkat cells was loaded on every gel and used for normalization within (2–3 Jurkat samples per gel) and among gels. The coefficient of variation of telomere length of Jurkat cells measured at different times was 10.6 % (n = 146). The qPCR method was carried out in triplicates. A conversion equation was generated between the TRF and the qPCR method based on the measurement of 130 samples by both methods with the correlation of R2 = 0.5. The rates of telomere length change were calculated by dividing telomere length differences over the corresponding time lag (in years) between the two subsequent samples for the 5-year follow-up and by the slope of data of three time-points for the 12-year follow-up. The reproducibility of telomere measurement was further examined by repeated measure of the same sample and the difference in telomere length between the two measurements was 52 ± 281 bp (mean ± S.D., n = 147) or 52 bp/year as one S.D. (average 5.4 ± 1.2 year). We postulated that changes within one S.D. (50 bp/year) could be considered as part of normal random variation and, thus, a rate of telomere length change between −50 bp/year and +50 bp/year was considered no measurable change. The yearly percentage of telomere length change over time was calculated by dividing the percentage of telomere length changes over the corresponding span of years between the two samples.
Telomerase activity measurement
The procedure for the telomerase assay was previously described [30]. A serial dilution of Jurkat cells (6–333 cell equivalents/PCR) was carried out for establishing the sensitivity and linearity of the telomerase assay. Under our conditions, telomerase activity (presented as the number of Jurkat cells) was detected at the lowest number of Jurkat cells as six and the linearity was determined by regression analysis (R2 = 0.96). The reproducibility of the telomerase activity measurement was verified by the repeated measurements of the Jurkat cells as well as the samples. The coefficient of variation of telomerase activity from the Jurkat cells measured at different times is 57.7 % (n = 27).
Statistical analysis
Figures were plotted as scatterplots with a linear regression line for telomere length and rates of change in these measures by age. The regression lines were tested using mixed effects linear regression on age to address the within-subject correlation with the repeated measurement with no adjustments. The inclusion of the time difference between the measurements did not affect the assessment. Multiple regression was used to examine a sequence of models of the association of telomere length and rate of change in length by age, telomerase and covariates that might affect the relationships. For model 4 in the Tables, only covariates were included that were found to be of importance in the model (P < 0.10) after backward elimination. The initial covariates included cancer status (or interval diagnosis), baseline body mass index (BMI), triacylglycerols (triglycerides), high-density lipoprotein (HDL), low-density lipoprotein (LDL), diabetes mellitus status and smoking status. To further examine the relationship of the telomere length with these variables, Bayesian Model Averaging [32] was utilized to identify which variables were most associated with telomere length. For the regression models, all tests were performed with a P < 0.05. For the multiple regressions with backward elimination, all variables were maintained in the parsimonious model with a P < 0.1.
Telomerase activity was not normally distributed with a high percentage of samples showing no activity and other data being quite high. We explored the distributional aspects of the high and zero values. The majority of the high values were in the youngest age-group, whereas the number of subjects with no activity increased with increasing age. We found no evidence that the highest values could be distributed by chance using a permutation test. Analyses relating telomerase activity to age and telomere length were modelled both using linear regression with permutation tests and considering the activity as a count variable using mixed effects negative binomial regression using the package glmmADMB in R [33]. The regression model using the negative binomial distribution fit the data better than the Poisson and the normal distributions. All statistical analyses were done using R version 2.12.1 (http://www.r-project.org). For general comparison, the Student’s t test was used.
RESULTS
Dynamic changes in telomere length in human blood lymphocytes and monocytes in vivo with age
We followed 216 participants from ~1000 active participants of the ongoing BLSA with an average follow-up of 5 years. The age range (23–91 years old; mean 71.1 years) and gender distribution (male = 53%; female = 47%) roughly reflected the whole BLSA study population (Supplementary Table S1). To determine the telomere length changes in vivo with age, we measured telomere lengths of PBMCs and isolated T-cells and B-cells and monocytes from PBMCs at the beginning and at 5-year follow-up. The analyses of telomere length as a function of age showed shortening of the telomeres in PBMCs, lymphocytes (T-cells and B-cells) and monocytes with age as has been reported (Supplementary Figure S1A) [7-9]. Telomere length was closely correlated between PB-MCs and T-cells as approximately half of the PBMCs are T-cells (R2 = 0.72, P < 0.01) (Figure 1A). Correlations between PBMCs and B-cells (R2 = 0.59, P < 0.01), T-cells and B-cells (R2 = 0.64, P < 0.01) and T-cells and monocytes (R2 = 0.57, P < 0.01) were significant but less tight than the correlations between PBMCs and T-cells. B-cells had the longest telomeres with an average of ~0.6 kb longer than the PBMCs (P < 0.01), T-cells (P < 0.01) and the monocytes (P < 0.01), whereas T-cells and monocytes had similar telomere length (P = 0.97) (Supplementary Figure S1B).
Figure 1. Telomere length in PBMCs, T-cells, B-cells and monocytes: correlation and change with age in vivo.
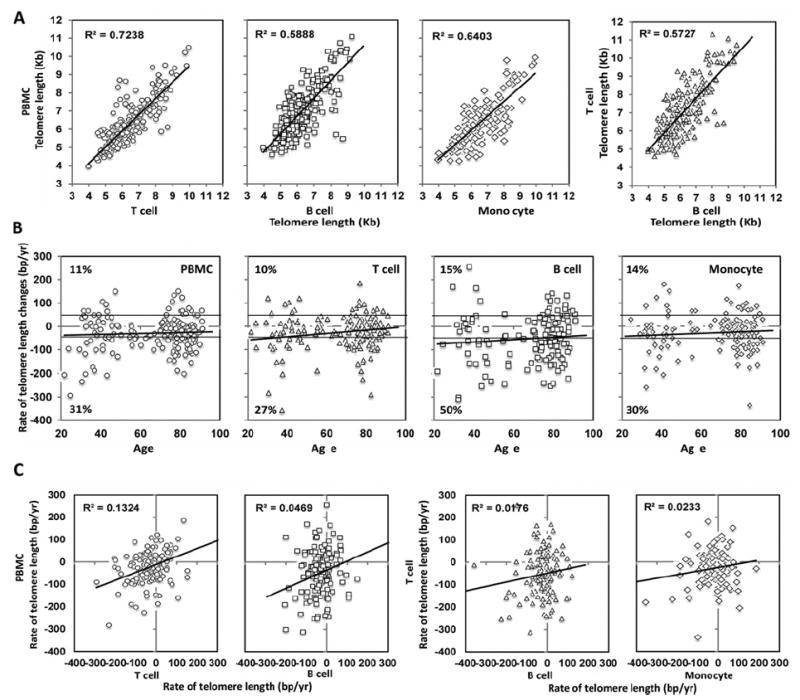
(A) Comparison of telomere length among PBMC, T-cells, B-cells and monocytes. (B) Change of telomere length in vivo in PBMCs (n = 201), T-cells (n = 193), B-cells (n = 143) and monocytes (n = 153) are displayed. Solid black lines at 50 bp/year and −50 bp/year were the boundary for defining categories of ‘decrease’, ‘no change’ and ‘increase’ in telomere length. The trend lines show the rates of telomere changes with age. The percentages of the subjects with either increased or decreased rates of telomere length are presented. (C) Comparison of the rates of telomere changes among PBMCs, T-cells, B-cells and monocytes.
To address how telomere length changes with aging in different types of cells in vivo , we calculated the rates of telomere length-change over time and found that the rates of telomere attrition in all four types of cells were reduced with age at slightly different rates (Figure 1B). Interestingly, the average rates of telomere loss computed from this longitudinal analysis (−28 bp/year in PBMC, −25 bp/year in T-cells, −52 bp/year in B-cells and −28 bp/year in monocytes) were comparable with those estimated from cross-sectional analyses (Supplementary Figure S2). To further study the distribution of telomere length changes in vivo, we considered that changes within 50 bp/year (see the Materials and methods section for details) could be attributed to chance error. Using this criterion, participants with the rates of telomere length change between <50 bp/year and >−50 bp/year were designated as no measurable change. Rates ≥50 bp/year were designated as an increase whereas rates less than or equal to −50 bp/year were designated as a decrease. We found that 31% of the subjects had decreased telomere length in PBMC (27% for T-cells, 50% for B-cells and 30% for monocytes) over 5 years whereas 58% of the subjects exhibited no measurable telomere length change in PBMC (63% for T-cells, 35% for B-cells and 56% for monocytes) (Figure 1B). However, we also observed that 11% of the subjects had increased telomere length in the PBMC (10% for T-cells, 15% for B-cells and 14% for monocytes; Figure 1B).
Although telomere lengths exhibited correlations among PB-MCs, T-cells and B-cells and monocytes in an individual, the rates of change of telomere length for PBMCs, T-cells, B-cells and monocytes were not correlated (Figure 1C). Furthermore, a proportion of individuals who did not have detectable changes in telomere length of total PBMCs did have significant changes in telomere lengths of their isolated T-cells and B-cells and monocytes (23%, 55% and 36% respectively) (Supplementary Figure S2). This suggests that different types of cells have different rates of telomere length changes with age in vivo and the rates changes across cell types do not always cluster in the same individual.
Alteration of telomere length in human PBMCs in vivo with age at ~12-year follow-up
The changes in the telomere length in PBMCs from individual subjects showed decrease or no change or increase during the initial 5-year follow-up based on the measurement at two visits. To examine the stability of telomere length change in an individual over a longer time, we extended telomere length measurement of PBMCs of the available participants (n = 158) to an average of 12 years based on the measurements at three or more visits. Comparing 5-year and 12-year follow-ups, the percentage of subjects who had a decrease in telomere length in PBMCs from baseline measurement increased from 30% to 46%; subjects with no change decreased from 59% to 47%; and subjects with increased telomere length decreased from 11% to 7%. In addition, the average rates of telomere length changes for the subjects who had a decreased telomere length were −105 bp/year at 5-year and −73 bp/year at 12-year follow-up and for the no change telomere length group were −10 bp/year and −32 bp/year, for 5- and 12-year follow-up respectively (Figure 2A). Interestingly, those with an increase in telomere length in the 5-year follow-up (average = 88 bp/year) showed an average decrease (−24 bp/year) at the 12-year follow-up (Figure 2A). This increase in the loss of telomere length over a longer time-period is further evident from higher telomere length attrition at the 12-year follow-up (−44 bp/year) than at the 5-year follow-up (−29 bp/year) in these 158 subjects.
Figure 2. Telomere length change of PBMC in vivo at ~5- and ~12-year follow-up.
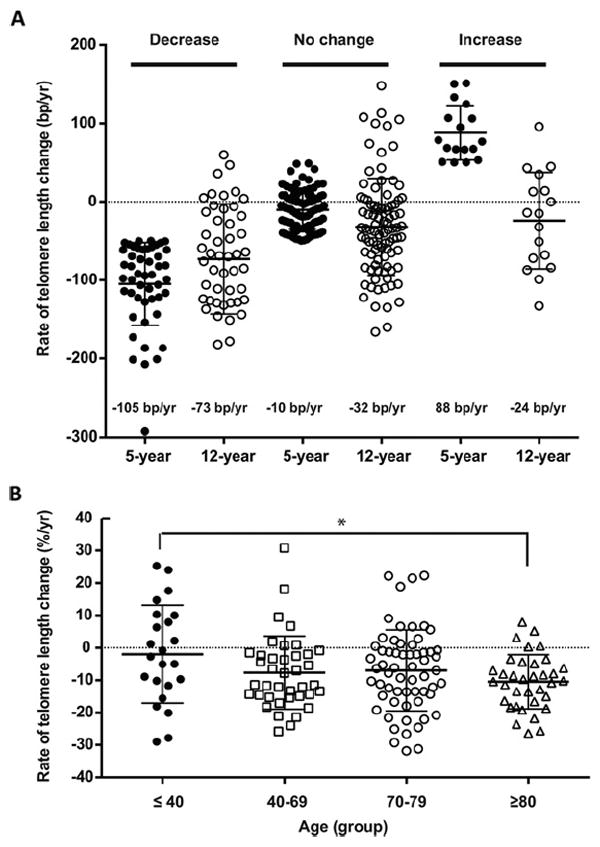
(A) Rates of telomere length changes with decrease, no change and increase groups in 5- and 12-year follow-up. The numbers of subjects within each group are: decreased (n = 45), no change (n = 98) and increased (n = 15). (B) Rates of telomere length change as a percentage of the initial telomere length in four age groups in the 5- and 12-year follow-up. The numbers of subjects within each age group are ≤40 (n = 23), 40–69 (n = 39), 70–79 (n = 61) and ≥80 (n = 35) based on time 0.
A previous study of cross-sectional telomere changes with age suggested that telomere length shortening accelerates in individuals of advanced age [34]. To test this finding in our longitudinal data, we compared the average rate of telomere loss in four age groups (≤40, 41–69, 70–79 and ≥80 years old). The average rates of telomere attrition for each age group were −21 bp/year, −43 bp/year, −42 bp/year and −61 bp/year respectively. Although the trend of telomere shortening with age appeared to increase among the different age groups, they did not reach statistical significance (Supplementary Figure S3). As older age is generally associated with shorter telomeres, loss of the same absolute amount of telomeres could have a higher percentage of loss from the initial telomere length and may thus have more severe consequences in those with short telomeres than those with long telomeres. To address this issue, we calculated the rates of telomere length change as a percentage of the initial telomere length. Indeed, the percentage of telomere loss was significantly greater in the ≥80 years old group than in the ≤40 years old young group (P < 0.05) (Figure 2B), suggesting that the affect of telomere loss at a similar rate may be significantly greater in subjects with short telomeres or in older adults than in long telomere or young adults.
Reduction in telomerase activity in T-cells and B-cells with age
To determine whether there is a change of telomerase activity with age, we measured the telomerase activity of resting and activated T-cells and B-cells at baseline and at the 5-year follow-up in participants. Quantification of telomerase activity is a challenging task as the coefficient of variation is high in the cells with high telomerase activity but measures obtained in the cells with low telomerase activity are quite reproducible. In good agreement with previous reports [18,22], we found that more than half of the subjects did not have detectable telomerase activity in resting T-cells and B-cells. Subjects who had detectable telomerase activity were more frequent among young subjects than old subjects. Overall, there was a significant decline of telomerase activity with age in both the resting T-cells and the resting B-cells (Figure 3A). These findings were further confirmed using different statistical methods (see the Materials and methods section for details). The rate of telomerase activity change appeared to be relatively stable across age groups from the second to the ninth decades in T-cells and B-cells (Figure 3B).
Figure 3. Telomerase activity change with age in T-cells and B-cells and its association with telomere length.
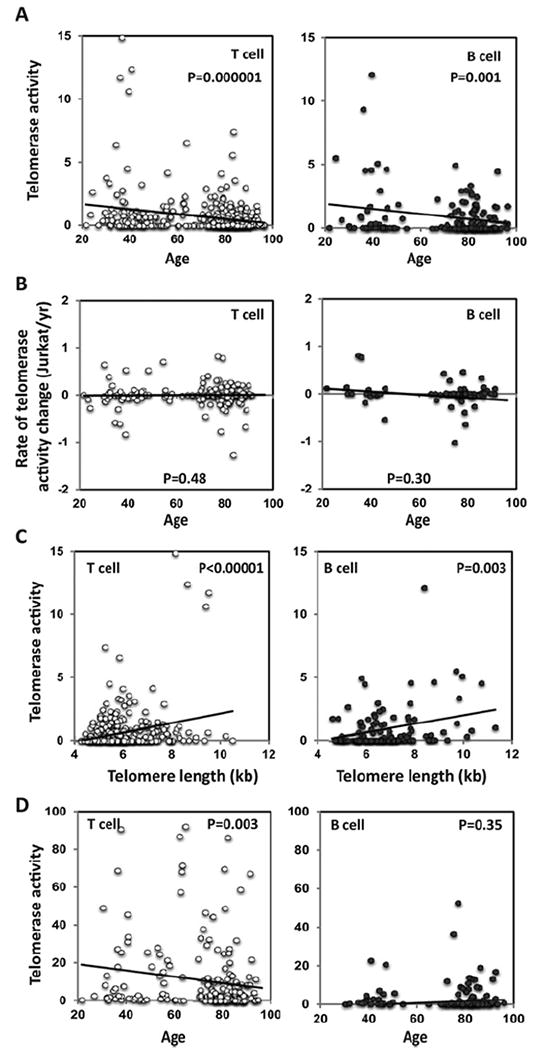
(A) Average telomerase activity in resting T-cells (n = 366) and B-cells (n = 174) in different age groups indicated on the x-axis. The trend lines show the telomerase activity change with age. (B) Rates of telomerase activity changes in resting T-cell (n = 183) and resting B-cell (n = 74). The differences in telomerase activity over time were calculated and presented as telomerase activity of the number of Jurkat cells/year. The trend lines show the rate of telomerase change with age. (C) Comparison of telomerase activity and telomere length in T-cells (n = 386) and B-cells (n = 138). The trend lines show the correlation between telomere length and telomerase activity. (D) Average telomerase activity in activated T-cells (n = 195) and B-cells (n = 148) in different age groups as indicated. The trend lines show correlation between the level of induced telomerase activity and age. The P values test the null hypothesis that the regression coefficient is equal to zero (no trend).
The relationship between telomerase activity and telomere length was further analysed and there was a significant correlation between telomerase activity and telomere length in resting T-cells and B-cells (Figure 3C). Activation-induced proliferation of lymphocytes is associated with the induction of telomerase [18,21]. To determine if induced telomerase during lymphocyte activation changes with age, we measured telomerase activity in isolated T-cells and B-cells stimulated in vitro by anti-CD3 plus IL-2 or PWM for 72 h respectively. We observed significant reduction in induced telomerase activity in activated T-cells but not in activated B-cells with age (Figure 3D).
Age-associated changes in lymphocytes and monocytes and their influence in telomere length measurement
As described, B-cells have longer telomeres than T-cells and naïve T-cells have longer telomeres than memory T-cells [6,7] and CD28− T-cells have shorter telomeres [35]. To determine the age-associated compositional changes of lymphocytes and monocytes in PMBCs, we analysed cross-sectional data and observed a decrease in the percentages of T-cells and B-cells but an increase in the percentage of monocytes in PBMCs with age (Figure 4A). The reduction in B-cells (long telomeres) and increase in monocytes (relatively shorter telomeres compared with B-cells) in PBMCs could contribute to the average telomere shortening in PBMCs with age. In lymphocytes, there was a dramatic decrease in naïve T-cells (−0.3%/year, P < 0.001), a significant increase in CD28− T-cells (0.24%/year, P < 0.001) and a significant decrease in naïve B-cells (−0.36%/year, P < 0.001) with age (Figure 4B). The decrease in naïve T-cells and increase in CD28− T-cells could also contribute to telomere shortening of total T-cells with age. From the longitudinal analysis, we observed a significant change in the rate of change in T-cells but not in B-cells, monocytes, naïve and CD28− T-cells or naïve B-cells (Figures 4C and 4D).
Figure 4. Change in lymphocytes and their subsets and monocytes with age.
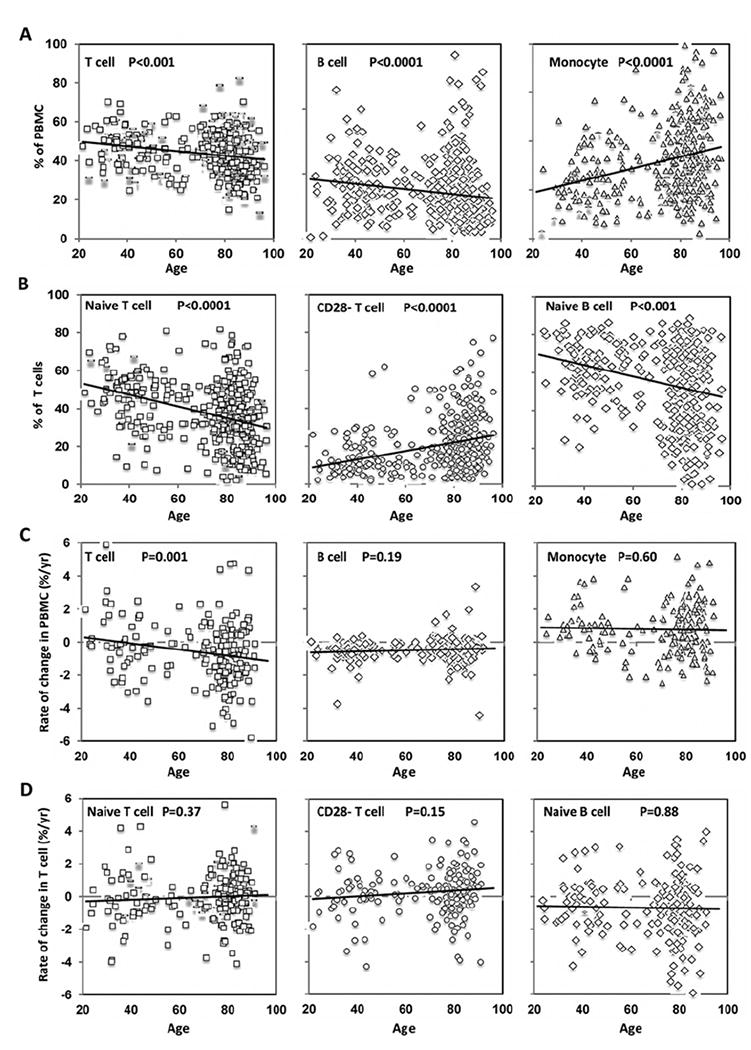
(A) Changes in percentages of T-cells, B-cells and monocytes in PBMCs. The number of samples are T-cell = 388, B-cell = 412 and monocyte = 372. (B) Changes in percentages of naïve, CD28− T-cells and naïve B-cells with age. The number of samples are naïve T-cell = 381, CD28− T-cell = 381 and naïve B-cell = 356. (C) Rates of change in T-cells, B-cells and monocytes in PBMCs with age. The number of samples are T-cell = 192, B-cell = 205 and monocyte = 186. (D) Rates of change in naïve and CD28− T-cells (n = 190) and naïve B-cells (n = 177). The trend lines and P values are presented.
Association of changes in naïve T-cell percentage and health parameters and changes in telomere length in T-cells
To further analyse the affect of the age-associated changes on the telomere length in T-cells, we used multiple linear regression models to test the hypothesis that telomere attrition attributed to aging could be explained, at least in part, by age-related changes in telomerase activity, lymphocyte subset composition and other physiological changes during aging. As expected, telomere length of T-cells was significantly associated with age (coefficient range from −0.61 to −0.40, P < 0.05) (Table 1, Models 1–4). Furthermore, telomere length of T-cells was positively correlated with the level of telomerase activity in resting T-cells (coefficient = 0.16 ± 0.04, P < 0.05) and with the percentage of naïve T-cells (coefficient = 0.23 ± 0.05, P < 0.05) but was negatively correlated with the plasma level of glucose and IL-6 (P < 0.05) (Table 1). Finally, using Bayesian Model Averaging [32], we found the probabilities that the coefficients for telomerase activity of resting T-cells and percentage of naïve T-cells were high (P = 0.7 and P = 1.0 respectively). Together, the changes in telomerase activity, naïve-cell percentage and certain physiological measurements account for approximately one-third of the telomere attrition in T-cells that was attributed to chronological aging in the unadjusted model.
Table 1.
Multiple linear regression factors associated with telomere length in T-cells
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Age | −0.61 ± 0.04* | −0.54 ± 0.04* | −0.47 ± 0.05* | −0.40 ± 0.07† |
| Sex (Male) | −0.19 ± 0.08 | −0.26 ± 0.08* | −0.19 ± 0.09 | – |
| Telomerase activity | – | 0.16 ± 0.04* | 0.19 ± 0.04* | 0.16 ± 0.07* |
| Naïve T-cells (%) | – | – | 0.23 ± 0.05* | 0.23 ± 0.07* |
| Glucose | – | – | – | −0.01 ± 0.005* |
| IL-6 | – | – | – | −0.05 ± 0.03* |
| R2 | 0.38 | 0.39 | 0.45 | 0.43 |
The β-coefficient values ± S.D. are shown.
P < 0.05.
The samples (including first and follow-up visits) used for model 1 = 364, model 2 = 320, model 3 = 275 and model 4 = 175. Model 4 included the following covariates which were removed by backward elimination: cancer, diabetes, BMI, smoking, triacylglycerol, LDL, HDL, fasting-glucose, IL-6 and body fat. R2 reflects the percentage of the variance explained by the model.
DISCUSSION
In the present longitudinal study, we showed that the percentage of subjects with a reduction in telomere length with age increased from 34% to 46%, the subjects with no obvious change in telomere length were reduced from 56% to 47% and the subjects with an increase in telomere length decreased from 10% to 7% in 5- to 12-year follow-up. These findings suggest that telomere attrition in PBMCs is relatively slow in vivo. However, even a slow rate of telomere attrition could have a significant affect on the old populations who have generally shorter telomeres than do younger subjects. In addition, we also found a small fraction of subjects with slightly increased telomere length in the 5- and 12-year follow-up, which was also reported [36-38]. It remains to be determined whether those subjects who did not have detectable telomere loss in the present study will continue to maintain unchanged telomere length or will display telomere attrition over a longer time follow-up.
Telomerase plays a critical role in the telomere length maintenance of haematopoietic cells [39]. However, it is unknown whether there is an age-associated decline of telomerase activity in lymphocytes and whether telomerase plays any role in age-associated telomere attrition. In the present study, we demonstrated that telomerase activity in both the resting T-cells and the resting B- cells is significantly decreased with age. The reason behind this age-associated loss of telomerase activity is not completely understood. Since mature thymocytes (CD4+CD8− and CD4−CD8+) express high levels of telomerase activity [18] and there are more new thymic emigrants (naïve T-cells) in the periphery in young than in old participants [40], it is possible that the significantly lower levels of telomerase in lymphocytes in elderly may be due to the reduction in new thymic emigrants the periphery. Interestingly, in contrast with the telomerase activity decline with age in the resting T-cells and B-cells, in vitro activation-induced telomerase activity in T-cells does appear to decline with age but activation-induced telomerase activity in B-cells does not decline at all. Clearly, more studies are needed to understand the difference in activation-induced telomerase activity in T-cells and B-cells with age.
Prospective epidemiological analyses of telomere length in leucocytes or PBMC have shown an association of short telomeres with certain diseases [12,41], lifespan and age [31,42], but their mechanistic relationships have not been firmly established. Since these studies are performed on telomeres obtained from mixed populations [white blood cell (WBC) or PBMC], they lack sufficient resolution to determine if the telomere length changes in all or some of the sub-populations of cells. Our findings, in the present study, show that telomere length in T-cells is correlated positively with the percentage of naïve T-cells in total T-cells and negatively with the percentage of CD28− T-cells in total T-cells, indicating that the composition of T-cell subsets constitutes one of the major factors of telomere length change in T-cells with age. It is, thus, necessary to further delineate whether lymphocyte composition change correlates with or may even contribute to the telomere length shortening associated pathologies suggested by epidemiological analyses.
Multiple factors can potentially influence changes of telomere length in lymphocytes in vivo with age. We demonstrated that telomerase activity, composition of lymphocytes and certain health conditions (plasma glucose and IL-6 levels) account for approximately 30% of the age-associated telomere length attrition in T-cells, presenting a more comprehensive picture of telomere length regulation in vivo with age. Obesity associated with shorter telomeres was reported from a cross-sectional analysis of telomere length and age-related changes [43]. IL-6 is a multi-functional cytokine and an elevated level of IL-6 in blood is a strong indicator of biological aging [44]. Although how these factors are regulated and contributed to the telomere length changes remains to be determined, our findings suggest that maintaining telomerase activity and/or lymphocyte composition (protecting naïve cells) can retain telomere length or at least minimize the loss of telomeres in lymphocytes. In addition, how changes in health conditions, such as alterations in metabolic rates and the presence of pathology, contribute to age-associated changes in telomere length, directly or indirectly through negative affect on telomerase and lymphocyte compositions, has not been directly assessed. An important next step will be extending this multi-parameter analysis and defining the contributions of these factors with improved resolutions of the measurements and longer follow-up times. Ultimately, this will lead to a better understanding of the mechanisms governing the regulation of telomere function and its role in human aging.
Supplementary Material
CLINICAL PERSPECTIVES.
The mechanism of age-associated decline in physiological functions, particularly the decline in immune functions, is mostly unknown. Telomere length serves an essential role in cellular replicative senescence and is considered as a bio-marker for aging.
Our study examines how telomere length changes with age in vivo and the resulting contributing factors in humans and reveals that telomere length change with age in vivo differs among individuals and in different cell types and is influenced by telomerase activity, naïve T-cell percentage and changes in physiological conditions.
These findings may provide a basis for developing potential therapeutic remedies and intervention against aging.
Acknowledgments
We thank Mark Mattson and Yie Liu for the critical reading and comments on the manuscript before submission; National Institute of Aging clinical core laboratory for collecting blood samples; all of the Baltimore Longitudinal Study of Aging participants in the present study; and Cindy Clark for reviewing the manuscript before submission.
FUNDING
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- BLSA
Baltimore Longitudinal Study of Aging
- BMI
body mass index
- HDL
high-density lipoprotein
- IL
interleukin
- LDL
low-density lipoprotein
- PBMC
peripheral blood mononuclear cell
- PWM
pokeweed mitogen
- qPCR
quantitative PCR
- TRF
terminal restriction fragment
Footnotes
AUTHOR CONTRIBUTION
Yun Lin performed most of the experiments with assistance from Huy Nguyen, Thai Truong, Kevin Najarro and Christa Morris. Amanda Damjanovic initiated the experiments. Jeffrey Metter and Luigi Ferrucci did the statistical analysis. Yun Lin, Ming Zhan, Luigi Ferrucci and Nan-ping Weng did the data analysis. Nan-ping Weng and Richard Hodes designed the study with input from Dan Longo and Luigi Ferrucci. Nan-ping Weng, Yun Lin, Luigi Ferrucci and Richard Hodes wrote the manuscript with input from the other co-authors.
References
- 1.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci U S A. 1998;95:90–92. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhao J, Zhu Y, Lin J, Matsuguchi T, Blackburn E, Zhang Y, Cole SA, Best LG, Lee ET, Howard BV. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes. 2014;63:354–362. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Njajou OT, Cawthon RM, Blackburn EH, Harris TB, Li R, Sanders JL, Newman AB, Nalls M, Cummings SR, Hsueh WC. Shorter telomeres are associated with obesity and weight gain in the elderly. Int J Obes. 2012;36:1176–1179. doi: 10.1038/ijo.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 8.Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrera E, Martinez-A C, Blasco MA. Impaired germinal center reaction in mice with short telomeres. EMBO J. 2000;19:472–481. doi: 10.1093/emboj/19.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng NP, Granger L, Hodes RJ. Telomere lengthening and telomerase activation during human B cell differentiation. Proc Natl Acad Sci U S A. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holly AC, Melzer D, Pilling LC, Henley W, Hernandez DG, Singleton AB, Bandinelli S, Guralnik JM, Ferrucci L, Harries LW. Towards a gene expression biomarker set for human biological age. Aging Cell. 2013;12:324–326. doi: 10.1111/acel.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with Age. J Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- 15.Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, Harley CB, Lansdorp PM. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 16.Cipriano C, Tesei S, Malavolta M, Giacconi R, Muti E, Costarelli L, Piacenza F, Pierpaoli S, Galeazzi R, Blasco M, et al. Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. J Gerontol A Biol Sci Med Sci. 2009;64:745–751. doi: 10.1093/gerona/glp048. [DOI] [PubMed] [Google Scholar]
- 17.Hathcock K, Hodes R, Weng NP. Analysis of telomere length and telomerase activity. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; 2004. pp. 10.30.1–10.30.27. [DOI] [PubMed] [Google Scholar]
- 18.Junge S, Kloeckener-Gruissem B, Zufferey R, Keisker A, Salgo B, Fauchere JC, Scherer F, Shalaby T, Grotzer M, Siler U, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37:3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 19.Norrback KF, Hultdin M, Dahlenborg K, Osterman P, Carlsson R, Roos G. Telomerase regulation and telomere dynamics in germinal centers. Eur J Haematol. 2001;67:309–317. doi: 10.1034/j.1600-0609.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- 20.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder MN, Nielsen A, John Sibert. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optimization Methods and Software. 2012;27:233–249. [Google Scholar]
- 21.Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 23.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8(+) T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–4245. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 25.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8 +counterparts. J Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 26.Greider CW. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 27.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spyridopoulos I, Hoffmann J, Aicher A, Brummendorf TH, Doerr HW, Zeiher AM, Dimmeler S. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–1372. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- 30.Weng N, Levine BL, June CH, Hodes RJ. Regulated expression of telomerase activity in human T lymphocyte development and activation. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cech TR. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 34.Allsopp RC, Harley CB. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 35.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 38.Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Statistical Sci. 1999;14:382–401. [Google Scholar]
- 39.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 40.Rufer N, Migliaccio M, Antonchuk J, Humphries RK, Roosnek E, Lansdorp PM. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood. 2001;98:597–603. doi: 10.1182/blood.v98.3.597. [DOI] [PubMed] [Google Scholar]
- 41.Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012;8:e1002696. doi: 10.1371/journal.pgen.1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marciniak RA, Johnson FB, Guarente L. Dyskeratosis congenita, telomeres and human ageing. Trends Genet. 2000;16:193–195. doi: 10.1016/s0168-9525(00)01984-3. [DOI] [PubMed] [Google Scholar]
- 43.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol. 2005;174:4019–4024. doi: 10.4049/jimmunol.174.7.4019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


