Abstract
The type 2 inositol 1,4,5-trisphosphate receptor (IP3R2) is the principal intracellular Ca2+ release channel in hepatocytes, and so is important for bile secretion and other functions. IP3R2 activity is regulated in part by post-translational modifications but little is known about transcriptional regulation of its expression. We found that both IP3R2 mRNA and protein levels in liver were increased during fasting. Treatment of hepatocytes with forskolin or 8-CPT-cAMP also increased IP3R2, and this was reduced by actinomycin D. Analysis of the IP3R2 promoter revealed five CREs, and CREB potently increased promoter activity. Mutation of CRE4 or CRE5 decreased induction by CREB, and ChIP assay showed recruitment of CREB to these sites. Adenylyl cyclase (AC) 6 and 9 were the principal AC isoforms detected in rat hepatocytes, and silencing either one decreased organic anion secretion, which depends on IP3R2. Secretion furthermore was increased by overnight but not acute treatment with forskolin or 8-CPTcAMP. These findings provide evidence that IP3R2 expression is transcriptionally regulated by cAMP via CREB binding to CRE elements in its promoter. The findings furthermore suggest that this mechanism is relevant for hormonal regulation of bile secretion.
Keywords: Type 2 inositol 1,4,5-trisphosphate receptor; calcium signaling; hepatocytes; bile secretion; cyclic AMP
INTRODUCTION
The inositol 1,4,5-trisphosphate receptor (IP3R) is an intracellular calcium release channel found in virtually every cell type [1] and is the principal intracellular calcium release channel in non-excitable cells [2]. There are three isoforms, which have distinct biophysical properties and which are expressed to different degrees and in distinct subcellular regions in each type of cell and tissue [3]. The type 2 isoform (IP3R2) is perhaps the least studied of the three [4]. It is the principal isoform in hepatocytes [5], and so may be important for calcium-mediated functions such as metabolism [6, 7], bile secretion [8, 9], and liver regeneration [10, 11]. Several factors phosphorylate this isoform of the receptor to modulate its calcium-release properties, including PKA [12], CaMK-II [13], and mTOR [14]. Cyclic AMP (cAMP) may directly interact with the receptor to modify its calcium-release properties as well [15]. Receptor expression is regulated in part by the transcription factors NFAT [16] and ETS-1 [17], and by miRNA-133a [18]. Given the central role of IP3R in mediating glucagon-induced hepatic glucose metabolism [6], plus the role of cAMP as an effector of glucagon [19], we investigated whether cAMP regulates hepatic expression of IP3R2.
MATERIALS AND METHODS
Animals and Materials
Male Sprague-Dawley rats weighing 180–250 grams were obtained from Charles River Laboratories (Wilmington, MA). All animal procedures were approved by the Yale Animal Care and Use Committee, according to criteria outlined in the NIH Guide for Care and Use of Laboratory Animals. Chemicals were purchased from Sigma (St. Louis, MO) unless otherwise specified. Cell culture media, fetal bovine serum (FBS), penicillin/streptomycin, trypsin, and phosphate-buffered saline were from Invitrogen (Carlsbad, CA). Collagen I was from BD Sciences (Bedford, MA). IP3R2 antibodies were kindly provided by Dr. Richard Wojcikiewicz (SUNY, Syracuse, NY). CMFDA was from Molecular Probes (Eugene, Oregon). Small interfering RNAs (siRNAs) were from Ambion (Austin, TX). Lipofectamine 2000 and FuGENE HD Transfection Reagents were obtained from Invitrogen, and Roche Applied Science (Indianapolis, IN), respectively.
Cell Culture
Human hepatoma HepG2 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin G and streptomycin, at 37°C, 5% CO2.
Isolation and Collagen Sandwich Culture of Rat Hepatocytes
Hepatocytes were isolated by collagenase perfusion, then maintained in culture on collagen-coated plates (BD Biocoat, Franklin Lakes, NJ) as previously described [5, 8, 9]. Cells were coated with a second layer of collagen 24 hr after plating and treated with forskolin+IBMX, actinomycin D, or 8-CPT-cAMP. Cells were used for experiments 12 hr after treatment. For knockdown of IP3R2, AC6, or AC9, hepatocytes (8×105 cells/35 mm dishes) were transfected with 100 nM IP3R2-siRNA, AC6-siRNA, AC9-siRNA or scrambled siRNA with lipofectamine 2000 (Invitrogen) prior to collagen overlay.
RNA Extraction and Quantitative RT-PCR Analysis
Total RNA was extracted from snap-frozen liver tissue or HepG2 cells using a RNAqueous phenol-free total RNA isolation kit (Ambion), and then converted to cDNA by reverse transcription using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Grand Island, NY). Gene mRNA expression was examined using TaqMan real-time PCR in an ABI 7500 Detection system (Applied Biosystems, Foster City, CA). The GAPDH and 18S genes were used to normalize gene expression. All primers/probes were TaqMan Gene Expression Assays from Applied Biosystems.
Measurement of organic anion secretion in hepatocytes
Isolated rat hepatocytes were plated onto collagen-coated coverslips for 2 hr at 37°C before being overlaid with a second layer of collagen to form a sandwich pattern. Sandwich-cultured rat hepatocytes were used 3–5 days after plating [8, 9, 20]. Coverslips seeded with the cells were washed, transferred to a perfusion chamber on the stage of a Zeiss LSM 710 DUO confocal microscope (Thornwood, NY), and then perifused with the organic anion 5-chloromethylfluorescein diacetate (CMFDA), which is cell permeant and nonfluorescent but is cleaved to form fluorescein in hepatocytes, which then is secreted into the canalicular lumen by the organic anion transporter MRP2. Canalicular fluorescence following perifusion of hepatocytes with CMFDA was monitored in the canalicular spaces between hepatocytes by time-lapse confocal microscopy [8, 9, 20]. The dye was excited at 488 nm and emission signals from 505–550 nm were collected. Increases in organic anion secretion were expressed as percent increase of canalicular fluorescence intensity and normalized by the baseline fluorescence.
Plasmid Constructs
A 2kb IP3R2 promoter including 321 bp of the 5′-UTR was amplified by PCR from a BAC clone (AC 024093) using forward and reverse primers (see Table 1). PCR was performed using Phusion Polymerase according to manufacturer’s instructions. The PCR product was purified using Qiagen PCR purification kit, A-tailed using Taq polymerase, and cloned into TA-cloning vector pCR2.1. Following sequencing verification, miniprep DNAs in the correct orientation were cloned after digestion with KpnI and XhoI and purification on an agarose gel into similarly digested pGL4 upstream of the luciferase reporter cDNA (Promega, Madison, WI). Miniprep DNAs from transformed colonies were verified by DNA sequencing. 0.5, 1.0 and 1.5 kb truncated fragments were similarly cloned using primers (see Table 1) in conjunction with reverse primer used for the 2-kb promoter. Two clones for each construct were grown as Maxipreps and purified DNAs were used in the transfections. Plasmid encoding cDNA for CREB was obtained from Addgene and used in the co-transfection experiment.
Table 1.
Primer sequences used in this study
| Primer name | Sequence 5′--------------------------3′ |
|---|---|
| ITPR2 promoter cloning: | |
| 2 kb: | |
| Forward | TCC CAG CTT CAA GCA ATT CTC |
| Reverse | GGA CTA CAG CGG CCA AGA G |
| 1.5, 1.0 and 0.5 kb: | |
| Forward -1.5 kb | CAA CAG AGA AGG CGT GGA AAA |
| Forward -1.0 kb | GGA CGG GTC GAC TTT CAT T |
| Forward – 0.5 kb | ACC TCT CCA GGG CTC CTC T |
| Mutation of the CRE’s : | |
| CRE1: | |
| Forward | ACC CGC TGC GGC TTT CGC AGC CGC CCG GC |
| Reverse | GCT CGC GTC CAC CTG GAA ATA AGC GGA CGG |
| CRE3: | |
| Forward | CCG TCC GCTTAT TTC CAG GTG GAC GCG AGC |
| Reverse | CCG TCC GCT TAT TTC CAG GTG GAC GCG AGC |
| CRE4: | |
| Forward | CAA AAA CTG GCT TGG CCA AGT aAa aTT TAC CTC CTT GGA GGC TCG C |
| Reverse | GCG AGC CTC CAA GGA GGT AAA ttT tAC TTG GCC AAG CCA GTT TTT G |
| Primers for ChIP : | |
| CRE1 ChIPF | CAC CTG GTC CAA AAA TTC CCC CTG A |
| CRE1 ChIPR | CTC CAA CAC CTT TGC TCC TCC |
| CRE2 ChIPF | CCA ACC GAT GTT TTT GAC CGA |
| CRE2 ChIPR | CCG AAC TGG AGA TGA AAA G |
| CRE3 ChIPF | GGC TGA AGG TTG GCT AAA AAC TG |
| CRE3 ChIPR | GGC GTT AAT GAA ACT CGA CCC GT |
| CRE2 ChIPF | CCC AGC TTT CAA GCA ATT CTG |
| CRE2 ChIPR | GGC CAA CAT GGC GAA ACC CCA T |
| CRE5 ChIPF | |
| CRE5 ChIPR | |
F – Forward; R- Reverse; ChIP: Chromatin Immunoprecipitation
Cell Transfection and Luciferase Reporter Assay
HepG2 cells were plated onto 24-well plates 24 hr prior to transfection and grown in EMEM supplemented with 5% heated inactivated FBS, and 1% penicillin-streptomycin. HepG2 cells were transfected with 0.5 μg of either pGL4-luciferase (empty vector), IP3R2WT-1.5 kb, IP3R2WT-1.0 kb, CRE4-mutant luciferase or CRE5-mutant luciferase, and co-transfected with CREB expression plasmid and 5 ng pRL-CMV (Renilla plasmid) using FuGENE HD Transfection Reagent. At 48 hr post-transfection, firefly and renilla luciferase activities were measured using a Dual-Luciferase Reporter Assay (Promega) in a Synergy2 microplate reader (BioTek, Winooski, VT). Promoter activity was calculated by normalizing the firefly luminescence to the renilla luminescence signal, and the ratio of promoter construct over control is presented.
Immunoblotting
Total protein was extracted from isolated hepatocytes or HepG2 cells and protease inhibitor cocktail was added to the lysis buffer just before use. Lysates were resolved by SDS-PAGE 4–20% TGX gels, then transferred to PVDF membranes (BioRad). Blots were then incubated overnight at 4°C with anti-IP3R2 antibody directed against the C-terminus [3], followed by horseradish peroxidase (HRP)-conjugated secondary antibody (Amersham). Chemiluminescence of band intensities was quantified using FOTODYNE Imaging and TL-100 image analysis software. Data were normalized to β-actin.
Chromatin Immunoprecipitation (ChIP)
Assays were performed using EZ-Magna ChiP HiSEns kit (Millipore-Upstate, Temecula, CA) according to manufacturer’s instructions. Briefly, HepG2 cells were co-transfected with empty pCMV vector or CREB/pCMV for 48 hr before treatment with forskolin 50 μM or vehicle (DMSO). Cells were fixed and cross-linked in 1% formaldehyde, quenched with 2.5 M glycine, then suspended in lysis buffer. Chromatin samples were sheared into fragments by sonication, followed by centrifugation at 10,000 rpm for 10 min at 4°C. Samples (Input) were precleared using protein G beads, then incubated overnight at 4°C with ChIP-grade rabbit anti-CREB polyclonal antibody (Santa Cruz), normal rabbit IgG as a negative control, or RNA polymerase antibody (Millipore) as a positive control. Antibody-chromatin complexes were pulled down with Magna Protein G Agarose beads, washed and reversed cross-linked to elute DNA. DNA was purified and PCR was performed to evaluate the ChIP-enriched DNA. The PCR reaction was electrophoresed through 2.5% agarose gel. Primers for the ChIP assay are listed in Table 1.
Statistical Analysis
Data are presented as mean±SEM. Data were analyzed using Graphpad Prism. Differences between experimental groups were assessed for significance with the two-tailed Student’s t-test for groups of two or else analysis of variance (ANOVA). Statistical significance is defined as p<0.05.
RESULTS
Fasting increases IP3R2 expression
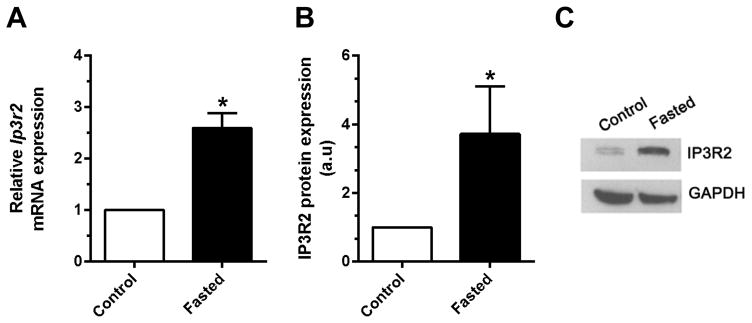
Fasting increases serum glucagon levels, and stimulation of the glucagon receptor increases cAMP in hepatocytes [21]. Therefore, we examined the effect of a 16-hr (overnight) fast on IP3R2 expression in hepatocytes. Fasting led to a 2.6-fold increase in Ip3r2 mRNA (p<0.01, n=3) and a 3.7-fold increase in IP3R2 protein levels (p<0.05, n=3) in rat hepatocytes (Figure 1). These data suggest that fasting increases Ip3r2 through both transcriptional and post-transcriptional mechanisms.
Figure 1. Fasting increases hepatic expression of IP3R2.
Rats were fasted overnight (16 hrs), then hepatocytes were isolated. (a) Ip3r2 mRNA was measured by qPCR and (b) IP3R2 was measured by densitometry of immunoblots; Representative immunoblot shown in (c). Measurements were made in triplicate (*p<0.05).
Cyclic AMP increases Ip3r2 transcription in rat hepatocytes
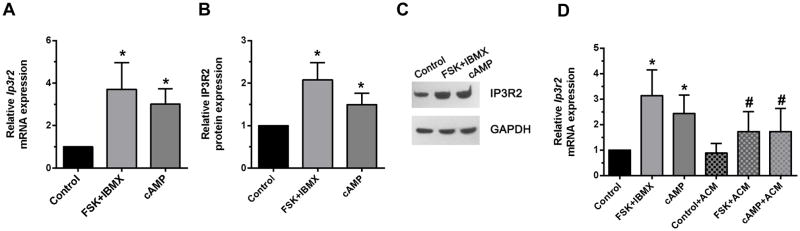
Because of the link between fasting, increased serum glucagon, and increased cAMP in hepatocytes, we investigated the effects of the adenylyl cyclase (AC) activator forskolin (FSK) and the cell permeant analog 8-CPT-cAMP on Ip3r2 transcription and expression. Rat hepatocytes were incubated for 12 hr with FSK (50 μM) plus IBMX (250 μM) to inhibit phosphodiesterase, or with 8-CPT-cAMP (0.2 mM). Treatment with FSK led to a 3.7±0.4-fold increase (p<0.0001, n=8), while 8-CPT-cAMP treatment led to a 3.0±0.3-fold increase (p<0.0001, n=8) in Ip3r2 message levels (Figure 2a). Similarly, treatment with FSK led to a 2.1+0.2-fold increase (p<0.001, n=5), while 8-CPT-cAMP treatment led to a 1.5+0.1-fold increase (p<0.001, n=5) in IP3R2 protein levels (Figure 2b,c). These data indicate that cAMP increases IP3R2 expression in rat hepatocytes. To verify that the increase in Ip3r2 mRNA levels was transcriptionally mediated, rat hepatocytes were incubated with or without the RNA synthesis inhibitor Actinomycin D (ACM) for 5 hr, then treated with FSK or 8-CPT-cAMP as above (Figure 2d). ACM significantly reduced the increase in Ip3r2 message induced by either FSK or 8-CPT-cAMP (p<0.05 by one-way ANOVA with Bonferroni’s post-tests). These results provide evidence that cAMP increases Ip3r2 transcription, although the residual increase in Ip3r2 mRNA seen in the presence of ACM might suggest additional potential mechanisms whereby cAMP could activate Ip3r2 message half-life.
Figure 2. Cyclic AMP increases hepatic expression of IP3R2.
Rat hepatocytes were isolated and then incubated with FSK (50 μM) plus IBMX (250 μM) or with 8-CPT-cAMP (0.2 mM) for 12 hrs. (a) Ip3r2 mRNA was measured by qPCR (*p<0.0001, n=8) and (b) IP3R2 was measured by densitometry of immunoblots (*p<0.001, n=5); Representative immunoblot shown in (c). (d) The increase in Ip3r2 message induced by cAMP was significantly lower in the presence of the mRNA synthesis inhibitor Actinomycin D (ACM; 1 μg/ml) for 5 hr (#p<0.05 by one-way ANOVA with Bonferroni’s post-tests, n=7).
Identification of cyclic AMP response elements (CRE) in the IP3R2 promoter
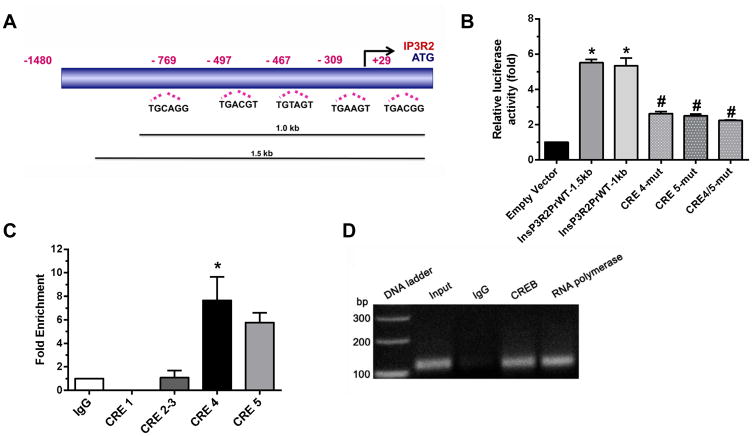
To understand the transcriptional regulation of the IP3R2/Ip3r2 gene, a 2 kb IP3R2 promoter including 321 bp of the 5′-UTR) was cloned upstream of the reporter gene luciferase in the pGL4-vector backbone. The BAC clone AC 024093 (RP11-791I2) was used with the forward and reverse primers listed in Table 1. Truncated fragments of the promoter of 1.5, 1.0 and 0.5 kb in length were also generated using additional internal primers (Table 1), in conjunction with the reverse primer. The sequence obtained from the 2kb promoter was analyzed with Matinspector (www.genomatix.de) to identify potential transcription factor sites within this region of the promoter. Identified sites included: (1) GC Box/Sp-1 (at +313, −151, −232, −218, −352), (2) CAAT box (at +286 3), (3) AP-1 (−24), (4) VDR/RXR (−29), (5) ER (at −29 and −376), (6) NFAT (at −268, −505, −605 −772) 7), (7) PPAR alpha/RXR (at −772 and – 1520), (8) PPARγ/RXR (−1577), and (9) Calcium response element (CARE) (at −1323). In addition, five CRE’s were identified within the 2 kb promoter (Figure 3a). These were located at (1) +81 (CRE1), (2) – 497(CRE2), (3) – 691(CRE3), (4) – 707(CRE4), and (5) −1423 (CRE5), respectively, with the Transcription Start Site (TSS) at +1.
Figure 3. CREB activates the IP3R2 promoter via binding to CRE4 and 5.
(a) Schematic illustrating the five putative CRE sites in the IP3R2 promoter. (b) HepG2 cells transfected with 1.5 kb or 1.0 kb segments of the IP3R2 promoter showed >20-fold greater activity when co-transfected with CREB, relative to empty vector (*p<0.0001, n=4), while mutation of CRE4, CRE5, or both markedly reduced activation by CREB (#p<0.001, n=4). There was no effect when CRE sites 1, 2 or 3 were mutated (not shown). (c) Summary of qPCR ChIP assay experiments shows that there was no enrichment in the CREB binding to CRE’s 1 and 2–3 over IgG control, but CREB binding was higher to CRE4 and 5. The increase was significant for CRE5 (*p<0.01, n=3) and marginally significant for CRE4 (0.1>p>0.05, n=3) compared to IgG. (d) Representative ChIP assay.
CREB activates the IP3R2 promoter via binding to CRE4 and 5
Cyclic AMP can activate IP3R2 via PKA-mediated phosphorylation [4, 12] but it is not known if cAMP can transcriptionally activate the IP3R2 gene via PKA-mediated phosphorylation of CREB. To understand the functional significance of the CRE sites on the IP3R2 promoter, HepG2 cells were transfected with 1.5 kb and 1.0 kb segments of the promoter and co-transfected with CREB. Both the 1.5 and 1.0 kb promoter constructs showed >20-fold greater activity when co-transfected with CREB, relative to empty vector (22.3±0.34–fold for the 1.5 kb and 21.3±0.25-fold for the 1.0 kb promoter, respectively; p<0.0001, n=4)(Figure 3b). The promoter constructs also showed enhancement of reporter gene activity relative to controls in the absence of CREB co-transfection, indicating considerable basal promoter activity (data not shown). To determine whether this activation by CREB involves the CRE’s within the promoter, the sites were mutated using site-directed mutagenesis. Mutation of CRE4 and CRE5 abolished activation by CREB (21.3±0.25 vs 8.1±0.16–fold in the wild-type vs. CRE-4 mutated; p<0.001, n=4, and 22.3±8.5–fold in the wild-type vs. CRE-5 mutated; p<0.001, n=4), while there was no effect when CRE sites 1, 2 or 3 were mutated (Figure 3b). Combined mutation of CRE4 and 5 showed synergism, suggesting that there was cooperation between these sites (22.3 ± 0.35 vs 7.4 ± 0.1 in the wild-type vs CRE-4,5 double-mutated, n=4, p<0.001). To confirm that CREB was recruited and bound to CRE4 and 5 we performed ChIP assays (Figure 3c,d) using specific primers flanking the CRE site (Table 1). Quantitation of CREB binding by qPCR showed there was no enrichment in CREB binding to CRE1 and 2–3 (a single primer set was used because CRE2 and 3 were close to each other) over IgG control. In contrast, CREB binding to CRE4 and 5 were higher compared to IgG, providing further evidence that only CRE4 and 5 are involved in activation of the IP3R2 promoter by CREB, and that the promoter is activated by recruitment of CREB to those sites.
Effects of cAMP on IP3R2-mediated bile secretion in rat hepatocytes
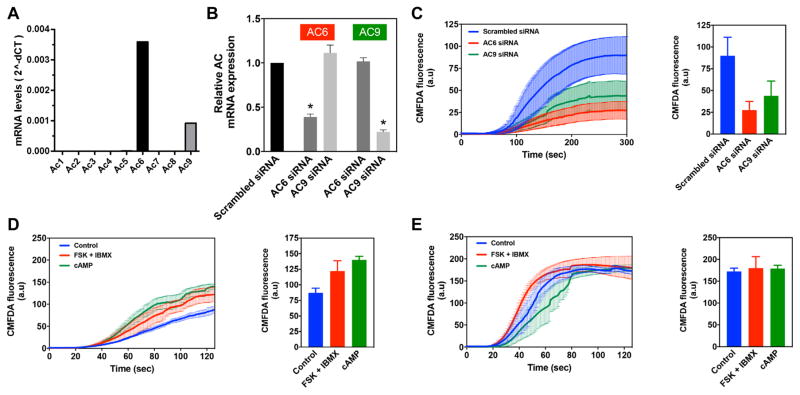
Because IP3R2 gene expression is increased by cAMP/PKA, we investigated the physiological effects of cAMP on IP3R2 function in rat hepatocytes. Hepatocytes secrete organic anions into bile via MRP2, and canalicular insertion and function of MRP2 depends on calcium release from IP3R2 [8]. The kinetics of secretion can be monitored by examining the accumulation of canalicular flourescence over time in hepatocytes in collagen sandwich culture incubated with CMFDA, which is converted to fluorescein and then secreted by MRP2 [8, 9, 20]. To validate the role of cAMP in this process, qPCR was used to determine that the only AC isoforms in primary rat hepatocytes are AC6 and AC9 (Figure 4a). AC6 was expressed at a four-fold higher level than AC9 (Figure 4a). Specific siRNAs were used to selectively reduce expression of AC6 or AC9 (by 61% or 78%, respectively; p<0.0001 by ANOVA with Dunnet’s post-test, n=10)(Figure 4b). Silencing either AC isoforom significantly reduced CMFDA-induced canalicular fluorescence (Figure 4c); silencing AC6 decreased canalicular fluorescence by 70%, while silencing AC9 decreased fluorescence by 51% (p<0.0001 by ANOVA with Dunnet’s post-test). This suggests that AC6- and AC9-mediated cAMP production each contributes to IP3R2-mediated secretion. Finally, to understand the relative roles of cAMP/PKA phosphorylation vs cAMP-mediated increased expression of IP3R2, secretion was compared in hepatocytes treated either acutely or for 12 hr with FSK+IBMX or CPT-cAMP (Figures 4d,e). Canalicular fluorescence was increased by 40–60% relative to untreated controls in hepatocytes treated for 12 hr (p<0.0001 by ANOVA). In contrast, fluorescence was increased by only 4% in hepatocytes in which cAMP was increased acutely (p<0.0001 by ANOVA). These findings suggest that the dominant physiological effect of cAMP on IP3R2-mediated bile secretion occurs through increased expression of IP3R2 rather than PKA phosphorylation of the receptor in this particular model system.
Figure 4. Effects of cAMP on IP3R2-mediated bile secretion.
(a) Quantitative PCR detected only two AC isoforms in rat hepatocytes, AC6 and AC9. (b) Specific siRNAs selectively silenced AC6 and AC9 (*p<0.0001 by ANOVA with Dunnet’s post-test, n=10). (c) Organic anion secretion is significantly reduced by knockdown of either AC6 or AC9 (p<0.0001 by repeated measures ANOVA with Dunnet’s post-test, n=5). Bar graph illustrates that peak fluorescence is reduced by 70% by treatment with AC6 siRNA and by over 50% by AC9 siRNA. Secretion in this and the next two panels was measured by time-lapse confocal microscopic measurement of canalicular accumulation of fluorescence in rat hepatocytes in collagen sandwich culture that were incubated with CMFDA. (d) Organic anion secretion is significantly increased by 12 hr of treatment to increase cAMP with either FSK+IBMX or 8-CPT-cAMP (p<0.0001 by repeated measures ANOVA with Dunnet’s post-test, n=3). Bar graph illustrates that peak fluorescence is increased by 40% by FSK+IBMX and by over 60% by 8-CPT-cAMP. (e) Organic anion secretion is altered by acute treatment to increase cAMP with either FSK+IBMX or 8-CPT-cAMP (p<0.0001 by repeated measures ANOVA with Dunnet’s post-test, n=4). However, the principal difference is in the kinetics as canalicular fluorescence increases. The bar graph illustrates that peak fluorescence is increased by only 4% by treatment with either FSK+IBMX or 8-CPT-cAMP.
DISCUSSION
IP3R2 is one of three isoforms of the IP3R. Each one is an IP3-gated calcium channel in the ER, but there is variability among the three isoforms in biophysical properties and subcellular and tissue distribution [1]. IP3R2 is important for secretion in the types of epithelial cells that express it. For example, missense mutations in IP3R2 in humans results in impaired secretory function in eccrine sweat glands, which in turn causes anhidrosis [22]. IP3R2 is the predominant IP3 receptor isoform in both rodent and human hepatocytes, where it is localized to the region of the ER beneath the canalicular membrane [5, 23]. This expression and localization of IP3R2 is important for membrane insertion and proper function of the bile acid export pump BSEP [9] and the organic anion transporter MRP2 [8], two of the most important transporters for bile formation. Either decreased expression or improper subcellular localization of IP3R2 results in impaired bile secretory activity, and IP3R2 expression is decreased in animal models of impaired bile secretion as well [9]. The current study takes advantage of a previously validated model system of primary hepatocytes in short-term culture that permits the proper expression and subcellular localization of IP3R2 to be directly related to its physiological effect as a regulator of bile secretion [8, 9, 20]. Although previous studies had shown that decreased IP3R2 expression results in impaired secretion, this model system also enabled us to provide evidence that increased IP3R2 expression may increase secretion. The observation that hepatic IP3R2 expression is increased by fasting furthermore suggests that this may be a common albeit previously unrecognized physiological mechanism for regulation of bile secretion.
Calcium signaling and thus IP3R function is important for regulation of hepatic glucose and lipid metabolism [6, 7, 19, 24]. Although IP3R2 accounts for ~80% of IP3Rs in hepatocytes [5], metabolism is minimally altered in IP3R2 KO mice [20]. IP3R1 accounts for the remaining ~20% of IP3R’s in hepatocytes [5], but this is the isoform that selectively couples to mitochondria in this cell type and therefore appears to be the one that is principally responsible for mediating metabolic effects such as lipid metabolism [24]. Both IP3R1 and IP3R2 are phosphorylated by PKA, and PKA phosphorylation of each isoform enhances calcium release [1, 4]. However, the PKA phosphorylation sites differ between the two isoforms, and this particular posttranslational modification is thought to enhance calcium release more in IP3R1 than IP3R2 [1, 4]. This difference in the effects of PKA between the two isoforms, combined with the difference in their subcellular distribution, may explain why phosphorylation of IP3Rs via cAMP/PKA strongly regulates the CREB co-regulator CRCT2 and thus hepatic glucose production [6], yet the effects of cAMP/PKA on bile secretion appear to result more from changes in expression rather than phosphorylation of IP3R (Figure 4). Cyclic AMP also can directly associate with IP3R2 to promote IP3-mediated calcium release, but this is thought to occur mostly at very high local concentrations of cAMP [15]. This effect furthermore may require co-localization of IP3R2 with AC6 [15], and this particular AC isoform is expressed in hepatocytes (Figure 4). However, it is not clear whether IP3R2 and AC6 co-localize in hepatocytes, and our results furthermore suggest that AC6 is no more effective than AC9 in modulating IP3R2-mediated secretion (Figure 4). In summary, the current study provides evidence for hormonal regulation of IP3R2 expression in hepatocytes, which in turn contributes to our understanding of IP3R isoform-specific effects of cAMP on liver physiology.
Supplementary Material
HIGHLIGHTS.
Fasting increases hepatic expression of the type 2 IP3 receptor
Cyclic AMP, via CREB, increases hepatic expression of the type 2 IP3 receptor
Increased expression of the type 2 IP3 receptor stimulates bile secretion
Acknowledgments
The authors express sincere gratitude to Christine Abu-Hanna, Program Manager of the Yale Liver Center, for administrative support on this project.
FUNDING
This work was supported by NIH grants P30 DK034989 (Morphology and Cellular/Molecular Cores), P01 DK057751, R01 DK045710, and R56 DK099470.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mikoshiba K. Role of IP3 receptor signaling in cell functions and diseases. Adv Biol Regul. 2015;57:217–227. doi: 10.1016/j.jbior.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. Molecular cell biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Wojcikiewicz RJ. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 4.Vervloessem T, Yule DI, Bultynck G, Parys JB. The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca(2)(+)-release channel. Biochim Biophys Acta. 2015;1853:1992–2005. doi: 10.1016/j.bbamcr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirata K, Pusl T, O’Neill AF, Dranoff JA, Nathanson MH. The type II inositol 1,4,5-trisphosphate receptor can trigger Ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485:128–132. doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nature medicine. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, Nathanson MH. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology. 2010;52:327–337. doi: 10.1002/hep.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruglov EA, Gautam S, Guerra MT, Nathanson MH. Type 2 inositol 1,4,5-trisphosphate receptor modulates bile salt export pump activity in rat hepatocytes. Hepatology. 2011;54:1790–1799. doi: 10.1002/hep.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra MT, Fonseca EA, Melo FM, Andrade VA, Aguiar CJ, Andrade LM, Pinheiro AC, Casteluber MC, Resende RR, Pinto MC, Fernandes SO, Cardoso VN, Souza-Fagundes EM, Menezes GB, de Paula AM, Nathanson MH, de Leite MF. Mitochondrial calcium regulates rat liver regeneration through the modulation of apoptosis. Hepatology. 2011;54:296–306. doi: 10.1002/hep.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagoudakis L, Garcin I, Julien B, Nahum K, Gomes DA, Combettes L, Nathanson MH, Tordjmann T. Cytosolic calcium regulates liver regeneration in the rat. Hepatology. 2010;52:602–611. doi: 10.1002/hep.23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betzenhauser MJ, Fike JL, Wagner LE, 2nd, Yule DI. Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J Biol Chem. 2009;284:25116–25125. doi: 10.1074/jbc.M109.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maxwell JT, Natesan S, Mignery GA. Modulation of inositol 1,4,5-trisphosphate receptor type 2 channel activity by Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated phosphorylation. J Biol Chem. 2012;287:39419–39428. doi: 10.1074/jbc.M112.374058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regimbald-Dumas Y, Fregeau MO, Guillemette G. Mammalian target of rapamycin (mTOR) phosphorylates inositol 1,4,5-trisphosphate receptor type 2 and increases its Ca(2+) release activity. Cell Signal. 2011;23:71–79. doi: 10.1016/j.cellsig.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Tovey SC, Dedos SG, Taylor EJ, Church JE, Taylor CW. Selective coupling of type 6 adenylyl cyclase with type 2 IP3 receptors mediates direct sensitization of IP3 receptors by cAMP. J Cell Biol. 2008;183:297–311. doi: 10.1083/jcb.200803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankar N, deTombe PP, Mignery GA. Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J Biol Chem. 2014;289:6188–6198. doi: 10.1074/jbc.M113.495242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W, Nurbaeva MK, Schmid E, Russo A, Almilaji A, Szteyn K, Yan J, Faggio C, Shumilina E, Lang F. Akt2- and ETS1-dependent IP3 receptor 2 expression in dendritic cell migration. Cell Physiol Biochem. 2014;33:222–236. doi: 10.1159/000356664. [DOI] [PubMed] [Google Scholar]
- 18.Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F, Sjaastad I, Liu N, Catalucci D, Mikoshiba K, Hisatsune C, Okkenhaug H, Andrews SR, Bootman MD, Roderick HL. Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J Cell Biol. 2012;199:783–798. doi: 10.1083/jcb.201111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, Wronska A, Chen BX, Marks AR, Fukamizu A, Backs J, Singer HA, Yates JR, 3rd, Accili D, Tabas I. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell metabolism. 2012;15:739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feriod CN, Nguyen L, Jurczak MJ, Kruglov EA, Nathanson MH, Shulman GI, Bennett AM, Ehrlich BE. Inositol 1,4,5-trisphosphate receptor type II (InsP3R-II) is reduced in obese mice, but metabolic homeostasis is preserved in mice lacking InsP3RII. American journal of physiology. Endocrinology and metabolism. 2014;307:E1057–1064. doi: 10.1152/ajpendo.00236.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klar J, Hisatsune C, Baig SM, Tariq M, Johansson AC, Rasool M, Malik NA, Ameur A, Sugiura K, Feuk L, Mikoshiba K, Dahl N. Abolished InsP3R2 function inhibits sweat secretion in both humans and mice. J Clin Invest. 2014;124:4773–4780. doi: 10.1172/JCI70720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata J, Guerra MT, Shugrue CA, Gomes DA, Nagata N, Nathanson MH. Lipid rafts establish calcium waves in hepatocytes. Gastroenterology. 2007;133:256–267. doi: 10.1053/j.gastro.2007.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feriod CN, Oliveira AG, Guerra M, Nguyen L, Mitchell-Richards K, Jurczak MJ, Ruan HB, Camporez JP, Yang X, Shulman GI, Bennett AM, Nathanson MH, Ehrlich BE. Hepatic Inositol 1,4,5 Trisphosphate Receptor Type I Mediates Fatty Liver. Hepatology Communications. 2017;1:23–35. doi: 10.1002/hep4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.