Abstract
Objective
It has been speculated that cancer survivors in Asia may have lower quality of life (QOL) compared with their western counterparts. However, no studies made international comparisons in QOL using a comprehensive measure. This study aimed to compare Chinese breast cancer survivors’ QOL with United States (US) counterparts and examine if demographic and medical factors were associated with QOL across groups.
Method
The sample consisted of 159 breast cancer patients (97 Chinese and 62 US) who completed the Functional Assessment for Cancer Therapy Breast Cancer Scale (FACT-B) before the start of radiotherapy in Shanghai, China and Houston, US.
Results
Higher income was associated with higher QOL total scores in both Chinese and American cancer patients, but QOL was not significantly associated with other factors including age, education, disease stage, mastectomy and chemotherapy. Consistent with hypotheses, compared to their US counterparts, Chinese breast cancer survivors reported lower QOL including FACT-G total scores and all four sub-dimensions including Functional Well-being (FWB), Physical Well-being (PWB), Emotional Well-being (EWB) and Social Well-being (SWB); they also reported more breast cancer specific concerns (BCS). Differences were also clinically significant for FACT-G total scores and the FWB subscale. After controlling for demographic and medical covariates, these differences remained except for the SWB and BCS. Furthermore, Chinese breast cancer survivors receiving chemotherapy reported significantly lower FACT-G scores than those who did not, but this difference did not emerge among US breast cancer survivors.
Discussion
Chinese breast cancer survivors reported poorer QOL on multiple domains compared to US women. Findings indicate that better strategies are needed to help improve the QOL of Chinese breast cancer survivors, especially those who underwent chemotherapy.
Keywords: quality of life, breast cancer, culture, country
Introduction
Cancer is one of the leading causes of mortality worldwide [1]. Asia represents 60% of the world’s population [2]. It is estimated to experience 45% of all new cancer cases in the world, and 50% of all cancer deaths in 2008 [3]. China is seeing a change in cancer rates [4] and currently observing a country-wide increase [5]. Breast cancer is among the most frequent types of cancer and alone accounted for 1,383,000 new cancer cases and 519,000 cancer related deaths in 2008 worldwide [1]. Since 1990, rates of breast cancer in China have increased 3% to 4% annually compared to a global annual increase of 0.5% [6]. As the effectiveness of cancer treatments continues to develop in China the number of breast cancer patients and survivors will continue to rise. As patients live longer, concern for psychological factors and quality of life (QOL) among this population has grown [7]. Although a growing number of studies have reported QOL in Asian populations, they focus on the validation of measurement and one population. Cross-country comparison of QOL can help to understand possible areas of intervention and how to design culturally sensitive interventions. However, no publications have compared the QOL between Asian and western breast cancer patients. This paper aims to compare differences in QOL between Chinese and United States (US) breast cancer patients.
In 1993, the World Health Organization (WHO) defined QOL as “individuals’ perceptions of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” [7]. This broad ranging concept is affected in a complex way by a person’s physical health, psychological state, level of independence, social relationships, and relationship to their environment [7]. Many methods have been documented in the literature for the purpose of evaluating the QOL in cancer patients. Of the twelve existing measures, the two most commonly used were the EORTC QLQ-C30 and the FACT [8]. The FACT-B was developed as a means to evaluate a spectrum of QOL components in breast cancer patients specifically. The FACT-B is validated in Chinese; however, no studies have directly compared responses on the FACT-B in Chinese populations to responses from US populations.
Despite the lack of studies comparing Chinese to US populations, there is reason to expect that Chinese cancer survivors may have lower QOL than Americans. For example, Asian American breast cancer survivors have reported lower QOL than their European counterparts [9, 10]; Chinese American survivors are more likely to experience poorer socioeconomic well-being than non-Hispanic White survivors [11]. Qualitative evidence has also shown that Chinese survivors describe more distress than Americans [12]. Based on these findings, we hypothesize that Chinese survivors may have lower QOL compared with the US population.
QOL has become a consistent index of adjustment and an end point in clinical trials in the West [8], but little research has characterized QOL issues in Chinese breast cancer patients. One study with newly diagnosed Chinese breast cancer patients found that income, time since diagnosis, marital status, and education were all independently associated with overall QOL [13]. Other studies in Chinese and US women have observed that younger age was associated with worse QOL in breast cancer patients [14–19]. Chinese breast cancer survivors reported that women who underwent breast conservation therapy had better body image compared to women who had mastectomy alone [20], consistent with results from studies with US women [21]. Patients who undergo chemotherapy have been found to report lower quality of life [22]. Other factors, e.g., stage of the disease, were also found to be associated with Chinese cancer survivors’ QOL [23–25]. Patients who undergo chemotherapy have been found to report lower quality of life [22], and this may be especially true for Chinese cancer patients. The present study therefore investigated how demographic and disease-related factors were associated with QOL in both countries.
This study was a secondary analysis of existing data from two intervention studies [26, 27]. The primary goal of this study was to compare Chinese breast cancer survivors’ QOL with US counterparts. The second goal was to examine how demographic and medical factors were associated with QOL across groups. We hypothesized that Chinese women would have lower QOL compared with the US women (i.e., hypothesis 1). Based on the literature reviewed above, we also hypothesized that lower income and education, younger age, later stage of diagnosis, and more aggressive treatment would be associated with worse QOL (i.e., hypothesis 2), independent of ethnicity. We finally explored whether medical factors differentially influenced QOL depending on ethnicity (Chinese vs. US). We hypothesized that having undergone chemotherapy prior to the start of radiotherapy (assessment point) and later stage of diagnosis would have a greater influence on QOL among Chinese than among US breast cancer survivors (i.e., hypothesis 3).
Methods
Participants
A total of 159 patients (97 Chinese and 62 American) participated in the study. Participants were recruited from two comparable intervention studies conducted in Shanghai, China and Houston, US. All the participants who enrolled in these studies were included in this study and met all inclusion and exclusion criteria of parent studies, which were the same criteria for this study. Detailed information on the study methods has been published previously [26, 27]. Eligible Chinese patients were identified by physicians and research nurses at the breast cancer clinic. These patients were scheduled for radiotherapy at Fudan University Shanghai Cancer Center (FUSCC) in Shanghai, China. Eligible US patients were identified through the CARES database, which is an institutional database that keeps track of patient schedules at MD Anderson Cancer Center. These patients were undergoing radiotherapy in the Department of Radiology Oncology, at MD Anderson Cancer Center. Inclusion criteria were 1) women 18 years or older, 2) with stage 0-III breast cancer, and 3) completed surgery and/or chemotherapy and had not started radiotherapy. Additional inclusion criteria were reading, writing and speaking fluency in Chinese for Chinese women or English for US women. The study excluded patients with any major psychiatric diagnoses and metastatic disease.
Procedures
Patients were recruited and provided written informed consent prior to the start of radiotherapy. All patients had completed surgery and/or chemotherapy prior to consent. In a Qigong intervention study, 123 Chinese patients were approached, and 100 patients consented and were randomized, and 96 completed the survey, yielding a response rate of 96%. In a Yoga intervention study, 137 of the US patients were approached, 81 consented, 71 were randomized and 61 completed the survey, resulting in a response rate of 75.3%. After patients consented to the study and before they were randomized to the experimental or control groups, a 45-minute battery of questionnaires was given at baseline to measure QOL and demographic information and medical data was extracted from patient charts and electronic medical record. The MD Anderson Institutional Review Board approved both studies and the Fudan University IRB approved the Chinese study.
Measures
QOL was measured by FACT-B –Version 4. This measure is validated for both Chinese and US breast cancer patients [28, 29]. Participants respond on a Likert scale ranging from 0 (not at all) to 4 (very much). The instrument has a total of 36 statements asking respondents to rate how true each statement is for the last seven days. One of the items in the social well-being sub-dimension asked about sexual satisfaction and was largely skipped by Chinese participants; therefore this item was excluded from the analysis in this paper. The FACT-B consists of the Functional Assessment for Cancer therapy general scale (FACT-G) [28], with the addition of breast cancer-specific questions. The FACT-G has four sub-scale scores of physical well-being (e.g., “I have nausea.”), functional well-being (e.g., “I am able to work, including work at home.”), emotional well-being (e.g., “I feel nervous.”), and social/family well-being (e.g., “I am satisfied with family communication about my illness.”) and summed for a total score with greater scores indicating higher QOL. The BCS subscale addresses breast cancer specific concerns (e.g., “One or both of my arms are swollen or tender.”), with higher scores on this dimension indicating less concerns and better QOL. In this current study, for group comparison, we reported the FACT-G subscale and total scores and BCS subscale separately so that future studies with non-breast and breast cancer survivors can compare the FACT-G score with our report. Prior literature demonstrates that the alpha coefficients of the whole scale are .92 and .90, and for each subscale ranges from .82 to .88 and from .82 to .85 in US and Chinese samples, respectively.
Data Analyses
In the preliminary analyses, descriptive statistics were computed within each of the cultural samples and cultural group comparisons of all the variables were conducted with ANOVAs or Chi-square tests. Correlation coefficients of all variables were computed with Pearson correlations, Spearman correlations, or cross-tabulations. For all the analyses below, we first used the FACT-G total score and the BCS score as the dependent variable. When group differences emerged in the FACT-G total score, each subscale of FACT-G was used as a dependent variable to further illustrate cultural differences in a particular domain of QOL.
To test hypothesis 1, ANOVAs were performed with cultural groups as an independent variable. To rule out the possibility that the findings were confounded with demographic and cancer-related characteristics, ANCOVAs were conducted controlling for all the demographic and medical variables including age, disease stage, surgery type (mastectomy vs. conservation breast surgery), chemotherapy (yes vs. no), income, and education. When statistically controlling for income, we used the relative income compared with the mean within the group, rather than the absolute value to adjust for country-related differences in income. To test hypothesis 2, regression analyses were used with QOL and subscales as dependent variables and with all demographic and medical variables (age, disease stage, surgery type, chemotherapy, income, and education) entered as independent variables. To test hypothesis 3, ANCOVAs were conducted to examine how disease stage and chemotherapy would separately interact with cultural groups in predicting QOL when controlling for all demographic and medical variables. For significant interaction effects, we conducted simple effect analyses to illustrate how these variables would be differently associated with QOL within each of the two cultural samples [30].
Results
Sample characteristics and country comparisons are shown in Table 1. Compared with the US sample, the Chinese sample was younger, poorer, less educated, and had a higher percentage of women that had undergone chemotherapy, even though there were no disease stage differences. ANOVAs for hypothesis 1 showed that Chinese breast cancer survivors reported lower scores for FACT-G total, all FACT-G subscales, and BCS than the US counterparts (Table 2). ANCOVA analyses revealed that after controlling for covariates including age, disease stage, mastectomy, chemotherapy, income, and education, the above cultural differences remained significant except for BCS and the SWB subscale.
Table 1.
Demographic and Cancer-related Characteristics of the Samples
| Total N=159 n (%) |
Chinese N=97 n (%) |
United States N=62 n (%) |
F/χ2 | df | P | |
|---|---|---|---|---|---|---|
| Age | 15.53 | 2 | <.001 | |||
| 25–45 years | 54 (34.0) | 38 (39.2) | 16 (25.8) | |||
| 46–55 years | 61 (38.4) | 43 (44.3) | 18 (29.0) | |||
| 56–68 years | 44 (27.7) | 16 (16.5) | 28 (45.2) | |||
| Annual Personal Income | 15.66 | 2 | <.001 | |||
| Below average | 9 (5.7) | 6 (6.2) | 3 (4.8) | |||
| Average | 50 (31.4) | 43 (44.3) | 7 (11.3) | |||
| Above average | 65 (40.9) | 33 (34.0) | 32 (51.7) | |||
| Missing | 35 (22.0) | 15 (15.5) | 20 (32.3) | |||
| Educational Attainment | 35.11 | 2 | <.001 | |||
| High school or lower | 51 (32.1) | 44 (45.4) | 7 (11.3) | |||
| College | 80 (50.3) | 47 (48.4) | 33 (51.3) | |||
| Graduate degree | 25 (15.7) | 4 (4.1) | 21 (33.9) | |||
| Missing | 3 (1.9) | 2 (2.1) | 1 (1.6) | |||
| Disease Stage | 1.11 | 3 | .78 | |||
| 0–I | 47 (29.5) | 28 (28.9) | 19 (30.7) | |||
| II | 62 (39.0) | 35 (36.1) | 27 (43.5) | |||
| III | 40 (25.2) | 24 (24.7) | 16 (25.8) | |||
| Missing | 10 (6.3) | 10 (10.3) | 0 (0) | |||
| Mastectomy | 2.16 | 1 | .14 | |||
| Yes | 79 (49.7) | 53 (54.6) | 26 (41.9) | |||
| No | 79 (49.7) | 44 (45.4) | 35 (56.5) | |||
| Missing | 1 (.6) | 0 (0) | 1 (1.6) | |||
| Chemotherapy | 15.73 | 1 | <.001 | |||
| Yes | 22 (13.8) | 92 (94.8) | 45 (72.6) | |||
| No | 137 (86.2) | 5 (5.2) | 17 (27.4) |
Notes. The cut-off points of average and low income are retrieved from government report for each cultural sample, which are $8,000 and $1,500 (currency rate: 6.34 Yuan = $1 USD) in the Chinese sample, and are $50,000 and $20,000 in the US sample.
Table 2.
Mean, Standard Deviation, Comparison of Quality of Life between Chinese and United States Breast Cancer Patients
| Chinese (N = 97) |
United States (N = 62) |
F | df | P | ηp2 | |
|---|---|---|---|---|---|---|
| FACT-G (26 items) | 72.45 (15.31) | 83.30 (12.25) | 22.10 | 1 | <.001 | .123 |
| PWB (7 items) | 19.73 (5.27) | 22.52 (4.13) | 12.46 | 1 | .001 | .073 |
| SWB (6 items) | 19.29 (4.07) | 21.34 (3.58) | 10.54 | 1 | .001 | .063 |
| EWB (6 items) | 17.68 (4.46) | 19.92 (3.27) | 11.58 | 1 | .001 | .069 |
| FWB (7 items) | 15.75 (5.19) | 19.53 (5.27) | 19.84 | 1 | <.001 | .112 |
| BCS (9 items) | 22.30 (4.88) | 24.13 (4.67) | 5.51 | 1 | .020 | .034 |
Notes. Abbreviation: Functional Well-being (FWB), Physical Well-being (PWB), Emotional Well-being (EWB) and Social Well-being (SWB); Breast cancer specific concerns (BCS).
Regression analyses for hypothesis 2 for the combined populations revealed that after controlling for other demographic and medical variables, income was positively associated with FACT-G total scores (β = .31, p =.001), and three subscales of FACT-G, including PWB (β = .21, p = .03), SWB (β = .38, p < .001), and FWB (β = .29, p = .002). FACT-G was not significantly associated with other factors including age, education, disease stage, mastectomy and chemotherapy. However, age was positively associated with BCS (β = .18, p = .04), and having chemotherapy was negatively associated with PWB (β = −.21, p = .04), after controlling for the other demographic and medical variables.
Analyses for hypothesis 3 found significant interactions between cultural group and chemotherapy predicting FACT-G total scores (F (1, 138) = 6.63, p =.01, ηp2 = .046) even after controlling for demographic and other medical covariate variables. Simple effect analysis demonstrated that Chinese breast cancer survivors receiving chemotherapy (M = 71.55, SD = 14.52) reported significantly lower FACT-G than those who did not (M = 86.20, SD = 12.44), F (1, 138) = 7.73, p = .006, but such difference did not emerge among American breast cancer survivors, F (1, 138) = 1.94, ns (see Figure 1). Subscale analyses revealed that cultural group × chemotherapy interaction effect were significant on PWB (F (1, 138) = 4.00, p =.047, ηp2 = .028) and EWB (F (1, 138) = 5.95, p =.016, ηp2 = .041). Chinese breast cancer survivors who had chemotherapy (M PWB= 19.44, SD = 5.21) reported significantly lower PWB than those who did not (M PWB = 25.00, SD = 3.32), F (1, 138) = 11.63, p =.001. However, US breast cancer survivors who had chemotherapy (M = 20.50, SD = 2.71) reported better EWB than those who did not (M = 18.50, SD = 3.87) (F (1, 138) = 4.87, p =.03).
Figure 1.
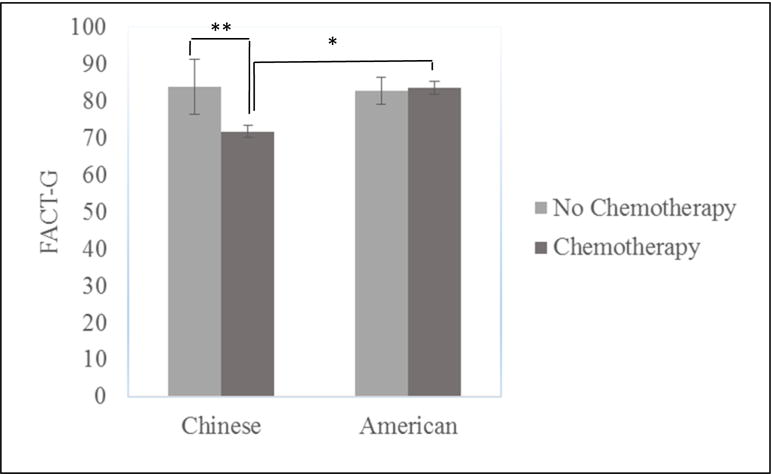
Interaction Effects between Chemotherapy and Cultural Group on FACT-G.
*p< .05, **p< .01
Because the Chinese sample had a significantly higher percentage (94.8%) undergoing chemotherapy compared with the US sample (72.6%) and only 5 Chinese women did not receive chemotherapy, we also compared QOL among those with chemotherapy controlling for other covariates. Chinese breast cancer survivors receiving chemotherapy had significantly lower FACT-G (F(1,119)=11.97, p =.001, ηp2 = .091), PWB (F(1,119)=5.81, p =.018, ηp2 = .047), EWB (F(1,119)=9.17, p =.003, ηp2 = .072), and FWB (F(1,119)=9.53, p =.003, ηp2 = .074) than did their US counterparts, and no group differences emerged for SWB (F(1,119)=3.05, ns) or BCS (F(1,119)=1.38, ns).
The cultural group × disease stage interaction was also significant for FACT-G even after controlling for demographic and other medical variables (F (2, 136) = 4.32, p =.05, ηp2 = .06; see Figure 2). Simple effect analysis revealed that Chinese breast cancer survivors with stage II (M = 66.58, SD = 2.40) had significantly lower FACT-G than those with stages 0-I (M = 78.86, SD = 12.30), F (2, 136) = 7.50, p = .001, but such difference did not exist in the US sample (F (2, 136) = 1.73, ns). The Chinese breast cancer survivors scored lower on FACT-G compared to the U.S. women if they were at stage II (F (1, 136) = 12.86, p < .001) and stage III (F (1, 136) = 5.05, p = .03) but not at stage 0-I (F (1, 136) = .52, ns). Subscale analyses showed that cultural group interacted with disease stage on EWB (F (2, 136) = 3.78, p =.03, ηp2 = .05) only. Simple effect analyses demonstrated that Chinese survivors at stage II (M = 15.97, SD = 4.49) displayed significantly lower EWB than those at stage 0-I (M = 19.54, SD = 3.04) (F (2, 136) = 7.59, p = .001), but such difference did not exist in the US sample. No significant group and disease stage interaction merged for BCS.
Figure 2.
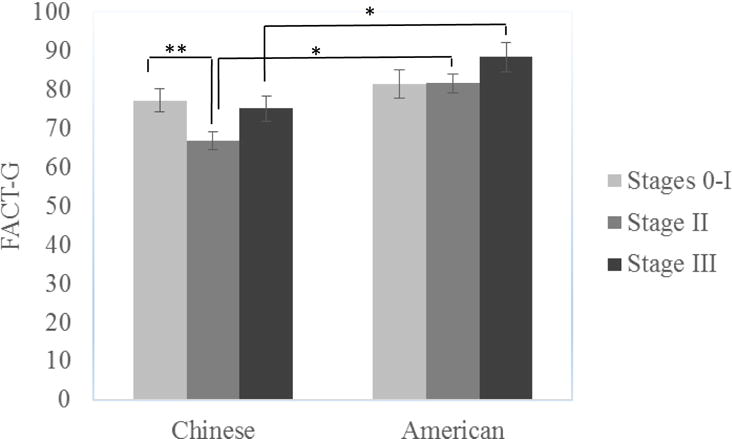
Interaction Effects between disease stage and Cultural Group on FACT-G
*p< .05, **p< .01
Discussion
Although the rates of breast cancer have been rising in Asian populations [5], there has been a lack of understanding of the QOL among Asian cancer survivors. Furthermore, previous studies have not compared Asian breast cancer survivors’ QOL with westerners. Studies that have been conducted separately, either in the US or China, are difficult to compare as a result of inconsistencies in the time points assessed, tools used for assessment, and the population of breast cancer patients examined. This is the first study that has compared responses on the FACT-B in Chinese and US women with breast cancer. Both populations were obtained from a similar group of patients and examined at the same time point (before the start of radiotherapy).
This study revealed that Chinese breast cancer survivors had lower overall FACT-G total scores compared to the US women. Furthermore, Chinese women reported lower levels of functional, physical, social, and emotional well-being and more breast cancer concerns than US women. A difference in FACT-G total scores of 5–7 points is indicative of clinically significant QOL changes/differences [31]. On average, Chinese women were 11 points lower on the FACT-G total score compared to the US, which is considered a clinically significant difference. Moreover, the differences remained pronounced in all multiple domains of QOL including functional, physical, and emotional domains even after controlling for age, disease stage, mastectomy, chemotherapy, income, and education. The more salient differences emerged for the functional well-being subscale (ηp2=.112), where differences were also clinically significantly different (>3).
The Chinese sample was poorer, younger, less educated, and more likely to have undergone chemotherapy compared with the US sample. The finding that Chinese women were younger on average than US women is consistent with prior research showing that Chinese women are being diagnosed with breast cancer at a younger age than US women [32]. Even after statistically controlling for these variables, the Chinese women still had worse quality of life. This suggests that perhaps symptom control strategies were not as aggressive for the Chinese as the US women. Nevertheless, it is still possible that income and greater use of chemotherapy could be reasons for country differences in QOL. Those who have undergone chemotherapy have been found to report lower quality of life [22]. It could be possible that Chinese patients undergo more aggressive treatment or take drugs that have more adverse side-effects. Yet, symptom control strategies may also be different, and these data were not collected. Further investigation is needed.
The interaction effect also provided some possible explanation. Chinese patients who underwent chemotherapy were at later cancer stages and had a much worse quality of life compared to their US peers, whereas Chinese patients who did not receive chemotherapy and were at an early cancer stage were similar to their US peers. These findings suggest that more attention needs to be paid to improve QOL among those with chemotherapy and those at more advanced cancer stages. We did not find surgery type was differentially linked to QOL. Future studies need to investigate symptom control strategies that may have contributed to the country differences in QOL.
Higher income was associated with higher QOL total score in both Chinese and US samples, a finding consistent with previous studies in Caucasian populations [10, 15, 33]. Past research also suggests that younger age and less education are associated with poor QOL [22, 34, 35]. We only found an association between younger age and worse QOL in the US breast cancer survivors. This may be a result of the small sample size, relative homogeneity of the samples, the fact that the Chinese women were significantly younger and less educated than the US women, and confounded by other medical and demographic facts known to be associated with QOL. Studies in Chinese populations have inconsistently found associations with stage of disease and some scales of the FACT-G [13, 36]. In some studies, FACT total score included the breast cancer concern sub-dimension and others did not include this sub-dimension. In order to easily make the comparisons between this study and other studies reported FACT scores, we calculated the FACT-G total separately from the BCS scale, and reported the four sub-dimensions and breast cancer concerns separately so that future studies can make comparisons with ours.
Comparison of responses on the FACT-B in Asian and US breast cancer patients has not been previously conducted. The FACT-B has been used in many studies in US breast cancer patients and even with the differences in the time QOL was assessed across studies [15, 19, 37], scores on the FACT-B subscale scores were similar to our US sample. A previous study validated the FACT-B in Chinese breast cancer inpatients at an Oncological Hospital in Yunnan providence [29]. The women in that study scored lower in all FACT-B scales compared to Chinese women in our study. The women in our study were treated in Shanghai at one of the best hospitals in China. If the women in our study have better QOL than Chinese women treated in other regions, the differences between Chinese women from regional hospitals and US women may even be larger.
Several caveats of the current study are worth mentioning. The study examined the country difference in QOL with two convenience samples, which limits the generalizability. However, the Chinese women in our study reported higher QOL than Chinese women in two other studies, suggesting that the major conclusion of the study that Chinese women had worse QOL could be generalized to Chinese women from other regions within China. Second, the small sample size limited our analyses of interactions between covariates and cultural groups. The cells had particularly smaller number for breast cancer survivors without chemotherapy in the analysis for cultural group by chemotherapy interaction. In addition, a limited number of covariates were examined. Other covariates that have been shown to be associated with QOL in both Chinese and US populations need to be included as well; these factors include marital status, time since diagnosis, co-morbidity factors, and social support [23–25, 36]. Other factors have also been shown to influence QOL, such as pain, fatigue and anxiety [18]. Future studies should examine the relationship between these factors and QOL in both groups. We were also not able to extract medical data related to symptom control strategies used for the women such as medications for nausea and vomiting, fatigue, and sleep disturbances. Differences in symptom control strategies may explain some of the QOL differences. Finally, although the FACT-B is validated in Chinese, it may not be completely comparable across populations and contain questions that introduce bias into study results. Future studies using a mixed paradigm with both qualitative and quantitative data may shed light into the cultural equivalence of the questions.
In sum, this study demonstrated that Chinese breast cancer survivors had worse QOL compared with US counterparts, and these difference were clinically significant. Treatment and cancer stage may have contributed to group differences. However, extra efforts are needed to help improve QOL of Chinese breast cancer patients. Future studies are warranted to further understand what contributed to country differences in QOL and how to design better behavioral and medical interventions to improve women’s lives in counties where QOL needs to be improved.
Acknowledgments
Support was provided in part by the United States National Cancer Institute (NCI) grants CA108084 and CA121503 (principal investigator, Lorenzo Cohen) and the American Cancer Society MRSGT-10-011-01-CPPB (principal investigator: Qian Lu). Partial support for Lorenzo Cohen was provided by the Richard E. Haynes Distinguished Professorship in Clinical Cancer Prevention.
Footnotes
Financial Disclosures: There are no financial disclosures from any authors.
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. World Population Prospects: The 2012 Revision, in Total Population - Both Sexes. 2012 [Google Scholar]
- 3.Centers of Disease Control and Prevention. Cancer Survivors — United States, 2007. MMWR. 2011;60:269–272. [Google Scholar]
- 4.Shin HR, et al. Cancer in Asia - Incidence rates based on data in cancer incidence in five continents IX (1998–2002) Asian Pac J Cancer Prev. 2010;11(Suppl 2):11–6. [PubMed] [Google Scholar]
- 5.Shin HR, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–85. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.WHOQOL group. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL) Qual Life Res. 1993;2:153–9. [PubMed] [Google Scholar]
- 8.Lemieux J, et al. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001–2009) J Natl Cancer Inst. 2011;103:178–231. doi: 10.1093/jnci/djq508. [DOI] [PubMed] [Google Scholar]
- 9.Ashing-Giwa KT, Lim J. Predicting physical quality of life among a multiethnic sample of breast cancer survivors. Quality Of Life Research. 2010;19(6):789–802. doi: 10.1007/s11136-010-9642-4. [DOI] [PubMed] [Google Scholar]
- 10.Ashing-Giwa KT, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncology Nursing Forum. 2009;36:79–88. doi: 10.1188/09.ONF.79-88. [DOI] [PubMed] [Google Scholar]
- 11.Wang JHY, et al. A mixed method exploration of survivorship among Chinese American and non-Hispanic White breast cancer survivors: the role of socioeconomic well-being. Quality of Life Research. 2013;22(10):2709–2721. doi: 10.1007/s11136-013-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JHY, et al. Physical distress and cancer care experiences among Chinese-American and non-Hispanic White breast cancer survivors. Gynecologic Oncology. 2012;124(3):383–388. doi: 10.1016/j.ygyno.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Y, et al. The long-term impact of medical and socio-demographic factors on the quality of life of breast cancer survivors among Chinese women. Breast Cancer Res Treat. 2004;87:135–47. doi: 10.1023/B:BREA.0000041620.76871.97. [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA, et al. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21:4184–93. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 15.Kwan ML, et al. Quality of life among women recently diagnosed with invasive breast cancer: the Pathways Study. Breast Cancer Res Treat. 2010;123:507–24. doi: 10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salonen P, et al. Telephone intervention and quality of life in patients with breast cancer. Cancer Nurs. 2009;32:177–90. doi: 10.1097/NCC.0b013e31819b5b65. quiz 191–2. [DOI] [PubMed] [Google Scholar]
- 17.So WK, et al. Age-related differences in the quality of life of Chinese women undergoing adjuvant therapy for breast cancer. Res Gerontol Nurs. 2011;4:19–26. doi: 10.3928/19404921-20101201-01. [DOI] [PubMed] [Google Scholar]
- 18.So WK, et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010;14:17–22. doi: 10.1016/j.ejon.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel LB, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–74. [PubMed] [Google Scholar]
- 20.Fung KW, et al. The impact of mastectomy, breast-conserving treatment and immediate breast reconstruction on the quality of life of Chinese women. ANZ J Surg. 2001;71:202–6. doi: 10.1046/j.1440-1622.2001.02094.x. [DOI] [PubMed] [Google Scholar]
- 21.Wainstock JM. Breast cancer: psychosocial consequences for the patient. Semin Oncol Nurs. 1991;7:207–15. doi: 10.1016/0749-2081(91)90034-m. [DOI] [PubMed] [Google Scholar]
- 22.Ganz PA, et al. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–87. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H, et al. Social support and quality of life among Chinese breast cancer survivors: Findings from a mixed methods study. European Journal of Oncology Nursing. 2013;17(6):788–796. doi: 10.1016/j.ejon.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 24.So WK, et al. Associations between social support, prevalent symptoms and health-related quality of life in Chinese women undergoing treatment for breast cancer: A cross-sectional study using structural equation modelling. European Journal of Oncology Nursing. 2013;17(4):442–448. doi: 10.1016/j.ejon.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 25.So WK, et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncology nursing forum. 2009;36(4):E205–14. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- 26.Chandwani KD, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43–55. [PubMed] [Google Scholar]
- 27.Chen Z, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer. 2013;119:1690–8. doi: 10.1002/cncr.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella DF, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 29.Wan C, et al. Validation of the simplified Chinese version of the FACT-B for measuring quality of life for patients with breast cancer. Breast Cancer Res Treat. 2007;106:413–8. doi: 10.1007/s10549-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 30.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park: SAGE; 1991. [Google Scholar]
- 31.Cella D, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:285–95. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 32.Kwong A, Chu AT. Experience of Southern Chinese: new challenges in treating young female breast cancer patients at child-bearing age–a call for multi-disciplinary collaboration. Asian Pac J Cancer Prev. 2012;13:3535–7. [PubMed] [Google Scholar]
- 33.Shimozuma K, et al. Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat. 1999;56:45–57. doi: 10.1023/a:1006214830854. [DOI] [PubMed] [Google Scholar]
- 34.Ganz PA, Lee JJ, Siau J. Quality of life assessment. An independent prognostic variable for survival in lung cancer. Cancer. 1991;67:3131–5. doi: 10.1002/1097-0142(19910615)67:12<3131::aid-cncr2820671232>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 35.Parker PA, et al. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003;12:183–93. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- 36.Lu W, et al. Impact of newly diagnosed breast cancer on quality of life among Chinese women. Breast Cancer Res Treat. 2007;102:201–10. doi: 10.1007/s10549-006-9318-5. [DOI] [PubMed] [Google Scholar]
- 37.Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23:3322–30. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]


