Abstract
Body weight–supported treadmill training (BWSTT) developed from animal studies of spinal cord injury (SCI). Evidence that spinal cats (i.e., cats that have a complete surgical transection of the cord) could regain the ability to step on a moving treadmill indicated a vast potential for spinal circuits to generate walking without the brain. BWSTT represented a means to unlock that potential. As the technique was adapted as a rehabilitation intervention for humans with SCI, shortcomings in the translation to walking in the real world were exposed. Evidence that BWSTT has not been as successful for humans with SCI leads us to revisit key animal studies. In this short review, we describe the task-specific nature of BWSTT and discuss how this specificity may pose limits on the recovery of overground walking. Also discussed are more recent studies that have introduced new strategies and tools that adapt BWSTT ideas to more functionally-relevant tasks. We introduce a new device for weight-supported overground walking in rats called Circular BART (Body weight supported Ambulatory Rat Trainer) and demonstrate that it is relatively easy and inexpensive to produce. Future animal studies will benefit from the development of simple tools that facilitate training and testing of overground walking.
Keywords: : locomotor function, recovery, spinal cord injury
Introduction
It has been nearly 30 years since Barbeau and colleagues first described a novel system for gait rehabilitation that included a weight support apparatus and a treadmill.21 The effects of body weight–supported treadmill training (BWSTT) have since been examined in hundreds of studies, yet its use in rehabilitation following spinal cord injury (SCI) and stroke remains controversial. There have been a number of reviews that provide differing perspectives on the clinical value of BWSTT.1–5 Here, we will review animal studies that gave rise to BWSTT. Given that BWSTT has not been as successful in humans, it is reasonable to ask, what did the pioneering animal studies tell us about stepping after a SCI? These studies were mostly performed in cats whose spinal cords were completely transected, and thus were known as the spinal cat studies. It is our hope that by revisiting these studies, we will be reminded how they changed our notions about spinal cord function and revealed astonishing plasticity inherent in the lumbar circuitry. We will explore the task-specific nature of BWSTT and how that has limited its therapeutic potential. Finally, we will take a look at strategies and tools that may be helpful in future animal studies. Rather than a comprehensive review, the focus here will be on key findings in the long history of BWSTT experiments. (More in-depth reviews can be found elsewhere.6–9) We hope the reader will excuse the interjection of some personal experiences working with spinal cats to bring a viewpoint that is left out of published articles.
The Tantalizing Promise of Treadmill Training
“…[T]he hindlimbs (of spinal kittens) can be put on a treadmill band while the forelimbs are standing on a platform…The hindlimbs then walk with a speed related to that of the treadmill…” Grillner (1975)10
A century ago, Sir Charles Sherrington reported that if the spinal cord was surgically transected at a low thoracic level, dogs were capable of “spinal stepping,” meaning their hindlimbs performed rhythmic alternating movements.10 Once in a while, if the hindquarters were positioned just right, the spinalized animals took a few steps before falling over. Years later, however, it was Sten Grillner, in studies of spinalized kittens, who realized the use of a treadmill facilitated the walking pattern. Grillner's team reported that the kittens spinalized 1–2 weeks after birth were capable of performing weight-bearing hindlimb stepping on the treadmill.10 Perhaps Grillner and colleagues were unaware at the time, but their techniques for triggering treadmill walking would go on to become part of standard practice for BWSTT in spinal animals for years to come. In particular, they suspended the cats over a treadmill with the forelimbs resting on a platform. Only the hindlimbs stepped on the treadmill. Because lateral stability of the trunk was poor, the tails of the cats were held to prevent the hindquarters from falling to a side. The tails also were used to stimulate walking. By squeezing the base of the tail in between the fingers and thumb, stepping was facilitated.11
Under these conditions, the spinal cats performed weight-bearing hindlimb walking that, based on kinematic and electromyography analyses, resembled walking patterns in normal cats. The spinal cats adjusted walking patterns to different locomotor conditions and perturbations. When the treadmill speed increased, the gait pattern changed from alternating stepping to a “hindlimb gallop.”12 When the spinal cats were challenged with walking on a split-treadmill belt walking, they successfully adjusted their hindlimb coordination patterns by performing fast stepping in one hindlimb and slow stepping in the other.12 These findings demonstrated the remarkable ability of spinal cats to generate locomotion. Here was proof that the spinal cord by itself was capable of generating walking. Grillner proposed a “spinal generator” for walking.10 These networks of neurons were responsible for the hindlimb walking that was observed in the spinal cats. The findings of these animal studies were both exciting and promising for people with spinal cord injuries who longed to walk again because it showed that even without connections to the brain, walking was possible.
The Birth of Weight-Supported Treadmill Training
“…[W]eight support was provided by the experimenter who held the tail…[T]he animal was led to progressively support more weight at different treadmill speeds…This was by achieved by a continual interaction between the animal and the experimenter who determined how much weight the animal could support…” Barbeau and Rossignol (1987)13
While the earlier studies were important for revealing the capabilities of the spinal cord, the role of training had not yet been appreciated until Hughes Barbeau and Serge Rossignol introduced their ideas on interactive treadmill training.13,14 Using a similar treadmill training set-up that Grillner developed, Barbeau and Rossignol's interactive training emphasized adjusting the amount of loading on the hindlimbs so as to enable stepping. The result was that the spinal cats regained weight bearing stepping in as little as four weeks after spinalization. This led Barbeau and Rossignol to conclude that not only was training important, but that the method of training (i.e. progressively increasing weight bearing) also was a critical factor.
At the same time, parallel studies were being performed by Lovely and colleagues in the laboratory of V. Reggie Edgerton.15 Again, weight support during training was adjusted with the goal of maximizing the amount of loading on the hindlimbs. They found that spinal cats that received regular treadmill training performed significantly better hindlimb stepping than untrained spinal cats.15 An important but often overlooked detail of these studies was the use of adult cats rather than spinal kittens. There had been some question as to whether adult spinal cats could perform as well as spinal kittens. Robinson and Goldberger demonstrated that the recovery of hindlimb locomotor function was superior in cats that were spinalized as newborns versus cats that were spinalized as adults.16 An “infant lesion effect” occurred in the kittens because the development of descending inhibitory pathways was interrupted by the spinalization.16,17 The work of Barbeau and Rossignol and also Edgerton and colleagues showed that, in fact, adult spinal cats were capable of weight bearing hindlimb stepping but training was essential.
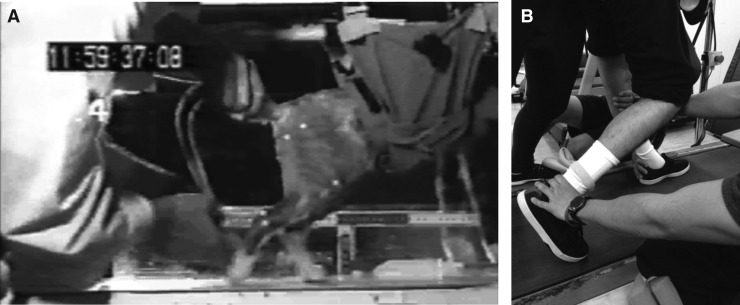
A characteristic of these early spinal cat studies was a reliance on tail stimulation. Grillner believed that providing “non-specific stimuli,” such as tail crimping or pinching, raised the level of excitation in the spinal cord, thereby facilitating stepping. The first author had the opportunity to work with spinal cats as a graduate student in the Edgerton laboratory at UCLA. The first time he saw a spinal cat walking on a treadmill he was fascinated, but believed the tail crimping and pinching could be avoided. It made more sense to utilize sensory feedback from the hindlimbs in order to drive hindlimb movements. Training spinal cats, then, became a team effort. One trainer lifted the tail for weight support and trunk stabilization while one or sometimes two trainers on either side of the cat moved the right and left hindlimbs in a forward stepping pattern. The team quickly learned tricks for eliciting stepping. For example, hip extension triggered forward swing as described by Grillner and Rossignol18 but the movement of the swinging limbs often was weak and insufficient to lift the paws. They found that tickling the ankle at the moment the hip reached its critical extension was effective in enhancing lift and uncurling the toes for plantar placement. Coordination between the two hindlimbs also was critical. It was important that a trainer place the contralateral hindlimb in a weight bearing position before the hip reached critical extension. In essence, they shifted weight to one hindlimb while extending the hip and assisting movement in the opposite hindlimb (Fig. 1A). These and other tricks were later referred to as “spinal rules of locomotion.”19 Spinal cats trained in this manner recovered the ability to walk without any trainer-added external stimulation (including tail stimulation) in 4–5 weeks after spinalization. Untrained spinal cats did not recover the same level of hindlimb stepping as occurred with training. The researchers therefore concluded that the spinal cord learned to step.20
FIG. 1.
Spinal rules of locomotion applied during body weight–supported treadmill training (BWSTT) in spinal cat (A) and a human with spinal cord injury (B). In (A), a trainer holds the tail to control weight bearing on the left hindpaw while another trainer is starting to assist the right hindlimb. Assistance is applied at the ankle and is timed to occur when the right hip has extended sufficiently to trigger swing. Note the forelimbs are resting on a platform above the treadmill and this creates more of an upright posture. In (B), a similar moment is shown during BWSTT in a human. While one trainer controls weight bearing in the left leg, another trainer is assisting swing in the right leg by manipulating the ankle. The assistance is applied when the hip is extended.
By this point in time, the basic techniques of BWSTT had emerged. The treadmill was essential for stimulating hindlimb movement, as was control over weight support. Manually assisting hindlimb movements also was important, but assistance in general should be reduced as independently-generated movements were recovered. Given the success of the spinal cat studies, it was only a matter of time that the techniques would be applied to human rehabilitation. Barbeau and colleagues first described a locomotor rehabilitation system that included a treadmill and a body weight support apparatus.21 Similar weight support and treadmill systems were used by other scientists who also were interested in applying the “spinal rules for locomotion” to gait rehabilitation.22–24
However, it was not until Behrman and Harkema's article that the parallels between the details of spinal cat training and training humans with SCI became obvious.25 As a post-doctoral student at the University of California, Los Angeles, Dr. Harkema periodically visited the spinal cat training sessions and joked with the first author that techniques used in the cats inspired the training methods she used with human subjects. In particular, two leg trainers assisted movements in the left and right legs while a hip trainer stabilized the trunk and hips. Great attention was paid to important parameters, such as, treadmill speed, leg loading, and muscle tendon stimulation, as they were critical for facilitating stepping. One aspect of training was especially interesting. To initiate swing, the trainers shifted the weight to the contralateral leg while assisting ankle movement in the swing leg. These were the same tricks used with spinal cats (Fig. 1B). Under these training conditions, remarkable locomotor activity was observed in humans with functionally complete SCI.22,23 It was apparent that the human spinal cord was capable of utilizing sensory input to generate locomotor output and just like in the cats, it did not need the brain to do this.
Apples and Oranges
“The hindquarters [of spinal kittens] are supported by the hindlimbs but often fall over in one direction or the other so that they only move 5 or 10 steps…” Grillner (1975)10
Given that trained spinal cats could walk quite well on a treadmill with their hindlimbs, it was a natural to ask whether they could also walk overground. Clearly, the treadmill was a powerful stimulus for walking. But how well could the spinal cats walk without the help of the treadmill? Could the sensory cues learned during treadmill training also be used to facilitate overground locomotion? For his thesis work, the first author worked with dozens of spinal cats and there were some who were clearly superior to others at treadmill walking. The “champion” cats performed nearly flawless hindlimb walking at all treadmill speeds. These cats' spinal cords had been trained so well that, literally and figuratively, turning on the treadmill flipped a “neural switch” and the spinal cats walked. When the animals were returned to their cages, however, their preferred method of getting around the cage was to use their forelimbs. The hindlimbs mostly slid on the floor. On occasion, a step occurred if the hindlimbs happened to be in a weight-bearing posture. As the cat pulled itself forward with its forelimbs, the hindlimb hip extended and triggered a step, again following a “spinal rule of locomotion.” Further steps were possible using the same strategies; however, the hindquarters eventually fell over just as Grillner described years earlier. It did not seem to matter how much treadmill training the spinal cats received (some were trained for 2 years)26; they never consistently used their hindlimbs to walk overground.
It was clear that getting spinal cats to walk outside of the treadmill environment was not going to be as straightforward as applying the spinal rules to overground walking. The spinal cord, which had been trained to perform a specific task, had become good at using sensory stimulation to generate hindimb treadmill stepping. Overground walking, however, was a different task and in many ways, it was a more demanding task. BWSTT did not sufficiently prepare the spinal cats to walk overground. There are many possible reasons for this, some of which are described as follows:
1. BWSTT did not train lateral stability and balance. Overground walking requires a degree of control over the trunk in order to maintain balance. During BWSTT, trunk control was assisted by holding the tail. Balance training had not been a primary focus of the spinal cat studies, perhaps because it was thought that spinal cats were not capable of trunk control. Grillner concluded that although the central rhythm of walking was programmed by the spinal cord, balance control resided elsewhere.10 Interestingly, a recent study by Oza and Giszter have incorporated trunk control training during BWSTT in rats that were spinalized as neonates. They have reported enhanced function and cortical plasticity arising from BWSTT with robotic trunk rehabilitation.27
2. BWSTT did not train hindlimb–forelimb coordination. Overground walking requires coordination of forelimb and hindlimb movements. During BWSTT, only hindlimb coordination improved since the forelimbs were stationary.
3. The spinal cats were trained to walk in an upright posture. During BWSTT, the cat's forelimbs stood on a platform that was raised above the treadmill (Fig. 1A). This had the effect of creating a posture that was more upright, compared with the posture during overground locomotion. Findings from a recent study provided evidence that the upright posture facilitated hindlimb walking.28 Rats that were spinalized as adults performed better walking when the angle of the body was closer to vertical than to horizontal. This may be due to the enhanced sensory feedback from load receptors on the paws. Changing the posture in this manner also extended the hip, which might further facilitate stepping. Hip extension during stance produces afferent drive that contributes to the initiation of swing.29,30 Previous findings do support a role for hip position in triggering forward swing in adult spinal cats.18
4. BWSTT did not train voluntary control over walking. Overground walking is purposeful and voluntary and thus involves descending control. BWSTT, however, trains the spinal cord to rely on sensory input to drive hindlimb locomotion. Descending control is not required (indeed, the first author has observed spinal cats falling asleep during long treadmill training sessions).
One could suppose that BWSTT would have a better chance for improving overground walking in animals that did not have a complete spinal cord injury. Based on a systematic review performed by Battistuzzo and colleagues, BWSTT has been shown to improve open field locomotion in rats with incomplete SCI.7 However, more recent findings lead to a different conclusion.31–33 For example, van den Brand and colleagues reported that BWSTT failed to improve overground locomotion in rats that received dual lateral hemisections of the spinal cord.33 Interestingly, the lack of effect was found even when pharmacological and spinal stimulation were added. Only when the rats underwent overground locomotor training was an improvement in overground locomotion observed. Other findings also point to a task-specific effect of locomotor training. Shah and colleagues reported that quadrupedal locomotion was improved by quadrupedal treadmill training but not bipedal treadmill training.31 Other evidence for a task-specific effect of training came from Kuerzi and colleagues, who used a clever way to provide weight support by training contused rats to walk in shallow water.34 The rats improved performance of shallow water walking but there did not appear to be an effect out of the water when open field locomotion was tested. These findings provided support for a task-specific effect of training on the spinal circuitry. In support of this, we reported years ago that the levels of GABAergic and glycinergic inhibition in the lumbar spinal cord were dependent on the type of training spinal cats received.35,36 Step training reduced inhibition, whereas stand training increased inhibition around various motor pools.
Strategies and Tools for Future Animal Studies in BWSTT
We have made the case that animal studies have not convincingly shown that BWSTT translated to improved overground walking. We are skeptical about this direct translation because of the task-specific nature of BWSTT and the different demands of overground walking. If the goal is to improve overground walking, then spinal cord injured animals will eventually need to be trained to perform the task of overground walking. Weight support, of course, will be necessary if the overground locomotor training is to be effective. This sounds obvious, but in fact, there have been few animal studies of weight-supported overground locomotor training. van den Brand and colleagues have developed an impressive neuroprosthetic system that, among other things, enables spinal cord injured rats to perform weight-supported overground walking.33 The rats received a dual lateral hemisection of the spinal cord, which interrupts supraspinal input but leaves some propriospinal connections intact. In addition to standard BWSTT, the researchers subjected rats to bipedal hindlimb training that included climbing stairs and walking over obstacles. These tasks were more challenging than treadmill walking and thus would “force the rats to actively use their hindlimbs.”33 Astonishingly, the paralyzed rats recovered voluntary control over hindlimb locomotion. Moreover, it was shown that standard BWSTT alone was not sufficient. Training that included walking overground, up stairs, and around obstacles was a key factor in regaining cortical control of the spinal circuits.
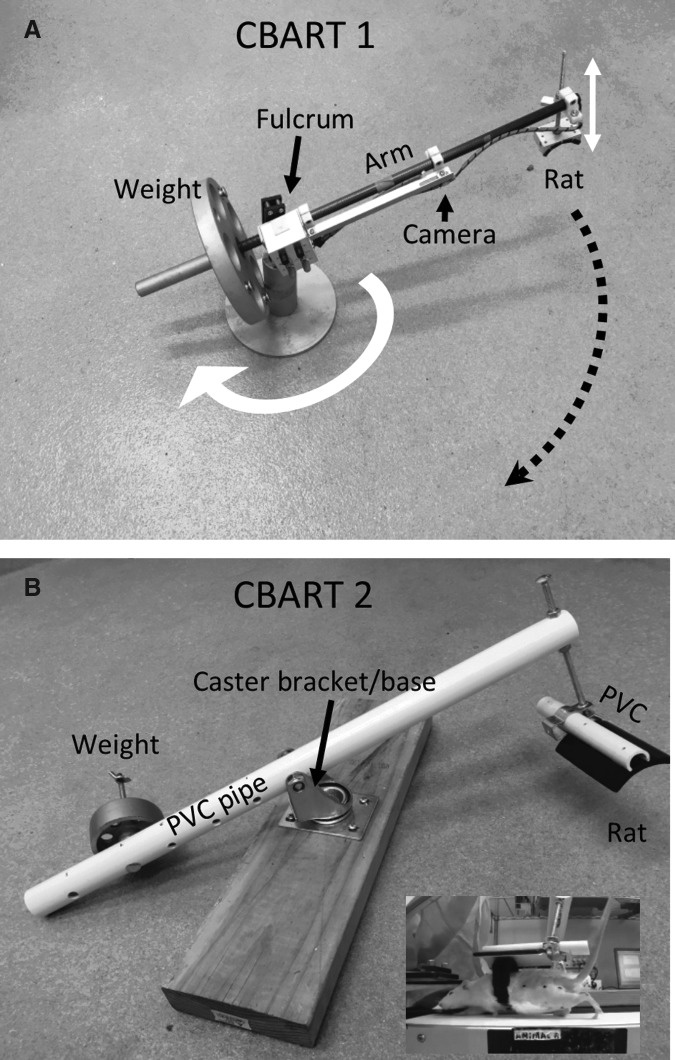
The results of van den Brand and colleagues' research reinforced the importance of incorporating overground training into animal studies. Robotic devices, however, are not easy to come by and we have been exploring ways to train overground locomotion in rats using simpler, non-robotic devices. Our initial device, the Body weight–supported Ambulatory Rat Trainer (BART) was developed in collaboration with David Reinkensmeyer.37 The device consisted of a weight support apparatus that slides on a rail above the rat.37 A pilot study demonstrated that the device was effective for facilitating quadrupedal overground walking in spinally contused rats. However, because of its short walking path, the BART device was not ideal for training multiple step cycles. We are currently testing another device called Circular BART (CBART; Fig. 2A). The CBART device consists of a lever arm that hinges on a fulcrum. The rat is placed at one end of the arm and is lifted by a weight placed on the other end of the arm. The amount of weight support is controlled by adding more weight or moving the location of the weight on the lever arm. The rats walk along a circular path because the arm rotates around a vertical axis (see white arrow and dashed black line and arrow in Fig. 2A). The force required to initiate rotation of the arm was low (i.e., 5% of body weight) and we have found that rats can easily move the arm while walking. A small camera on the arm is used to video record the rat during locomotion (see Fig. 2A).
FIG. 2.
Tools for weight supported overground and treadmill locomotion. In (A), the Circular Body weight supported Ambulatory Rat Trainer (CBART) 1 device, is shown. The rat is attached to one end of a lever arm. The lever arm pivots around a fulcrum. The rat is lifted (see vertical white arrows) by the weight on the opposite end of the lever arm. The amount of weight support is adjusted by moving the location of the weight on the lever arm. A small camera on the arm is used to video record the rat during locomotion. The lever arm also rotates around a vertical axis indicated by the white arrow. This allows the rat to walk in a circular path indicated by the black, dashed line and arrow. In (B), a second CBART device is shown. This CBART 2 device uses the same mechanical principles as the device in (A), but is made from parts (i.e., PVC pipe, caster) obtained from a hardware store. The inset shows a spinally contused rat undergoing treadmill training using the CBART 2 device (Supplementary Video 1; see online supplementary material at www.liebertpub.com).
We recently made a second, simpler CBART device (Fig. 2B). This also has low friction (approximately 1% of body weight) and SCI rats can easily move the arm while walking (Fig. 2B; Supplementary Video 1, see online supplementary material at www.liebertpub.com). An advantage of the CBART 2 device is that it is made entirely from parts obtained from a hardware store and thus it is relatively inexpensive and easy to assemble (Supplementary Video 2; see online supplementary material at www.liebertpub.com).
Simple devices like CBART will be necessary tools for future animal studies of BWSTT. First, if we are to more rigorously test the effects of BWSTT, then testing overground walking is essential. A recent review of training interventions in SCI animals found that while most studies reported positive effects, objective assessments of overground walking were rare (i.e., approximately 7% of the studies that were reviewed).7 The authors also correctly pointed out that this included the classic spinal cat studies, which despite their lack of overground testing, formed the basis for human BWSTT rehabilitation. We contend that objective overground testing in animals has been lacking in part because the tools for assessment were not available. The CatWalk was developed to address this need and it has been shown to be effective for kinematic analyses in SCI rats.38 Clearly, however, factors such as weight bearing and lateral stability heavily influence hindlimb stepping and should be taken into account when assessing overground locomotion. There are robotic systems such as van den Brand and colleagues' neuroprosthetic system33 and Oza and Giszter's trunk rehabilitator27 that succeed in this manner. Simpler systems like the BART and CBART may lack some of the control features of these robotic systems, but because they are easy to construct, they have the potential to be more widely used in rodent studies to assess overground walking.
Secondly, given the task-specific nature of training, the recovery of overground walking will require training the animals to walk overground. The majority of BWSTT studies in animals have focused only on the effects of BWSTT on hindlimb stepping. The early spinal cat studies, for example, were designed to study spinal control of stepping. Those studies did not deliberately set out to develop gait training techniques. Later, of course, this changed and there was increasingly greater interest in rehabilitation and functional recovery after SCI. The problem was that many animal studies continued to focus on training the hindlimbs only.
Recently, however, there has been an important shift in the training paradigm from bipedal hindlimb training to quadrupedal training. Shah and colleagues trained quadrupedal treadmill walking in spinally hemisected rats and reported better forelimb-hindlimb coordination in quadrupedal trained rats than in bipedal trained rats.31 Likewise, Ward and colleagues reported that quadrupedal treadmill training improved a number of locomotor outcomes, including open field locomotion in spinally contused rats.39
One strategy for future animal studies is to combine multiple types of weight-supported training. The first stage would involve traditional BWSTT to improve rhythmic hindlimb movements on the treadmill. The next stage would involve training weight supported quadrupedal walking on the treadmill. This intermediate stage would be used to improve forelimb–hindlimb coordination and strengthen propriospinal connections within the spinal cord. The final stage would be focused on functional recovery of quadrupedal, overground walking. The goal in this stage would be to improve lateral stability and balance. Tools that can be used for all these types of training are not available to most researchers. What CBART demonstrates is that basic requirements for improved training and testing can be achieved without complicated and expensive hardware and software. We believe that the continued development of these and other simple devices are essential if we are to more rigorously examine BWSTT.
Conclusions
To conclude, we return to the question of what we learned from the pioneering animal studies. Because of the work by scientists in the field such as Grillner, Barbeau, Rossignol, and Edgerton, we know of the incredible capabilities of the spinal cord and the role that BWSTT plays in enhancing these capabilities. It is important to remember spinally-generated locomotion was the focus of early spinal cat studies. From this perspective, the animal studies have been quite successful. There is still much to be done, however, if rehabilitation is the goal. More recent animal studies are discovering ways to improve functional recovery after SCI and not surprisingly, BWSTT plays a role. With the help of new assessment and training tools, BWSTT principles and techniques are adapting and evolving. This means that animal studies will continue contributing to the development of effective rehabilitation strategies.
Supplementary Material
Acknowledgment
Supported by NIH Grant R15NS082711 to R.D. de Leon.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Morawietz C. and Moffat F. (2013). Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch. Phys. Med. Rehabil. 94, 2297–2308 [DOI] [PubMed] [Google Scholar]
- 2.Mehrholz J., Kugler J., and Pohl M. (2012). Locomotor training for walking after spinal cord injury. Cochrane Database Syst. Rev. 11, CD006676. [DOI] [PubMed] [Google Scholar]
- 3.Wessels M., Lucas C., Eriks I., and de Groot S. (2010). Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J. Rehabil. Med. 42, 513–519 [DOI] [PubMed] [Google Scholar]
- 4.Harkema S.J., Hillyer J., Schmidt-Read M., Ardolino E., Sisto S.A., and Behrman A.L. (2012). Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 93, 1588–1597 [DOI] [PubMed] [Google Scholar]
- 5.Dobkin B.H. and Duncan P.W. (2012). Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil. Neural Repair 26, 308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubli M. and Dietz V. (2013). The physiological basis of neurorehabilitation–locomotor training after spinal cord injury. J. Neuroengineering Rehabil. 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battistuzzo C.R., Callister R.J., Callister R., and Galea M.P. (2012). A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J. Neurotrauma 29, 1600–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy R.R., Harkema S.J., and Edgerton V.R. (2012). Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch. Phys. Med. Rehabil. 93, 1487–1497 [DOI] [PubMed] [Google Scholar]
- 9.Rossignol S., Martinez M., Escalona M., Kundu A., Delivet-Mongrain H., Alluin O., and Gossard J.-P. (2015). The “beneficial” effects of locomotor training after various types of spinal lesions in cats and rats. Prog. Brain Res. 218, 173–198 [DOI] [PubMed] [Google Scholar]
- 10.Grillner S. (1975). Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol. Rev. 55, 247–304 [DOI] [PubMed] [Google Scholar]
- 11.Forssberg H. and Grillner S. (1973). The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 50, 184–186 [DOI] [PubMed] [Google Scholar]
- 12.Forssberg H., Grillner S., Halbertsma J., and Rossignol S. (1980). The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol. Scand. 108, 283–295 [DOI] [PubMed] [Google Scholar]
- 13.Barbeau H. and Rossignol S. (1987). Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95 [DOI] [PubMed] [Google Scholar]
- 14.Barbeau H., Julien C., and Rossignol S. (1987). The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Res. 437, 83–96 [DOI] [PubMed] [Google Scholar]
- 15.Lovely R.G., Gregor R.J., Roy R.R., and Edgerton V.R. (1986). Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 92, 421–435 [DOI] [PubMed] [Google Scholar]
- 16.Robinson G.A. and Goldberger M.E. (1986). The development and recovery of motor function in spinal cats. I. The infant lesion effect. Exp. Brain Res. 62, 373–386 [DOI] [PubMed] [Google Scholar]
- 17.Robinson G.A. and Goldberger M.E. (1986). The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Exp. Brain Res. 62, 387–400 [DOI] [PubMed] [Google Scholar]
- 18.Grillner S. and Rossignol S. (1978). On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 146, 269–277 [DOI] [PubMed] [Google Scholar]
- 19.Wernig A., Müller S., Nanassy A., and Cagol E. (1995). Laufband therapy based on “rules of spinal locomotion” is effective in spinal cord injured persons. Eur. J. Neurosci. 7, 823–829 [DOI] [PubMed] [Google Scholar]
- 20.de Leon R.D., Hodgson J.A., Roy R.R., and Edgerton V.R. (1998). Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 79, 1329–1340 [DOI] [PubMed] [Google Scholar]
- 21.Barbeau H., Wainberg M., and Finch L. (1987). Description and application of a system for locomotor rehabilitation. Med. Biol. Eng. Comput. 25, 341–344 [DOI] [PubMed] [Google Scholar]
- 22.Dietz V., Colombo G., Jensen L., and Baumgartner L. (1995). Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 37, 574–582 [DOI] [PubMed] [Google Scholar]
- 23.Dobkin B.H., Harkema S., Requejo P., and Edgerton V.R. (1995). Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J. Neurol. Rehabil. 9, 183–190 [PubMed] [Google Scholar]
- 24.Wernig A. and Müller S. (1992). Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia 30, 229–238 [DOI] [PubMed] [Google Scholar]
- 25.Behrman A.L. and Harkema S.J. (2000). Locomotor training after human spinal cord injury: a series of case studies. Phys. Ther. 80, 688–700 [PubMed] [Google Scholar]
- 26.Hodgson J.A., Roy R.R., de Leon R., Dobkin B., and Edgerton V.R. (1994). Can the mammalian lumbar spinal cord learn a motor task? Med. Sci. Sports Exerc. 26, 1491–1497 [PubMed] [Google Scholar]
- 27.Oza C.S. and Giszter S.F. (2015). Trunk robot rehabilitation training with active stepping reorganizes and enriches trunk motor cortex representations in spinal transected rats. J. Neurosci. Off. J. Soc. Neurosci. 35, 7174–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sławińska U., Majczyński H., Dai Y., and Jordan L.M. (2012). The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J. Physiol. 590, 1721–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiebert G.W., Whelan P.J., Prochazka A., and Pearson K.G. (1996). Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J. Neurophysiol. 75, 1126–1137 [DOI] [PubMed] [Google Scholar]
- 30.Whelan P.J., Hiebert G.W., and Pearson K.G. (1995). Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp. Brain Res. 103, 20–30 [DOI] [PubMed] [Google Scholar]
- 31.Shah P.K., Garcia-Alias G., Choe J., Gad P., Gerasimenko Y., Tillakaratne N., Zhong H., Roy R.R., and Edgerton V.R. (2013). Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain J. Neurol. 136, 3362–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A., Balasubramanian S., Murray M., Lemay M., and Houle J. (2011). Role of spared pathways in locomotor recovery after body-weight-supported treadmill training in contused rats. J. Neurotrauma 28, 2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Brand R., Heutschi J., Barraud Q., DiGiovanna J., Bartholdi K., Huerlimann M., Friedli L., Vollenweider I., Moraud E.M., Duis S., Dominici N., Micera S., Musienko P., and Courtine G. (2012). Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336, 1182–1185 [DOI] [PubMed] [Google Scholar]
- 34.Kuerzi J., Brown E.H., Shum-Siu A., Siu A., Burke D., Morehouse J., Smith R.R., and Magnuson D.S.K. (2010). Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp. Neurol. 224, 178–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillakaratne N.J.K., de Leon R.D., Hoang T.X., Roy R.R., Edgerton V.R., and Tobin A.J. (2002). Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. Off. J. Soc. Neurosci. 22, 3130–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Leon R.D., Tamaki H., Hodgson J.A., Roy R.R., and Edgerton V.R. (1999). Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J. Neurophysiol. 82, 359–369 [DOI] [PubMed] [Google Scholar]
- 37.Hamlin M., Traughber T., Reinkensmeyer D.J., and de Leon R.D. (2015). A novel device for studying weight supported, quadrupedal overground locomotion in spinal cord injured rats. J. Neurosci. Methods 246, 134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamers F.P.T., Koopmans G.C., and Joosten E.A.J. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J. Neurotrauma 23, 537–548 [DOI] [PubMed] [Google Scholar]
- 39.Ward P.J., Herrity A.N., Smith R.R., Willhite A., Harrison B.J., Petruska J.C., Harkema S.J., and Hubscher C.H. (2014). Novel multi-system functional gains via task specific training in spinal cord injured male rats. J. Neurotrauma 31, 819–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.