Dear Editor:
It is controversial whether antibodies in Graves' ophthalmopathy (GO) patients bind to both thyrotropin (TSH) receptors (TSHRs) and insulin-like growth factor-1 (IGF-1) receptors (IGF-1Rs), or whether GO pathogenesis involves TSHR/IGF-1R cross-talk initiated by binding to TSHRs only. In a recent review, Smith and Janssen (1) concluded “it remains possible although unproven that two discrete antibodies generated in Graves' disease are at play in the pathogenesis of [thyroid-associated ophthalmopathy]. This theoretical construct involves one antibody directed at TSHR and the other at IGF-IR.” They argued against our conclusion that it is TSHR/IGF-1R cross-talk initiated by TSHR-stimulating antibodies (TSAbs) (2). However, Smith and Janssen misinterpreted one of our findings and failed to consider another. First, they suggested that partial inhibition of TSAb stimulation by an IGF-1R kinase inhibitor necessitates IGF-1R phosphorylation following TSAb binding to IGF-1R, even though we found no evidence for IGF-1R phosphorylation by the monoclonal TSAb M22 or by immunoglobulins from 57 GO patients (2). They stated “that the Western blot assay [we] used for monitoring IGF-IR phosphorylation failed to detect what might have been low-level but physiologically important receptor activation.” We used a highly sensitive Bio-Plex MAGPIX assay, not Western blot, and found no evidence of IGF-1R phosphorylation. We suggest the kinase inhibitory effect may be caused by changing IGF-1R conformation (3) so that it can no longer interact with TSHR. We postulated this mechanism (2) to explain how one inhibitory anti-IGF-1R antibody 1H7 antagonized TSHR/IGF-1R cross-talk while another AF305 did not. Second, Smith and Janssen failed to acknowledge that AF305, which blocks binding to IGF-1R, had no effect on TSAb stimulation.
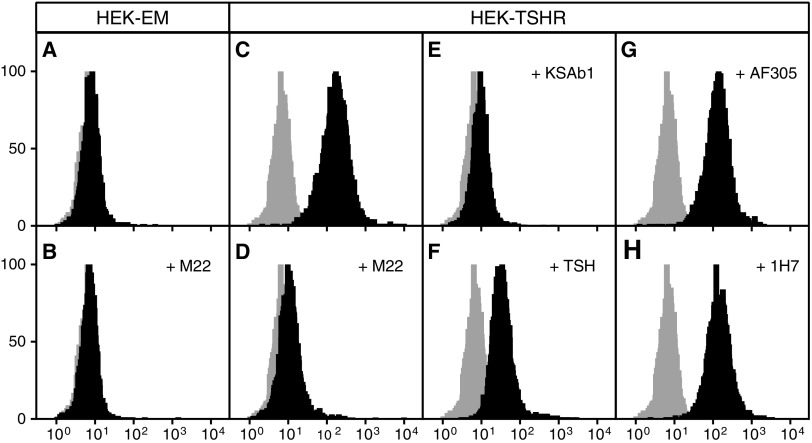
To address whether a TSAb must bind to IGF-1R to cause cooperation between TSHR and IGF-1R, we fluorescently tagged M22, a monoclonal TSAb that stimulates GOFs (4) in part via an IGF-1R-dependent mechanism (2), and measured binding of Alexa-647-M22 by fluorescence analysis. We used HEK-TSHR cells that stably express transfected TSHRs and endogenously express IGF-1Rs and parental HEK-EM cells that do not express TSHRs but express IGF-1Rs. Figure 1 illustrates that HEK-EM cells exhibit no specific Alexa-647-M22 binding, even though they express IGF-1Rs, as we demonstrated by specific radiolabeled IGF-1 binding (not shown). In contrast, Alexa-647-M22 binds specifically to TSHRs on HEK-TSHR cells because binding is competed by TSABs M22 and KSAb1 (kindly provided by Dr. Paul Banga), and partly competed by TSH, but not to IGF-1Rs because it is not competed by IGF-1 or the IGF-1R antibodies AF305 or 1H7.
FIG. 1.
Competitive binding of thyrotropin (TSH) receptor (TSHR) and insulin-like growth factor-1 receptor (IGF-1R) antibodies versus Alexa-M22-647. Binding assays on parental HEK-EM cells that do not express TSHRs but endogenous IGF-1Rs and on HEK-TSHR cells were performed by FACS competitive binding of Alexa-647-M22 by incubating cells with 1:25 ratio of Alexa-647-M22 to unlabeled TSHR antibodies M22 and KSAb1, TSH, and IGF-1R antibodies AF305 and 1H7 and IGF-1. Competing ligands were added at 4°C 15 min prior to the addition of Alexa-647-M22 and incubation for 1 h at 4°C. Gray histograms represent auto-fluorescence. Solid-black histograms show Alexa-647-M22 fluorescence intensity. (A) and (B) Alexa-647-M22 non-specifically bound to HEK-EM cells. (C)–(F) The >10-fold peak shift in Alexa-647 fluorescence in HEK-TSHR cells (C) was markedly reduced by unlabeled TSHR antibodies M22 (D) and KSAb1 (E), and partially reduced by TSH (F). (G) and (H) Anti-IGF-1R antibodies AF305 (G), 1H7 (H), and IGF-1 (not shown) did not reduce peak fluorescence.
We conclude that a TSAb is capable of stimulating TSHR/IGF-1R cross-talk by binding only to TSHRs. Although the binding experiment we report can only be performed with a monoclonal antibody, our findings demonstrate that a TSAb does not need bind to IGF-1R to cause TSHR/IGF-1R signaling. The concept that there are no IGF-1R stimulatory antibodies in GO patients is supported by our findings that GO immunoglobulins exhibited pharmacologic properties similar to those of M22 (2). Lastly, to our knowledge, a stimulatory IGF-1R antibody that causes disease has not been found in any patient.
Author Disclosure Statement
C.C.K., S.N., and M.C.G. have filed a patent pertaining to drug combinations targeting TSHR and IGF-1R.
References
- 1.Smith TJ, Janssen JAMJL. 2017. Building the case for insulin-like receptor-1 involvement in thyroid-associated ophthalmopathy. Front Endocrinol 7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger CC, Place RF, Bevilcqua C, Marcus-Samule B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. 2016. TSH/IGF-1 receptor cross talk in Graves' ophthalmopathy pathogenesis. J Clin Endocrinol Metab 101:2340–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kavran JM, McCabe JM, Byrne PO, Connacher MK, Wang Z, Ramek A, Sarabipour S, Shan Y, Shaw DE, Hristova K, Cole PA, Leahy DJ. 2014. How IGF-1 activates its receptor. eLife 3:e03772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieger CC, Gershengorn MC. 2014 A modified ELISA accurately measures secretion of high molecular weight hyaluronan (HA) by Graves' disease orbital cells. Endocrinology 155:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]