Abstract
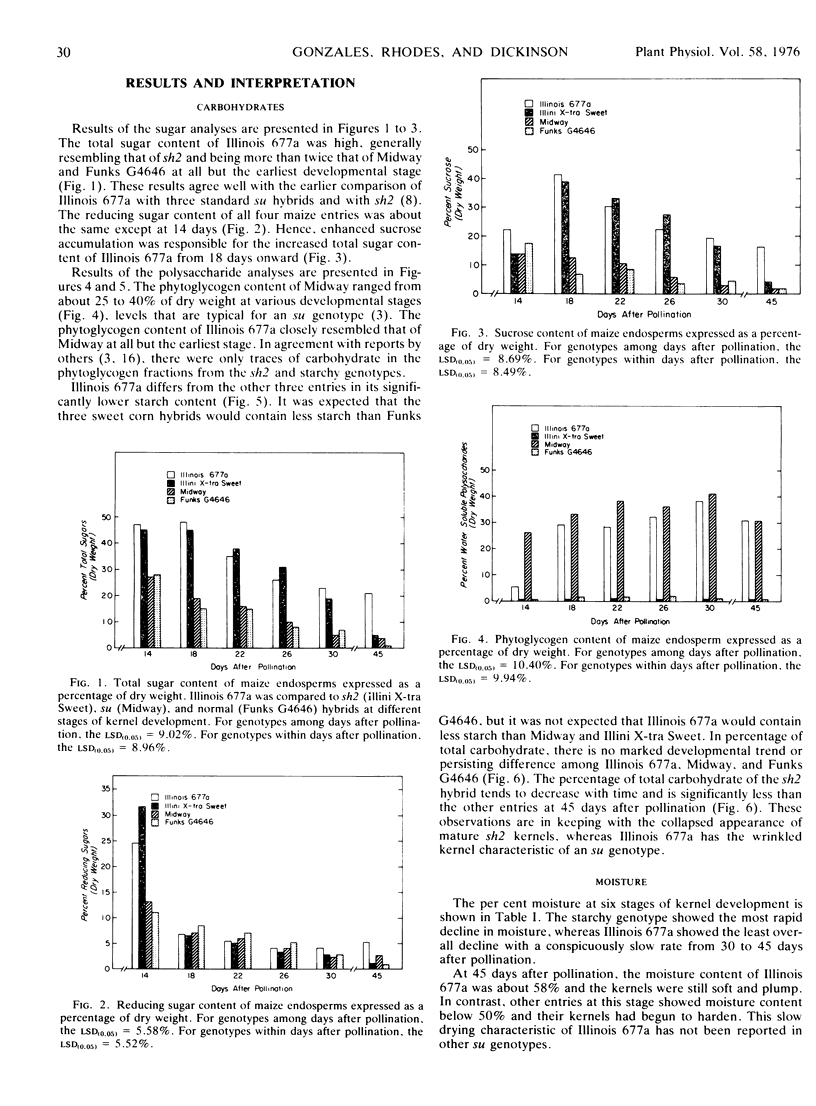
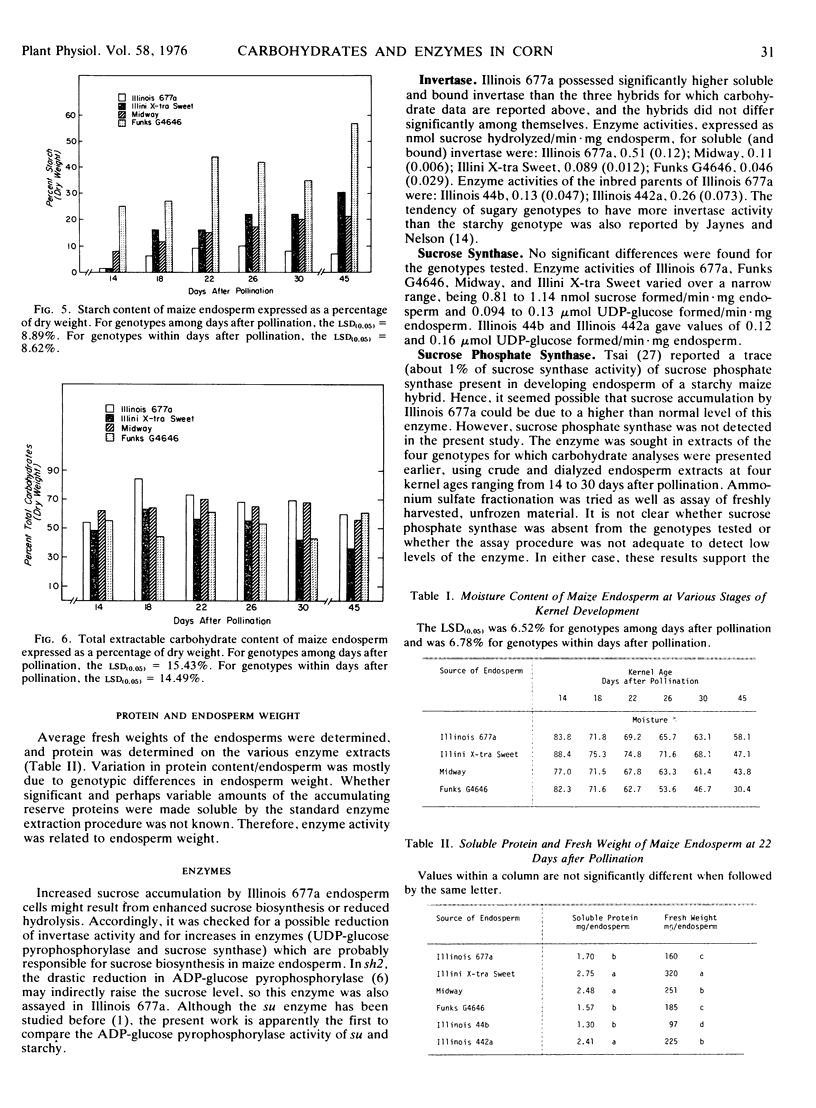
Reserve carbohydrates were determined on developing endosperm of a new line of sugary maize (Zea mays L.). Other entries, included for comparative purposes, were Midway (sugary), Funks G4646 (starchy), and Illini X-tra Sweet (shrunken-2). Sucrose in the new line, Illinois 677a, was more than twice that of Midway at most stages of development, and reached a maximum of 40% of dry weight at 18 days after pollination. Appreciable phytoglycogen accumulated in Illinois 677a, reaching 30% or more of dry weight as endosperm tissue matured. Thus, Illinois 677a is a typical sugary maize concerning phytoglycogen content, but it resembles shrunken-2 concerning the extent of sucrose accumulation.
Enhanced sucrose accumulation by Illinois 677a was not accounted for by altered in vitro activities of invertase, sucrose synthase, UDP-glucose pyrophosphorylase, or ADP-glucose pyrophosphorylase. Its normal level of ADP-glucose pyrophosphorylase set Illinois 677a apart from shrunken-2 in which the enzyme was drastically reduced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir J., Cherry J. H. Purification and properties of adenosine diphosphoglucose pyrophosphorylase from sweet corn. Plant Physiol. 1972 Jun;49(6):893–897. doi: 10.1104/pp.49.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. The Regulatory Properties of Purified Phaseolus aureus Sucrose Synthetase. Plant Physiol. 1972 Oct;50(4):469–472. doi: 10.1104/pp.50.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. B., Preiss J. Presence of ADP-Glucose Pyrophosphorylase in Shrunken-2 and Brittle-2 Mutants of Maize Endosperm. Plant Physiol. 1969 Jul;44(7):1058–1062. doi: 10.1104/pp.44.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H. F., Creech R. G., Loerch J. D. Biosynthesis of phytoglycogen in maize endosperm. The branching enzyme. Biochim Biophys Acta. 1969 Jul 8;185(1):70–79. doi: 10.1016/0005-2744(69)90283-6. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Dickinson D. B. Partial purification and sugar nucleotide inhibition of UDP-glucose pyrophosphorylase from Lilium longiflorum pollen. Arch Biochem Biophys. 1972 Feb;148(2):523–535. doi: 10.1016/0003-9861(72)90171-3. [DOI] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. An invertase inactivator in maize endosperm and factors affecting inactivation. Plant Physiol. 1971 May;47(5):629–634. doi: 10.1104/pp.47.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes T. A., Nelson O. E. Invertase Activity in Normal and Mutant Maize Endosperms during Development. Plant Physiol. 1971 May;47(5):623–628. doi: 10.1104/pp.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidby D. K. Activation of a plant invertase by inorganic phosphate. Plant Physiol. 1966 Sep;41(7):1139–1144. doi: 10.1104/pp.41.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laughnan J. R. The Effect of the sh(2) Factor on Carbohydrate Reserves in the Mature Endosperm of Maize. Genetics. 1953 Sep;38(5):485–499. doi: 10.1093/genetics/38.5.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer S., Lee E. Y.C., Whelan W. J. Multiple forms of starch synthetase in maize varieties as revealed by disc-gel electrophoresis and activity staining. FEBS Lett. 1973 Feb 15;30(1):129–132. doi: 10.1016/0014-5793(73)80634-9. [DOI] [PubMed] [Google Scholar]
- Shannon J. C., Creech R. G. Genetics of storage polyglucosides in Zea mays L. Ann N Y Acad Sci. 1973 Feb 9;210:279–289. doi: 10.1111/j.1749-6632.1973.tb47579.x. [DOI] [PubMed] [Google Scholar]


