Abstract
Background
This study was designed to investigate the effect of lycorine (LY) on the AMPK-mTOR-S6K signaling pathway and to clarify its role in autophagy and apoptosis.
Material/Methods
Various concentrations of LY were used to treat non-small cell lung carcinoma A549 cells. The MTT assay was used to measure cell viability and acridine orange staining was used to detect cell morphology changes. Western blot analysis was used to test the effect of LY on the expression levels of LC3, caspase 3, and other proteins involved in the AMPK-mTOR-S6K signaling pathway.
Results
The half maximal inhibitory concentration (IC50) of LY after 24-h treatment was 8.5 μM, with stronger inhibitory effect of 24-h LY treatment over 12-h LY treatment. Morphological observation showed that lower doses (4 μM and 8 μM) of LY treatment induced A549 cell death mainly caused by autophagy, whereas the higher dose (16 μM) of LY treatment induced A549 cell death, mainly caused by apoptosis. Furthermore, 8 μM LY caused the highest conversion of LC3-II from LC3-I. All LY treatments activated caspase-3. LY treatment also promoted AMPK phosphorylation (Thr172) and inhibited the phosphorylation of mTOR and S6K.
Conclusions
LY induced apoptosis of A549 cells by regulating the AMPK-mTOR-S6K signaling pathway. Lower levels (4~8 μM) of LY-induced autophagy contributed to LY-induced apoptosis.
MeSH Keywords: Apoptosis, Autophagy, MAP Kinase Signaling System
Background
Lung cancer is one of the malignant tumors with highest incidence and mortality. Clinical surgery and radiotherapy and chemotherapy are less effective for patients with advanced lung cancer. With the development of traditional Chinese medicine (TCM), it has become an effective method for the treatment of lung cancer [1]. Natural medicine has become one of the main sources of anti-cancer drugs because of its rich resources and a variety of pharmacological effects. Lycoris (LY) is an isoquinoline alkaloid isolated from bulbs of Monocotyledones amaryllidaceae plants. Recent studies have shown that LY (chemical structure shown in Figure 1) can be used in treatment of viral infection [2,3], inflammation [4], malaria [5], osteoporosis [6], and cancer [7].
Figure 1.

Chemical structure of lycorine.
During autophagy, the cell’s own proteins and organelles are swallowed into vesicles and combined with lysosomes to form autophagic lysosomes to degrade the contents and provide material and energy required for cell metabolism. Autophagy helps to maintain cell viability, but excessive autophagy may cause cell death. During tumorigenesis, autophagy regulates cell survival and death [8]. Apoptosis is programmed cell death, which maintains cell homeostasis [9,10]. Autophagy has been reported to be closely associate with apoptosis: (1) autophagy is one of the upstream processes required for apoptosis, and inhibition of autophagy can effectively inhibit apoptosis [11]; (2) autophagy can reduce the rate of apoptosis via inhibition of apoptosis [12]; and (3) autophagy and apoptosis can synergistically promote cell death [13], and regulation of both autophagy and apoptosis can switch between these 2 kinds of death [14]. Therefore, the effect of drugs on the regulation of autophagy and apoptosis of tumor cells is particularly important.
AMPK are kinases present in eukaryotic cells and they regulate energy metabolism. Inhibition of AMPK activity may effectively activate mTOR, thereby regulating cell growth and metabolism [15]. In situations of low blood sugar, hypoxia, and other stress conditions, the AMP/ATP ratio rises to activate AMPK α subunit, the latter in conjunction with the kinase LKB1 promotes phosphorylation in Thr172 [16, 17]. Phosphorylation of AMPK activates the tuberous sclerosis complex (TSC), thereby inhibiting downstream substrates of mammalian target of rapamycin (mTOR) [18]. Reports in the literature indicate that the AMPK-mTOR-S6K signaling pathway is closely linked to autophagy and apoptosis. Research in mammalian systems found that inhibition of mTOR activated autophagy [19]. In addition, AMPK can change the conformational of pro-apoptotic protein Bax to maintain mitochondrial transmembrane potential (ΔΨm), blocking the release of cytochrome C and activation of caspase-3, which in turn inhibits apoptosis [20].
In the present study, human lung adenocarcinoma A549 cells were used as research subjects, and the commonly used anti-cancer drugs cisplatin and curcumin were included [21–23] as positive controls to explore the effect of LY on the autophagy and apoptosis of A549 cells to provide a scientific basis for new ideas and directions for the clinical treatment of lung cancer.
Material and Methods
Material
Lycorine (purity >98%) was obtained from Nanjing Purun Tech Co. and cisplatin (purity >98.5%) was obtained from Dalian Meilun Biotech. Curcumin (purity >98%) was purchased from Shanghai Jianglai Biotech and non-small cell lung cancer cell line A549 was from Nanjing Lishizi Biotech (Nanjing, China). Fetal bovine serum was purchased from Hyclone (Logan City, UT). DMEM were from Shanghai Feili i Biotech (Shanghai, China). Cell culture flasks, cell culture dishes, and 96- and 6-well plates were purchased from Tianjin Ruishina biotech (Tianjin, China). MTT and trypsin was bought from Nanjing Zhongshan Biotech (Nanjing, China). Wortmannin was purchased from Selleck (Houston, TX). Protein extraction kit and BCA protein quantification kit were from Shijiazhuang Haisen Chemical (Shijiazhuang, China). Mouse anti-human β-actin (Item No. 3700), rabbit anti-human AMPK (Item No 5831), rabbit anti-human mTOR (Item No. 2983), rabbit anti-human S6K (Item No. 2708), rabbit anti-human LC3 (Item No. 3868), rabbit anti-human caspase3 (Item No. 9662), rabbit anti-human phospho AMPK (Item No. 5256), and rabbit anti-human S6K (Item No. 9234) antibodies were bought from Cell Signaling Technology (Danvers, MA). Goat anti-rabbit IgG-HRP (sc-2004) and goat anti-mouse IgG-HRP (sc-2005) secondary antibodies were from Santa Cruz Biotechnology (Dallas, TX).
Methods
MTT assay
LY was dissolved in DMSO (≥99%) and diluted in DMEM medium. A549 cells were inoculated at a concentration of 3000/well into 96-well plates. Various concentrations of LY solution (0.5 μM, 1 μM, 2 μM, 4 μM, 8 μM, 16 μM, and 32 μM) were added into cell culture medium and cultured at 37°C for 12 h or 24 h before 10 μL of MTT solution (5 mg/mL, pH=7.5) was added to each well. Cells were incubated for another 4 h, then 150 μL of DMSO (≥99%) was added to each well to stop the reaction. The absorbance value of each well at 490 nm wavelength was measured using a microplate reader. Cell viability was assessed as (OD value of blank control − OD value of treatment group)/(OD value of blank control − the OD value of control group) ×100%.
Morphology observation
A549 cells were inoculated into 6-well plates at a density of 3×104/mL. Various concentrations of LY (0.5 μM, 1 μM, 2 μM, 4 μM, 8 μM, and 16 μM) were added and the cells were cultured for 24 h. Acridine orange (final concentration of 1 μg/mL) was used to stain the cells for 15 min, and the cells were observed under a fluorescence microscope. There were 3 control groups: 4 μM and 8μM of lycorine and 100 nM autophagy inhibitors wortmannin were simultaneously added to the culture medium and incubated for 24 h, or cells were treated with 16 μM of LY for 24 h, then 100 nM wortmannin was added.
Western blot detection of protein expression
Cells were pelleted and RIPA buffer was used to lyse the cells. Protein concentrations were measured by use of a BCA protein quantification kit. We loaded 20 μg of each sample and resolved it by SDS-PAGE. Proteins were then transferred to PVDF membranes. After blocking (5% skim milk) for 2 h, anti-LC3, caspase3, AMPK, mTOR, S6K, phosphor-AMPK, phosphor-mTOR, phosphor-S6K, and β-actin antibodies (1: 1000) were added and incubated at 4°C overnight. After washing with TBST 3 times, horseradish peroxidase-conjugated secondary antibody (1: 5000) was added and incubated at 37°C for 2 h. After washing, ECL was used to develop it in a gel imaging system (GE, ImageQuant, model LAS4000). Image J software (National Institutes of Health, Bethesda, MA) was used to quantify the bands.
Statistical analysis
All data were analyzed using GraphPad prism5.0 statistical software (Ventura, CA). Data are expressed as means ± SD, differences between groups were analyzed by ANOVA or t-test, and P <0.05 was considered statistically significant.
Results
Lycorine inhibited A549 proliferation
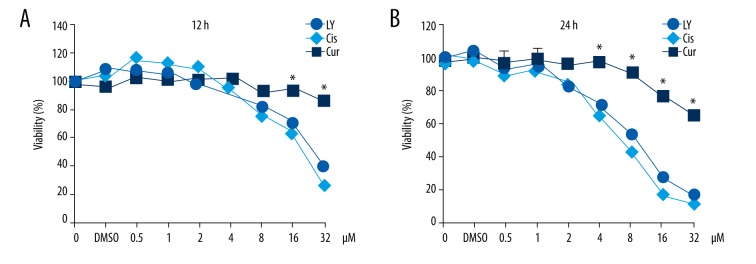
MTT assay results showed that the survival rate of A549 cells decreased with the increase of concentration and treatment time of LY, indicating that LY dose- and time-dependently suppressed A549 cell activity. IC50 of 12-h LY treatment and 14-h LY treatment were 27 μM and 8.5 μM, respectively, which was much better than that of curcumin (12 h and 24 h of IC50 >32 μM), and comparable with that of cisplatin (12 h and 24 h of IC50, 24 μM and 7.5 μM) (Figure 2).
Figure 2.
Effects of 3 compounds on cell viability of A549 cells. A549 cells were inoculated in 96-well plates followed by addition of different concentrations of LY solution (0.5 μM, 1 μM, 2 μM, 4 μM, 8 μM, 16 μM, and 32 μM) and then cultured at 37°C for 12 h (A) or 24 h (B) for analysis of cell viability by MTT assay. LY – Lycorine; Cis – cisplatin; Cur – curcumin. * P<0.05, compared with curcumin group and cisplatin group.
Lycorine promoted autophagy
Acridine orange can stain cytoplasm and nuclei to dark red and bright green, and stains acidic vesicles to bright red. In late autophagy, autophagic vacuoles combine with acidic lysosomes to form autophagy lysosomes; therefore, acridine orange stained acidic vesicles can be used as one of the signs of late autophagy. Wortmannin, as a PI3K kinase inhibitor, can inhibit early autophagy. Acridine orange staining results showed that compared with controls, low-dose (1 and 2 μM) LY treatment did not significantly induce morphology changes (Figure 3A–3C). Medium-dose (4–8 μM) LY treatment resulted in cytoplasmic vacuolization and increase of acidic vesicles but no significant change of the nucleus (Figure 3D, 3E), and 100 nM wortmannin treatment effectively reduced the number of acidic vesicles (Figure 3G, 3H). Nuclear condensation was caused by 16 μmol/L of LY treatment (Figure 3C–3F) but 100 nM wortmannin treatment did not induce further morphology change (Figure 3C–3I). These data suggested that at certain concentrations (from 0 to 8 μM), autophagy was increased with the increase of LY concentration. When LY concentration reached 16 μM, the cells began to show morphology changes and apoptosis.
Figure 3.
Morphology changes of A549 cells induced by LY (acridine orange staining). A549 cells were inoculated into 6-well plates followed by addition of different concentrations of LY and cultured for 24 h. Acridine orange (final concentration of 1 μg/mL) was used to stain the cells for 15 min, and the cells were observed under a fluorescence microscope (×200). (A) 0 μM LY; (B) 1 μM LY; (C) 2 μM LY; (D) 4 μM LY; (E) 8 μM LY; (F) 16 μM LY; (G) 4 μM LY with 100 nM wortmannin; (H) 8 μM LY with 100 nM wortmannin; (I) 16 μM LY for 24 h, then added 100 nM wortmannin.
Effect of LY on the expression levels of autophagy- and apoptosis-related proteins
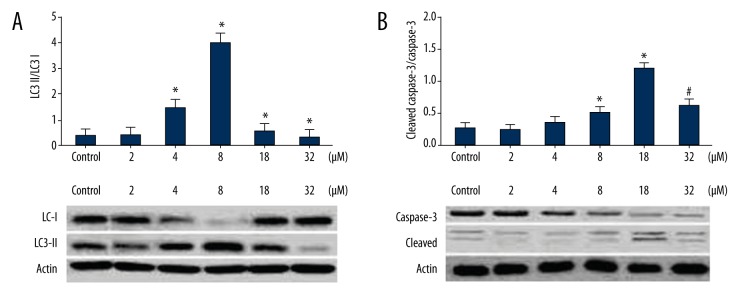
Western blot results showed that the levels of LC3-II were increased along with the increase of LY concentration and reached the highest level at LY concentration of 8 μM, indicating that LY promoted autophagy. The ratio of LC3-II and LC3-I was lower at 16 μM LY. The levels of caspase-2 were increased along with the increase of LY concentration and reached the highest level at an LY concentration of 16 μM. These results suggested that low-dose LY (<8 μM) stimulated autophagy of A549 cells, and a high concentration of LY (>16 μM) caused apoptosis of A549 cells (Figure 4).
Figure 4.
Effect of 24-h LY treatment on LC3 and caspase-3. A549 cells were inoculated into 6-well plates followed by addition of various concentrations of LY and cultured for 24 h. Then proteins were extracted for analysis of expression of LC3 and caspase-3 by Western blot. (A) Effect of 24-h LY treatment on LC3 (B) Effect of 24-h LY treatment on caspase-3. * P<0.05, compared with the control group; # P<0.05, compared with the 8 μmol/L LY group.
LY effect on AMPK, mTOR, and S6K
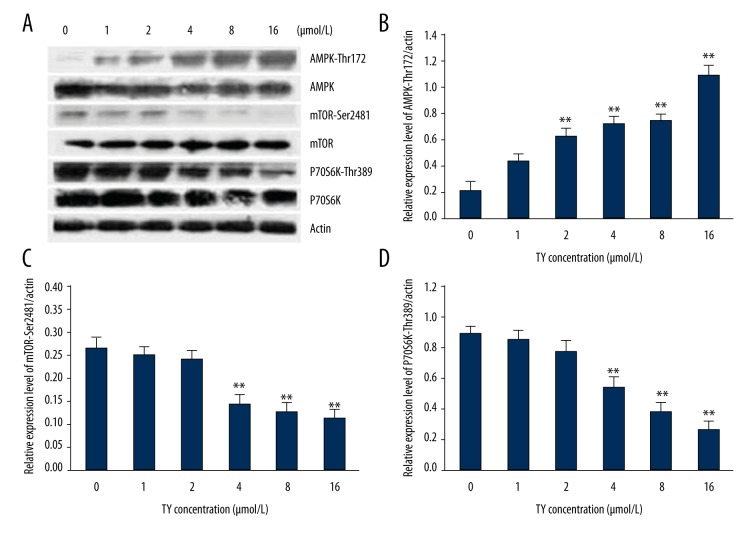
Western blot results showed that increased LY concentration caused increased phosphorylation of AMPK but decreased phosphorylation of mTOR and S6K in Ser2481 and Thr389, suggesting that LY promoted phosphorylation of AMPK to suppress the phosphorylation of mTOR and S6K to inhibit cell proliferation and stimulate autophagy (Figure 5).
Figure 5.
Effect of 24-h LY treatment on the AMPK-mTOR-S6K signaling pathway. A549 cells were inoculated into 6-well plates followed by addition of various concentrations of LY and cultured for 24 h. Then proteins were extracted for analysis of AMPK, mTOR, or P70S6K by Western blot. (A) Western blot results. (B) Analysis of phosphorylation of AMPK. (C) Analysis of phosphorylation of mTOR. (D) Analysis of phosphorylation of P70SK histogram. ** P<0.01, compared with control group.
Discussion
Autophagy and apoptosis play important roles in the maintenance of normal body functions. Induction of apoptosis of tumor cells is used for cancer treatment. The resistance of tumor cells in the treatment process is the main factor restricting anti-tumor therapy. As another type of programmed cell death, autophagy has become a popular new focus in cancer therapy. Autophagy is a dynamic equilibrium process that maintains the body’s cell metabolism and circulation. Upsetting this dynamic equilibrium causes autophagic cell death or apoptosis. Anti-tumor drugs regulate autophagy depending on cell type, drug concentration, and drug treatment time. Studies have found the presence of both autophagy and apoptosis in the treatment of tumor cells with curcumin [24–27]. In the present study, various concentrations of LY were used to treat A549 cells for 12 h and 24 h. Results showed that LY inhibited the growth of A549 cells in a time- and dose-dependent manner. Lower doses of LY (4 μM and 8 μM) treatment promoted autophagy of A549 cells, while the higher concentration of LY (16 μM) treatment induced apoptosis of A549 cells.
As a member of the ATG8 protein family, LC3 is now recognized as the most important marker of the autophagic signaling pathway. There are 2 types of LC3: LC3-I (18 kDa) and LC3-II (16 kDa). Under normal cell state, LC3 is cleaved by Atg4 at the carboxyl terminus to produce LC3-I. In autophagy, LC3-I is processed by ubiquitin-like systems, including Atg7 and Atg3, to produce LC3-II and is translocate to the autophagic body. Excessive autophagy can promote cell apoptosis. It has been shown that ionizing radiation can induce autophagy in human breast cancer cells, and promote the progression of apoptosis [28]. In our study, we found that low concentrations of LY increased the ratio of LC3-II/LC3-I. The 8-μM LY treatment showed the highest ratio of LC3-II/LC3-I. Higher concentration of LY (>8 μM) decreased the ratio of LC3-II/LC3-I. Caspase-3 is a member of the cysteine-aspartic protease family, which is generally considered a marker of the apoptosis signaling pathway. In normal cells, caspase-3 is mainly presented as an inactive zymogen (Pro-caspase-3) form. In the process of apoptosis, Pro-caspase-3 is hydrolyzed at Asp28~Ser29 and Asp175~Ser176 to form P17 (29~175) and P10 (182~277) fragments. The latter 2 can re-organize together to form caspase-3, which promotes the implementation of the apoptosis pathway. We found that caspase-3 was activated by LY treatment in a dose- and time-dependent manner. Thus, we believe that the low concentration of LY treatment (24 h) induced autophagy of A549, but the high concentrations of LY treatment (24 h) promoted apoptosis of A549 cells.
The results of this experiment showed that LY treatment of A549 cells for 24 h enhanced the phosphorylation of AMPK at Thr172 in a dose-dependent manner. Phosphorylation of AMPK can significantly reduce the activity of mTOR (Ser2481) and S6K (Thr389), thereby blocking downstream signaling. Inhibition of mTOR activity also promoted autophagy. The inhibitory effect of LY on A549 cell viability was stronger than that of curcumin (12 h and 24 h of IC50 >32 μM), and was comparable to that of cisplatin (12 and 24 h IC50: 24, 7.5 μM). These data suggest that within a certain range of concentrations and treatment times, LY-induced autophagy can promote apoptosis of A549 cells, primarily by enhancing AMPK activity and suppressing phosphorylation of mTOR and S6K.
Conclusions
Lycorine inhibited A549 cells proliferation by enhancing activation of AMPK and suppressing the mTOR-S6K signaling pathway. Lower concentrations of LY (4~8 μmol/L) can activate autophagy, and a higher concentration of LY (16 μmol/L) can induce apoptosis. These results provide a scientific basis for studying the effect of LY on tumor cell death, the relationship between autophagy and apoptosis, and the use of autophagy enhancers or inhibitors in cancer therapy.
Footnotes
Disclosure of conflict of interest
None.
Source of support: This work was supported by Supported by the National Natural Science Foundation of China (NO. 31071001 and 31271226)
References
- 1.Xue D, Han S, Jiang S, et al. Comprehensive geriatric assessment and traditional Chinese medicine intervention benefit symptom control in elderly patients with advanced non-small cell lung cancer. Med Oncol. 2015;32:114. doi: 10.1007/s12032-015-0563-5. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Wang Y, Cao L, et al. A conserved inhibitory mechanism of a lycorine derivative against enterovirus and hepatitis C virus. Antimicrob Agents Chemother. 2015;60:913–24. doi: 10.1128/AAC.02274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Cai J, Cheng J, et al. Design, synthesis and structure-activity relationship optimization of lycorine derivatives for HCV inhibition. Sci Rep. 2015;5:14972. doi: 10.1038/srep14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen JW, Ruan Y, Ren W, et al. Lycorine: A potential broad-spectrum agent against crop pathogenic fungi. J Microbiol Biotechnol. 2014;24:354–58. doi: 10.4014/jmb.1310.10063. [DOI] [PubMed] [Google Scholar]
- 5.Toriizuka Y, Kinoshita E, Kogure N, et al. New lycorine-type alkaloid from Lycoris traubii and evaluation of antitrypanosomal and antimalarial activities of lycorine derivatives. Bioorg Med Chem. 2008;16:10182–89. doi: 10.1016/j.bmc.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Jin G, Huang KM, et al. Lycorine suppresses RANKL-induced osteoclastogenesis in vitro and prevents ovariectomy-induced osteoporosis and titanium particle-induced osteolysis in vivo. Sci Rep. 2015;5:12853. doi: 10.1038/srep12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Cui EH. Study on effect of lycorine in inducing apoptosis of pulmonary carcinoma cell A549. Zhongguo Zhong Yao Za Zhi. 2015;40:3278–82. [PubMed] [Google Scholar]
- 8.Di Fazio P, Waldegger P, Jabari S, et al. Autophagy-related cell death by pan-histone deacetylase inhibition in liver cancer. Oncotarget. 2016;7(20):28998–9010. doi: 10.18632/oncotarget.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek SH, Lee JH, Ko JH, et al. Ginkgetin blocks constitutive STAT3 activation and induces apoptosis through induction of SHP-1 and PTEN tyrosine phosphatases. Phytother Res. 2016;30:567–76. doi: 10.1002/ptr.5557. [DOI] [PubMed] [Google Scholar]
- 10.Genov M, Kreiseder B, Nagl M, et al. Tetrahydroanthraquinone derivative (+/−)-4-deoxyaustrocortilutein induces cell cycle arrest and apoptosis in melanoma cells via upregulation of p21 and p53 and downregulation of NF-kappaB. J Cancer. 2016;7:555–68. doi: 10.7150/jca.13614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Sun K, Wang H, Dai Y. Knockdown of retinoblastoma protein may sensitize glioma cells to cisplatin through inhibition of autophagy. Neurosci Lett. 2016;620:137–42. doi: 10.1016/j.neulet.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Gui Y, Ren J, et al. Metformin protects against cisplatin-induced tubular cell apoptosis and acute kidney injury via AMPKalpha-regulated autophagy induction. Sci Rep. 2016;6:23975. doi: 10.1038/srep23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Hong S, Seong GJ, Kim CY. Cigarette smoke extract causes injury in primary retinal ganglion cells via apoptosis and autophagy. Curr Eye Res. 2016;4:1–6. doi: 10.3109/02713683.2015.1119856. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Tan M, Xie Z, et al. Inhibiting ROS-STAT3-dependent autophagy enhanced capsaicin-induced apoptosis in human hepatocellular carcinoma cells. Free Radic Res. 2016;50(7):1–31. doi: 10.3109/10715762.2016.1173689. [DOI] [PubMed] [Google Scholar]
- 15.Lin CY, Hsu SC, Lee HS, et al. Enhanced expression of glucose transporter-1 in vascular smooth muscle cells via the Akt/tuberous sclerosis complex subunit 2 (TSC2)/mammalian target of rapamycin (mTOR)/ribosomal S6 protein kinase (S6K) pathway in experimental renal failure. J Vasc Surg. 2013;57:475–85. doi: 10.1016/j.jvs.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Sanli T, Steinberg GR, Singh G, Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther. 2014;15:156–69. doi: 10.4161/cbt.26726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han D, Li SJ, Zhu YT, et al. LKB1/AMPK/mTOR signaling pathway in non-small-cell lung cancer. Asian Pac J Cancer Prev. 2013;14:4033–39. doi: 10.7314/apjcp.2013.14.7.4033. [DOI] [PubMed] [Google Scholar]
- 18.Cardnell RJ, Feng Y, Mukherjee S, et al. Activation of the PI3K/mTOR pathway following PARP inhibition in small cell lung cancer. PLoS One. 2016;11:e0152584. doi: 10.1371/journal.pone.0152584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie J, Tao ZH, Zhao J, et al. Glucose regulated protein 78 (GRP78) inhibits apoptosis and attentinutes chemosensitivity of gemcitabine in breast cancer cell via AKT/mitochondrial apoptotic pathway. Biochem Biophys Res Commun. 2016;474(3):612–19. doi: 10.1016/j.bbrc.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu GQ, Chai KQ, Zhu XM, et al. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget. 2016;7(18):26535–50. doi: 10.18632/oncotarget.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilicay E, Karahaliloglu Z, Hazer B, et al. Concanavaline A conjugated bacterial polyester-based PHBHHx nanoparticles loaded with curcumin for breast cancer therapy. J Microencapsul. 2016;33(3):274–85. doi: 10.3109/02652048.2016.1169325. [DOI] [PubMed] [Google Scholar]
- 23.Bollu VS, Barui AK, Mondal SK, et al. Curcumin-loaded silica-based mesoporous materials: Synthesis, characterization and cytotoxic properties against cancer cells. Mater Sci Eng C Mater Biol Appl. 2016;63:393–410. doi: 10.1016/j.msec.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Jia YL, Li J, Qin ZH, Liang ZQ. Autophagic and apoptotic mechanisms of curcumin-induced death in K562 cells. J Asian Nat Prod Res. 2009;11:918–28. doi: 10.1080/10286020903264077. [DOI] [PubMed] [Google Scholar]
- 25.Aoki H, Takada Y, Kondo S, et al. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 26.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isik A, Peker K, Gursul C, et al. The effect of ozone and naringin on intestinal ischemia/reperfusion injury in an experimental model. Int J Surg. 2015;21:38–44. doi: 10.1016/j.ijsu.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Kuger S, Corek E, Polat B, et al. Novel PI3K and mTOR inhibitor NVP-BEZ235 radiosensitizes breast cancer cell lines under normoxic and hypoxic conditions. Breast Cancer (Auckl) 2014;8:39–49. doi: 10.4137/BCBCR.S13693. [DOI] [PMC free article] [PubMed] [Google Scholar]