Abstract
Background
Alginate is a natural polysaccharide obtained from brown algae and has been shown to have numerous applications in biomedical science, such as wound healing, delivery of bioactive agents, and cell transplantation. Ovalbumin (OVA) peptide 323–339 has been reported to be involved in immune response.
Material/Methods
This work investigated the use of alginate particles as a carrier and adjuvant for the immune therapy of cancer. Alginate particles loaded with OVA peptide were produced via emulsion. A tumor model was established in C57BL/6J mice via subcutaneous injection of 3×105 B16-OVA tumor cells. The effect of alginate/OVA peptide on cell viability was analyzed by use of the CCK-8 assay kit. Activation of macrophages was examined by checking cell surface makers CD40 and CD86 by FACs.
Results
Alginate/OVA peptide inhibited tumor progression more effectively than using the peptide alone. The viability and uptake study illustrated that this particle is safe and non-toxic. The activation study demonstrated that alginate particles can promote the activation of surface markers on macrophages. ELISA assay showed that the particles with peptide can promote the secretion of inflammatory and effector cytokines from macrophages.
Conclusions
This study demonstrated that alginate has dual functions in immune therapy of cancer, serving both as a carrier and an adjuvant.
MeSH Keywords: Alginates, Cytokines, Macrophage Activation, Ovarian Neoplasms
Background
Biomaterials have been attractive in addressing challenging diseases such as cancer, infectious disease, and injuries [1]. Biomaterials have several unique advantages for these applications, such as biosafety, low cost, and easy accessibility [2]. Due to these advantages, biomaterials have been employed in biological system for drug delivery, tissue repair, and augmentation in the body [3, 4]. Alginate is a negatively charged polysaccharide usually extracted from seaweed [5]. Alginate is bio-compatible, low-cost, and non-toxic. In particular, alginate can interact with Ca2+ ions to form hydrogels. These hydrogels have a structure similar to extracellular tissues, and have been employed for wound healing [6,7]. In addition, alginate materials have also been used to encapsulate living, cells such as islet cells, for diabetic treatment [8,9].
In the interface of immunology and materials, alginate have been used to interact with immune cells for different biomedical applications. For example, alginate and poly-L-lysine have been assembled into thin structures to interact with macrophages and lymphocytes, where increased levels of IL1-β and TGF-β were stimulated from the cells [10]. Similarly, another study demonstrated that alginate can stimulate lymphocytes proliferation and promote IL-1 and TNF-α. In addition, other studies also demonstrated that alginate can promote both innate and adaptive immunity in a time- and dose-dependent manner [12]. Together, these studies demonstrated that alginate materials can affect immune cell functions. Many biologically active materials have been found to have anti-tumor effects [13], but sometimes they need to be delivered to the tumor site through carriers.
Ovalbumin (OVA) mainly exists in egg white and has been widely studied in allergy research. Several OVA peptides have been used for studies of immune response, such as 257–264 and 323–339 [14]. It has been demonstrated that OVA 257–264 peptides can be used to detect a strong CD8+ cytolytic T cell response. OVA 323–339, which encompasses an allergenic and antigenic epitope of the ovalbumin protein, has been used to study both T cell and B cell responses [15,16], but no studies have been performed to assess the effect of OVA 323–339 peptide in innate immune response.
In this work, alginate was used as a carrier to deliver OVA 323–339 peptide (NP/OVA) for cancer treatment. The alginate materials were synthesized into particle forms (nanoparticles, NP) and used in vitro and in vivo. The studies demonstrated that alginate particles can promote cytokines production in vitro, activate macrophages, and inhibit tumor progression. Our study indicates that alginate might be a bio-safe carrier for delivery of biological agents to target locations.
Material and Methods
Materials
Low viscosity alginate (medical grade) was from VWR. Ca2Cl2·2H2O was obtained from Sigma. Phosphate-buffered saline (PBS 1×) was from VWR. ACK (Ammonium-Chloride-Potassium) cell Lysing Buffer was obtained from Thermo scientific. DAPI (4′,6-diamidino-2-phenylindole) was from Invivogen. Cell culture medium RPMI and fetal bovine serum (FBS) were from VWR. Penicillin and streptomycin were from Invivogen.
Animals and cells
All the studies that involved use of animal followed local and governmental regulations on animal protections. Macrophages were isolated from mouse spleens. Briefly, spleens were dissected, ground, and filtered through a 40-μm nylon strainer. A single splenic cell suspension then was obtained. F4/80-positive and CD11c-negative cells were acquired by FACS sorting and cultured in complete RPMI1640 medium supplemented with 10% FBS at 37°C.
Alginate particle preparation
Alginate particles were prepared by ionic gelation method. Briefly, 5 mL 0.5 mM CaCl2 was added into 15 mL alginate solution drop by drop. The mixture was stirred at 800 rpm at room temperature for 2 h. The particles were then collected by centrifugation (17 500 G, 25 min). Extra-large particles were removed by filtration (40 μm porous filter, STEMCELL, USA). The particles were then washed twice with DI water, lyophilized, and stored at −80°C before use.
Tumor model
C57BL/6J mice were raised in the pathogen-free mouse room at the Medical School at Shanghai Jiaotong University. We injected 3×105 B16-OVA tumor cells into the flanks of mice through subcutaneous injection. Tumor dimensions were measured daily using a caliper. Mice were sacrificed upon tumor size reaching 1.5 cm2.
Viability study
The cells were seeded and analyzed using CCK-8 assay (YEASEN) following the manufacturer’s instructions. The Cell Counting Kit-8 (CCK-8) allows very convenient assays by utilizing Dojindo’s highly water-soluble tetrazolium salt. WST-8 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) produces a water-soluble formazan dye upon reduction by dehydrogenase in mitochondria in the presence of an electron carrier. Color depth is proportional to the cell proliferation. The absorbance value at 450 nm wavelength by enzyme standard instrument indirectly reflects the number of living cells.
Macrophage activation tests
Activation of macrophages was tested by assessing the expression of cell surface markers. Fluorescence-labeled antibodies against cell surface markers CD40+ (FITC) CD86+ (APC) were used to stain the cells by following the manufacturer’s instruction. Briefly, the cells were detached from the plates and washed with PBS 3 times. Then, cells were treated with CD40 and CD86 antibodies. The cells were then analyzed with flow cytometry (BD FACSCanto II Analyzer, BD Bioscience).
ELISA
Cell culture medium was collected after treatment and the level of cytokines IL-8, IL-1β, and G-CSF in the medium were tested using an ELISA kit (R&D Systems) by following the producer’s instructions. Costar 96-well ELISA plates were coated with specific antibodies overnight (4°C) and blocked for 1 h, RT. Supernatants diluted 1: 10 and standard concentration of cytokines (100 μL/well) were incubated overnight at 4°C, washed 3 times, and incubated with detection antibodies. After further triple washing, substrates were added for machine reading.
Result
The study began with producing the alginate nanoparticles loaded with peptide. Dynamic light scattering was also used to assess particle sizes. The particles had a diameter of 135±27 nm (Figure 1A). The particles were first imaged under a scanning electron microscope. Under a microscope, the particles have a spherical shape (Figure 1B). Loading of the OVA peptide is around 15.6 μg OVA per mg of carrier.
Figure 1.
Characterization of alginate particles carrying OVA peptide (NP/OVA). (A) Dynamic light scattering showing the size distribution of alginate particles with OVA peptide. The particles have a diameter of about 135 nm. (B) Scanning electron microscopy showing the morphological features of alginate particles carrying OVA peptide.
The particles with OVA peptide were then used for in vivo anti-cancer tests. For this study, the mice were immunized with different formulations on day 0 and day 14. On day 21, the mice were induced with B16-OVA tumor by implanting 3×105 tumor cells. Tumor sizes in 3 different groups (CTRL, NP, and NP/OVA) were monitored daily. The mice treated with NP/OVA had more dramatic inhibition of tumor progression compared to the other groups. The empty NP had a slight impact on tumor progression compared to CTRL (Figure 2A). Similar results were also revealed on the survival information: the mice treated with NP/OVA had the longest survival time compared to the other 2 groups; there was a slight but not dramatic, difference in the other 2 groups (CTRL and NP) (Figure 2B). In both studies, the OVA in soluble form also inhibited tumor progression and extend mouse survival time compared to CTRL and empty NP, indicating the anti-cancer capacity of this soluble peptide (Figure 2A, 2B).
Figure 2.
In vivo assessment of the anti-cancer functions of alginate particles carrying OVA peptide in a B-16 OVA mouse tumor model. Four groups of mice were used in this study: Control (mice with no treatment), NP (mice treated with alginate nanoparticles), OVA in soluble form (mice treated with soluble OVA), and NP/OVA (mice treated with alginate particles carrying OVA). (A) Tumor size over time in the 4 groups of mice. (B) Survival curve of the 4 groups of mice.
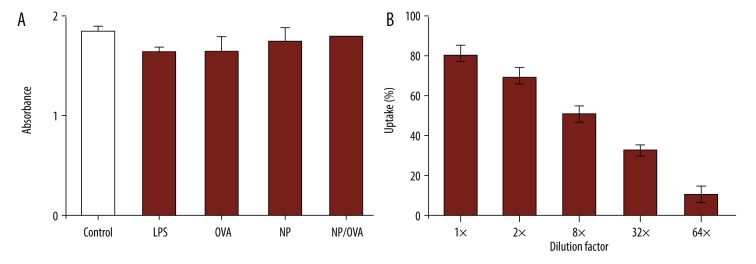
To determine the anti-cancer mechanism from NP/OVA, in vitro tests were performed. The particles were first interacted with macrophages to test the impact on cell viability. The macrophages isolated from mouse spleens were incubated with macrophages for 48 h for the viability tests. Our tests showed that nanoparticles loaded with OVA peptide did not affect cell viability (Figure 3A). In the viability tests, LPS, which is a macrophage stimulator, was used as positive control. Untreated cells were used as controls. We observed no difference between the NP/OVA and untreated groups in cell viability (Figure 3A). This study indicated that NP/SIIN are bio-safe for the in vitro tests. The NP/OVA were then used to interact with macrophages for the uptake tests. The tests began by using 400 μg NP/OVA-rhodamine to interact with the cells. A series dilution (1×, 2×, 8×, 8×, and 16×) was used to test the uptake. The flow cytometry study showed that the ratio of cells with alginate nanoparticles depended on the number of particles fed to the cells (Figure 3B). This result indicated that the macrophages can take up the particles very effectively.
Figure 3.
In vitro assessment of the effect of alginate particles carrying OVA on macrophage cell viability and uptake. (A) Cell viability after different treatments. (B) uptake of alginate particles by macrophages.
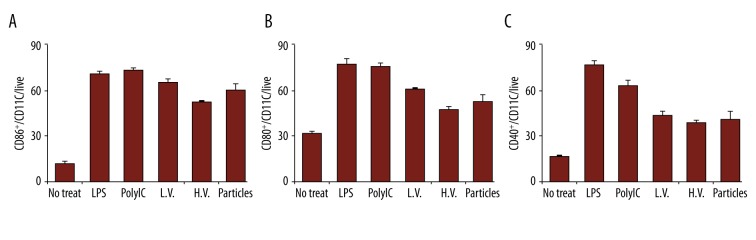
The next in vitro test in this study was the macrophage activation. Macrophages were treated with different formulations for 24 h, and then harvested for activation tests. Two surface markers, CD86 and CD40, were selected for this study. Flow cytometry assay showed that the particles can activate the 2 surface markers compared to cells with no treatment; the peptide alone has slight functions in activating these 2 markers. The NP/OVA kept the marker activation functions (Figure 4A, 4B). In both studies, LPS was selected as a control sample to indicate the successful activation of these markers.
Figure 4.
Activation of macrophage surface markers from different treatments. Activation of (A) CD86+ and (B) CD40+ cells.
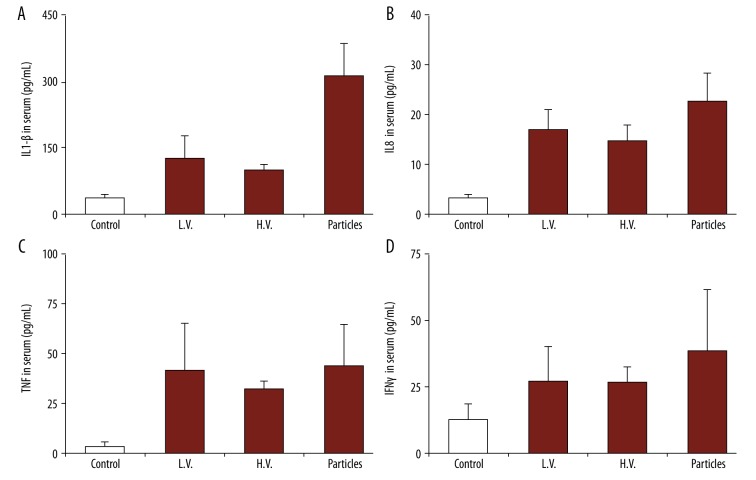
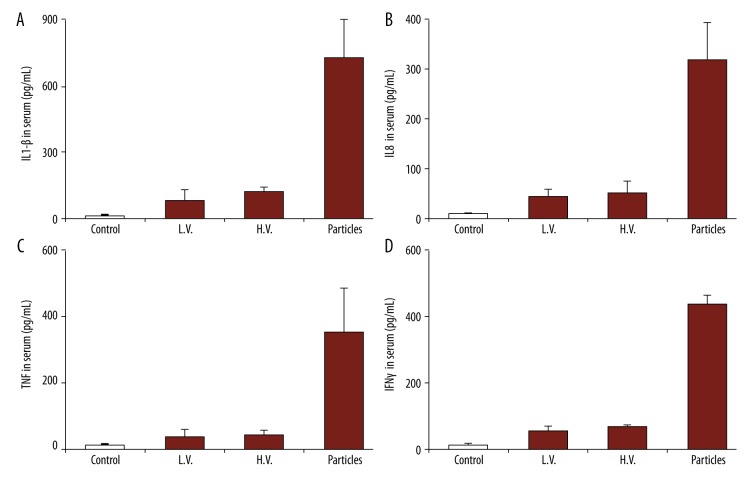
After the in vitro uptake tests, the particles with peptide were then used to treat the mice in vivo. Peripheral blood was collected from the mice 48 h after the treatment to test the level of serum. We first tested the production of cytokines IL-8 and IL-1β (Figure 5A). Compared to the mice with no treatment (CTRL), there was an obvious increase in IL-8 and IL-1β levels (Figure 5A). We also observed that the naked particles (particles with no peptide loading) also promoted the production of these 2 cytokines, and the levels were lower than with particles with peptide loading. We also tested cytokine G-CSF effector cytokines, which are responsible for anti-cancer immune responses (Figure 5B). We observed that the particles, either with or without the peptide, promoted the production of these cytokines. Our study also revealed that the levels of these 2 cytokines were higher than in the positive control (LPS), indicating alginate nanoparticles could be useful as vaccine adjuvants.
Figure 5.
Production of cytokines from macrophages with different treatments: no treatment, LPS, OVA (in soluble form), empty NP, and NP/OA (alginate particles carrying OVA). Secretion of (A) IL-8 and IL-1β, and (B) G-CSF.
Discussion
Alginate is a naturally occurring material that is being studied for a variety of biomedical applications. In addition to its dramatic advantages, such as biocompatibility and low-toxicity, another feature of this material is that, upon encountering divalent cations (e.g., Ca2+), alginate can form hydrogel or particles. In tissue engineering, this material has been exploited for wound-healing applications. Recent studies used alginate to deliver antigen for immune therapies [17]. For example, diphtheria toxoid, which is used against diphtheria, has been encapsulated into alginate particles in an anti-infection study. Upon interacting with the immune system, this material induced potent humoral response. In another relevant study, it was reported that alginate material can promote the production of inflammatory cytokines from macrophages [12].
Melanoma, a type of cancer that develops from the pigment-containing cells, is the most dangerous type of skin cancer. High-risk melanomas may require adjuvant therapy other than surgery, such as chemotherapy and immunotherapy. In this work, we studied the use of alginate materials for delivering OVA peptide for anti-cancer research. In vivo, we found that NP/OVA can inhibit tumor progression more effectively than soluble OVA, and extended the survival time of mice with tumors (Figure 2A, 2B). To investigate the anti-tumor mechanism, a variety of in vitro studies were performed, which revealed that macrophages, which are important in both innate and adaptive immune responses, played vital roles. The viability study confirmed this material is bio-safe and did not induce observable toxicity upon their uptake (Figure 3A, 3B).
To further explore the immunity from NP/OVA and macrophage interactions, we investigated the activation of macrophages and production of cytokines. For the activation study, 2 surface markers (CD86 and CD40) were assessed through flow cytometry. We found both surface markers are expressed at relatively high levels compared to CTRL. Comparing the activation of these markers in cells treated with OVA peptide, we conclude that the activation was mainly from the alginate particles (Figure 4A, 4B).
Similarly, the production of IL-8 and G-CSF cytokines also confirmed that alginate particles can promote cytokine secretion (Figure 5A, 5B). These results indicated that alginate particles can serve as an adjuvant to treat tumors, not only for melanomas, but also other tumors such as ovarian cancers, for which several biomarkers have been recently found [18–23]. We only investigated macrophages in this study, but during the immune responses to vaccination, other cells, especially dendritic cells, B cells, and natural killer cells, also have important functions. The roles of these cells will be studied in our future research.
Conclusions
Our work indicated that alginate particles are nontoxic, promote the activation of macrophages, and enhance the secretion of inflammatory and effector cytokines against cancer. Alginate particles loaded with OVA peptide inhibit tumor progression more effectively than using alginate or OVA peptide individually. This study provides a preliminary basis for using alginate as a carrier and adjuvant for anti-cancer therapy. Future studies needed to be done to clarify the underlining mechanism of alginate/OVA peptide, since our study only focused on the effect on macrophages. Other cells, such as dendritic cells, natural killer cells, and B cells, may also be involved in the anti-cancer effect.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Anhui Science Foundation (Grant #:1308085MC51) and Anhui Science Foundation for National Institutes and Colleges (KJ2012A034)
References
- 1.Weber JS, Mule JJ. Cancer immunotherapy meets biomaterials. Nat Biotechnol. 2015;33(1):44–45. doi: 10.1038/nbt.3119. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8(1):15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson B, Korsgren O, Lambris JD, Ekdahl KN. Can cells and biomaterials in therapeutic medicine be shielded from innate immune recognition? Trends Immunol. 2010;31(1):32–38. doi: 10.1016/j.it.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali OA, Mooney DJ. Immunologically active biomaterials for cancer therapy. Curr Top Microbiol. 2011;344:279–97. doi: 10.1007/82_2010_69. [DOI] [PubMed] [Google Scholar]
- 5.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliver Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 7.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs-Tulleneers-Thevissen D, Chintinne M, Ling Z, et al. Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient. Diabetologia. 2013;56(7):1605–14. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]
- 9.Soon-Shiong P, Heintz RE, Merideth N, et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343(8903):950–51. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 10.Robitaille R, Dusseault J, Henley N, et al. Inflammatory response to peritoneal implantation of alginate-poly-L-lysine microcapsules. Biomaterials. 2005;26(19):4119–27. doi: 10.1016/j.biomaterials.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Orive G, Tam SK, Pedraz JL, Halle JP. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27(20):3691–700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Jones KS. Effect of alginate on innate immune activation of macrophages. J Biomed Mater Res A. 2009;90(2):411–18. doi: 10.1002/jbm.a.32096. [DOI] [PubMed] [Google Scholar]
- 13.Long F, Wang T, Jia P, et al. Anti-tumor effects of atractylenolide-I on human ovarian cancer cells. Med Sci Monit. 2017;23:571–79. doi: 10.12659/MSM.902886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150(4):1212–22. [PubMed] [Google Scholar]
- 15.Bickert T, Wohlleben G, Brinkman M, et al. Murine polyomavirus-like particles induce maturation of bone marrow-derived dendritic cells and proliferation of T cells. Med Microbiol Immunol. 2007;196(1):31–39. doi: 10.1007/s00430-006-0026-x. [DOI] [PubMed] [Google Scholar]
- 16.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: Regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182(9):5188–92. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarei F, Dounighi NM, Zolfagharian H, et al. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J Pharm Sci. 2013;75(4):442–49. doi: 10.4103/0250-474X.119829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong L, Che H, Li M, Li X. Sam68 is overexpressed in epithelial ovarian cancer and promotes tumor cell proliferation. Med Sci Monit. 2016;22:3248–56. doi: 10.12659/MSM.899980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong L, Hui L. HOTAIR promotes proliferation, migration, and invasion of ovarian cancer SKOV3 cells through regulating PIK3R3. Med Sci Monit. 2016;22:325–31. doi: 10.12659/MSM.894913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Xu Y, Qiu W, et al. Tissue miR-193b as a novel biomarker for patients with ovarian cancer. Med Sci Monit. 2015;21:3929–34. doi: 10.12659/MSM.895407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu TT, Xu H, Gao WP, et al. SET and MYND domain-containing protein 3 (SMYD3) polymorphism as a risk factor for susceptibility and poor prognosis in ovarian cancer. Med Sci Monit. 2016;22:5131–40. doi: 10.12659/MSM.898095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Ma L, Rao Q, et al. MiR-1271 inhibits ovarian cancer growth by targeting cyclin G1. Med Sci Monit. 2015;21:3152–58. doi: 10.12659/MSM.895562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yiwei T, Hua H, Hui G, et al. HOTAIR interacting with MAPK1 regulates ovarian cancer skov3 cell proliferation, migration, and invasion. Med Sci Monit. 2015;21:1856–63. doi: 10.12659/MSM.893528. [DOI] [PMC free article] [PubMed] [Google Scholar]