Abstract
Nitrification inhibitors (NIs) have been used extensively to reduce nitrogen losses and increase crop nitrogen nutrition. However, information is still scant regarding the influence of NIs on nitrogen transformation, nitrous oxide (N2O) emission and nitrogen utilization in plastic-film-mulched calcareous soil under high frequency drip-fertigated condition. Therefore, a field trial was conducted to evaluate the effect of nitrapyrin (2-chloro-6-(trichloromethyl)-pyridine) on soil mineral nitrogen (N) transformation, N2O emission and nitrogen use efficiency (NUE) in a drip-fertigated cotton-growing calcareous field. Three treatments were established: control (no N fertilizer), urea (225 kg N ha-1) and urea+nitrapyrin (225 kg N ha-1+2.25 kg nitrapyrin ha-1). Compared with urea alone, urea plus nitrapyrin decreased the average N2O emission fluxes by 6.6–21.8% in June, July and August significantly in a drip-fertigation cycle. Urea application increased the seasonal cumulative N2O emission by 2.4 kg N ha-1 compared with control, and nitrapyrin addition significantly mitigated the seasonal N2O emission by 14.3% compared with urea only. During the main growing season, the average soil ammonium nitrogen (NH4+-N) concentration was 28.0% greater and soil nitrate nitrogen (NO3--N) concentration was 13.8% less in the urea+nitrapyrin treatment than in the urea treatment. Soil NO3--N and water-filled pore space (WFPS) were more closely correlated than soil NH4+-N with soil N2O fluxes under drip-fertigated condition (P<0.001). Compared with urea alone, urea plus nitrapyrin reduced the seasonal N2O emission factor (EF) by 32.4% while increasing nitrogen use efficiency by 10.7%. The results demonstrated that nitrapyrin addition significantly inhibited soil nitrification and maintained more NH4+-N in soil, mitigated N2O losses and improved nitrogen use efficiency in plastic-film-mulched calcareous soil under high frequency drip-fertigated condition.
Introduction
As a kind of potential greenhouse gas (GHG), nitrous oxide (N2O) causes climate change and indirectly contributes to stratospheric ozone depletion [1], and its global warming potential (GWP) is about 298 times that of carbon dioxide (CO2) over a 100 yrs time horizon [2]. In China, agricultural intensification and heavy fertilizer use have led to an increase in N2O emission losses, the contribution of croplands to the national total of fertilizer N-induced N2O (FIE-N2O) increased from 79% to 92% only in the period of 1980–2000 [3]. Thus, reducing N2O emissions from agricultural sources constitutes a critical research challenge.
Agricultural N2O emission has long been studied, however, whether N2O is mainly derived from nitrification process under aerobic conditions, or from denitrification process under either anaerobic or aerobic conditions is still inconclusive. The relative contribution of each process to N2O is largely dependent upon soil environmental conditions (e.g. soil organic matter, pH, soil moisture, soil N status, etc.). For instance, several previous studies suggest that nitrification accounts for most N2O emission in well-aerated soil below 60% water-filled pore space (WFPS), whereas denitrification reaction accounts for most N2O emission when soil moisture exceeds 70% WFPS [4–5]. Recent studies show that denitrification prevails over nitrification during N2O production for irrigated dryland soils or more humid soils initially [6–7].
Many researches demonstrate that N2O emission can be substantially mitigated by nitrification inhibitors (NIs) through reducing the rate of ammonium nitrogen (NH4+) oxidation into nitrate nitrogen (NO3-) during nitrification, and subsequently decreasing the substrate (NO3-) concentration for denitrificationin different agro-ecosystems [8–11]. Otherwise, NIs have been used to improve crop N status [8, 12] and increase soil mineral N retention [13]. As one of the effective nitrification inhibitors, nitrapyrin (2-chloro-6-(trichloromethyl)-pyridine) exhibited some effectiveness on reducing nitrate leaching and N2O emission losses, improving yield and N retention in the grain and corn field or under the simulated experimental condition [13–16]. We previously showed that nitrapyrin was more effective than dicyandiamide (DCD) in controlling nitrification process, and nearly as effective as 3, 4-dimethylpyrazole phosphate (DMPP) in calcareous soil with sandy, loam and clay texture [17]. Although numerous studies have been undertaken on the influences of different NIs on N2O emission and nitrogen transformations, how repeated supply of nitrapyrin with urea via fertigation impacts soil N2O emissions, nitrogen transformation and utilization under drip-irrigated condition is still unknown. Therefore, it is necessary to clarify the influences of repeated addition of nitrapyrin on soil N2O emissions and its major affecting factors in plastic-film-mulched drip-fertigated cotton field.
Xinjiang, located in northwest of China, is an important agricultural area. Due to the arid climate with annual precipitation of 160.6 mm on average, conventional agriculture in this region depends heavily on irrigation. To save fresh water resource and improve water utilization efficiency, a combination of plastic film-mulching with drip irrigation has been extensively adopted in this region during the last two decades. In this system, N fertilizer is applied about 8–10 times coupling with water via the irrigation system during a growing season. As a consequence, the re-wetting of the topsoil frequently occurs, and soil N transformation process in drip irrigation condition substantially differs from that in conventional cultivation condition (i.e. flooding irrigation and single fertilizer application).
The objectives of this study were to explore the effects of nitrapyrin added to the urea solution on N2O emission, soil mineral nitrogen transformations and nitrogen use efficiency in the plastic-film-mulched and high frequency drip-fertigated cotton field, and ascertain the major factors impacting on N2O emissions in this system. The results will provide a basis for decreasing nitrogen losses and promoting efficient utilization of nitrogen fertilizer through adding nitrapyrin to urea solution in drip-fertigated cropland in arid regions.
Materials and methods
Study site and soil characteristic
The field trial was set up at an experiment station of Agricultural College, Shihezi University, Xinjiang, China (44°18′N, 86°02′E) in April 2013. This site has a typical temperate continental climate and the annual precipitation varies between 125.0 and 207.7 mm.
The soil at the site is a Calcaric Fluvisals consisting of 24.7% sand, 20.1% silt and 48.8% clay. The initial physicochemical properties are: organic matter content, 16.2 g kg-1; total N content, 0.92 g kg-1; ammonium nitrogen content, 28.0 mg kg-1; nitrate nitrogen content, 40.9 mg kg-1; soil available phosphorus, 11.0 mg kg-1 and available potassium, 257.5 mg kg-1. The soil is alkaline (pH 7.96) with bulk density of 1.42 g cm-3 (0–15 cm layer). The groundwater is generally used for irrigation in this region.
Experimental design and field management
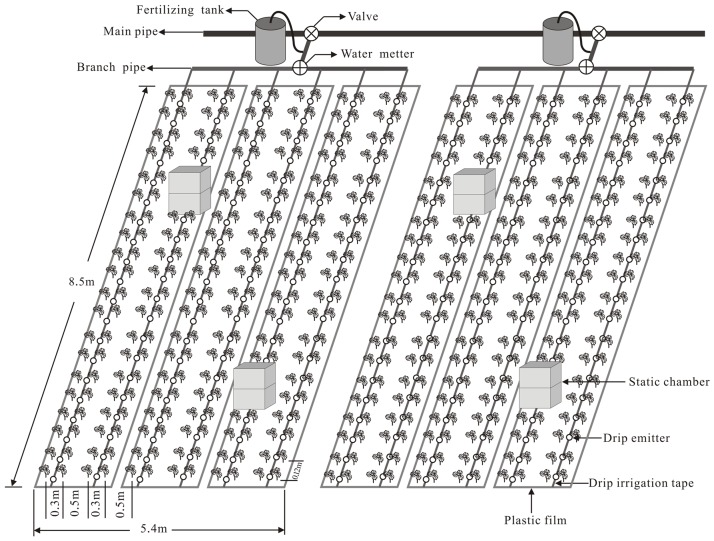
This experiment consisted of a randomized block field experiment with three treatments: (i) control (no N fertilizer), (ii) urea and (iii) urea+nitrapyrin. Each treatment was replicated three times. The area of each plot was 45.9 m2 (i.e. 5.4 m × 8.5 m), and 12 rows of cotton plants (Gossypium hirsutum cv. Xinluzao 45) were grown in each plot and the row spacing was 30 cm– 50 cm– 30 cm, and there was a 12 cm space between plants within a row (Fig 1). For each plot, a drip irrigation tape was placed in the middle of two rows, and six drip irrigation tapes were connected to a branch pipe controlled by a valve with a small fertilizing tank and a water meter installed to precisely control the irrigation volume and fertilizer rate.
Fig 1. Schematic diagram of the experimental plot layout with plastic-film-mulched and drip fertigation system.
A total of 465 mm irrigation water was applied to each plot in 10 split application times mainly in June, July, and August (Table 1). The nitrogen, phosphorus, and potassium fertilizers (urea and potassium dihydrogen phosphate) were dissolved in the irrigation water and applied during the first eight irrigation events. No urea was added to the irrigation water in the control treatment. The urea and urea+nitrapyrin treatments both received a total of 225 kg N ha-1, and all the treatments received a total of 90 kg ha-1 phosphorus pentoxide (P2O5) and 60 kg ha-1 potassium oxide (K2O) during the cotton growing season. Nitrapyrin, which was supplied by Zhejiang Aofutuo Chemical Ltd, P. R. China, was mixed into the irrigation water in the urea+nitrapyrin treatment at a rate of 1% urea-N. More detailed information about the schedule and rate of irrigation and fertilization was shown in Table 1.
Table 1. Drip fertigation schedule and the amounts of water and fertilizers.
| Fertigation time (d-m) |
13–06 | 23–06 | 04–07 | 12–07 | 23–07 | 04–08 | 12–08 | 22–08 | 30–08 | 04–09 |
|---|---|---|---|---|---|---|---|---|---|---|
| Water (mm ha-1) | 45 | 52.5 | 52.5 | 60 | 60 | 52.5 | 45 | 45 | 30 | 22.5 |
| N (kg ha-1) | 7.5 | 22.5 | 37.5 | 45 | 45 | 37.5 | 22.5 | 7.5 | 0 | 0 |
| P2O5 (kg ha-1) | 7.5 | 10.5 | 12 | 15 | 15 | 15 | 9 | 6 | 0 | 0 |
| K2O (kg ha-1) | 5 | 7 | 8 | 10 | 10 | 10 | 6 | 4 | 0 | 0 |
| Nitrapyrin (kg ha-1) | 0.075 | 0.225 | 0.375 | 0.45 | 0.45 | 0.375 | 0.225 | 0.075 | 0 | 0 |
The N rates are only for the urea and the urea+nitrapyrin treatments, nitrapyrin rates are only for the urea+nitrapyrin treatment, the rates of water, P2O5 and K2O are for all the treatments.
N2O sampling and measurement
Gas samples for N2O analysis were collected using static chambers consisting of top and middle chamber part, each being 60 cm×50 cm×55 cm for L×W×H in size to adapt to the height of the growing plants, and the final effective height reaching 110 cm. There were two chambers in each plot, the base of each chamber being inserted 10 cm deep into the soil to prevent air leakage, and each chamber having two fans to mix the air. The chambers were centred over two drip irrigation emitters and covered two rows of cotton plants (Fig 1). Gas samples were collected between 9:00 and 11:00 am on the 1, 2, 3, 4, 5 and 7 d after drip fertigation in June, July and August (fertilization season); four gas samples were collected from each chamber using 50 ml disposable syringes at 0, 10, 20 and 30 min after the chambers’ coverage. Meanwhile, soil temperature was measured using soil thermometers inserted to a depth of 5 cm inside the chambers.
The N2O concentrations in the gas samples from the field were determined within 12 h of collection using an Agilent 7890A gas chromatograph (Agilent Technologies Ltd, USA) equipped with a 2-mm ID stainless steel column, 3-m long and packed with Porapak Q (80/100 mesh), fitted with an electron capture detector (ECD) set at 300°C. The column temperature was maintained at 40°C and the carrier gas was N2 (99.999% purity) at a flow rate of 30 ml min-1.
Soil sampling and measurement
The determination of the initial soil organic matter, total N, available phosphorus, available potassium and pH was based on Lu [18]. In parallel with the sampling of N2O gas, surface soil sampling (0–15 cm in depth) was collected for the determination of soil mineral N (NH4+-N and NO3--N) concentrations and soil moisture. The soil samples were collected at six points randomly and mixed together to form one composite sample for each plot. The soil was sieved (2 mm mesh) and 5.00 g of fresh soil was extracted with 50 ml 2 M KCl solution for 1 h by shaking on a reciprocating shaker, then the extracts were filtered through N-free quantitative filter papers and frozen at -18°C for the determination of NH4+-N and NO3--N concentrations automatically on AA3-HR Continuous Flow Analytical System (SEAL Analytical Ltd, Germany). Soil water content was obtained by gravimetric determination after oven-drying of the samples at 105°C for 24 h to calculate WFPS.
Nitrogen determination and yield measurement in cotton plants
Five cotton plants were randomly sampled from the harvested cotton plants and separated into stems, leaves and reproductive organs (buds, flowers, bell shells, seeds and fibres). The plant organs were oven-dried at 105°C for half an hour, and then at 75°C for another 72 hours prior to dry matter measurement. The dried samples were crushed and sieved (0.5 mm mesh) for N determination. The content of N was determined on Kjeltec 8400 Automatic Kjeldahl Nitrogen Determination Apparatus (FOSS Analytical Ltd, U.S.A.). Cotton yield was measured by weighing all of the cotton (fibres and seeds) in each plot at maturity, and the lint yield (only fibres) was calculated according to the cotton yield multiplied by lint percent.
Estimations
N2O emission was calculated using Eq (1):
| (1) |
Where F (μg N m-2h-1) is N2O emission flux, M is the molecular weight of N2 in the N2O molecule (28 g N2O-N mol−1 for N2O), V is the mole volume (22.4 L mol−1) at 273 K and 1.013×105 Pa. H (m) is the effective chamber height (1.10 m in this experiment). P is air pressure in static chamber (Pa), P0 is the ambient air pressure at the experimental site (1.013×105 Pa), P/P0≈1. T is the temperature in the chamber (°C). c (ppbv) is the volume concentration of N2O, t (h) is the time of chamber closure, and dc/dt (ppbv h-1) is the rate of change in the N2O volume concentration in the chamber enclosure [19].
Cumulative N2O emission was calculated by Eq (2):
| (2) |
Where CE is N2O accumulative emission rate (kg N ha-1), F is N2O emission flux (μg N m-2h-1), i represents the ith times of N2O determination, (ti+1 − ti) is the time interval between two determinations, and n is the total determination times.
The calculation of the N2O emission factor (EF, %) was according to Eq (3):
| (3) |
Whereis the cumulative emissions of N2O in the urea or urea+nitrapyrin treatment (kg N ha-1), is the cumulative emissions of N2O in the control treatment (kg N ha-1), and N applied is the total N amount applied to urea or urea+nitrapyrin treatment (225 kg N ha-1 in this experiment).
Soil water-filled pore space (WFPS) was calculated based on Eq (4):
| (4) |
Where SWC is the soil water content (%), DB is the soil bulk density (g cm-3) and DP is the soil particle density (g cm-3). The particle density is assumed to be 2.65 g cm-3, and the soil bulk density in this experiment is 1.42 g cm-3.
The nitrogen use efficiency (NUE) was calculated according to Eq (5):
| (5) |
Where N uptake(treament) is the N uptake in cotton plants based on total aboveground biomass in the urea or urea+nitrapyrin treatment, N uptake(control) is the N uptake in cotton plants based on total aboveground biomass in the control treatment, N input is fertilizer N application rate (225 kg N ha-1 in this experiment).
Statistical analysis
The significance of differences among the different treatments in N2O, NH4+-N, NO3--N, N uptake, yield, EF of N2O and NUE were tested via Two-way ANOVA and Duncan multiple-comparison test using SPSS 13.0 software (IBM SPSS Statistics, Inc., USA). The direct and indirect effects of the driving factors on N2O emissions were performed through Pearson correlation and path analysis using SPSS 13.0.
Results and discussion
N2O emission in a drip-fertigated cycle
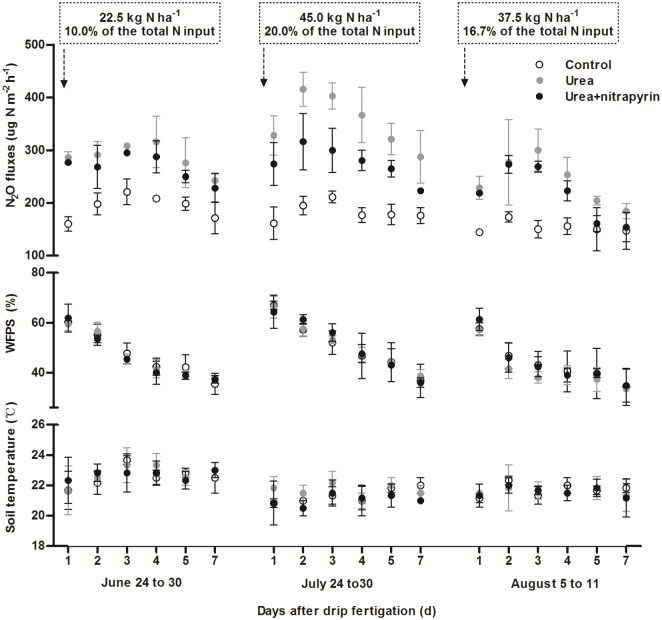
Under the drip-fertigated condition, some distinctive features occurring on N2O emissions were attributed to frequently re-wetting and repeatedly supplying of N fertilizer, thus leading to cycling of oxi-reduction and typical featuring of N2O dynamics (Fig 2). It was found that soil N2O fluxes increased to a maximum between 2 to 4 d after fertigation and then gradually declined within each fertigation cycle across all the three months (fertilizer season) tested, which is in line with the previous reports [20–21]. The greatest emissions of N2O occurred in July due to the majority of water and fertilizer N addition. More N application and lower N2O emission were observed in August than in June, suggesting that the nearly mature cotton plants absorbed N more quickly after fertigation in August, with the result such that less N was available for conversion to N2O [22–23].
Fig 2. Dynamics of soil N2O emissions, water-filled pore space (WFPS) and soil temperature at the 5 cm depth.
Error bars represent standard deviation.
A marked variation in N2O emissions was observed in three treatments as shown in Fig 2. The average fluxes of soil N2O kept the lowest values of 193.0±7.9, 183.2±13.9 and 153.6±8.6 μg N m-2h-1 in June, July and August for the control treatment in seven days after drip fertigation. In the urea treatment, the average fluxes of N2O were increased to 286.8±4.6, 353.6±21.1 and 241.5±5.8 μg N m-2h-1, respectively, and these values were reduced to 267.8±6.1, 276.4±13.1 and 216.5±8.5 μg N m-2h-1, respectively, in urea+nitrapyrin treatment. In a fertigation cycle, nitrapyrin addition decreased N2O fluxes by 6.6% (P<0.05), 21.8% (P<0.01) and 10.3% (P<0.01), respectively, in June, July and August compared with urea only. This shows that urea plus nitrapyrin can significantly reduce soil N2O emission in a drip-fertigated cycle.
Cumulative N2O emission during the main growing season of cotton plants
As shown in Table 2, cumulative N2O emissions in the urea treatment were significantly higher than in the control treatment (P<0.001). Compared with the urea treatment, application of urea with nitrapyrin (urea+nitrapyrin treatment) reduced cumulative N2O emissions by 7.1% (P<0.05), 21.9% (P<0.01) and 11.6% (P<0.01) in the three months tested accordingly. Urea application increased cumulative N2O emission by 2.4±0.1 kg N ha-1 during the main growing season, while nitrapyrin addition mitigated N2O emission by 14.3% (P<0.01) at the rate of 225 kg N ha-1.
Table 2. Cumulative soil N2O emission during the main growing season of cotton plants.
| Treatments | N2O emissions rates (kg N ha-1) | Total | ||
|---|---|---|---|---|
| June | July | August | ||
| Control | 1.13 ± 0.05 c | 1.06 ± 0.07 C | 0.86 ± 0.07 C | 3.05 ± 0.05 C |
| Urea | 1.76 ± 0.05 a | 2.18 ± 0.14 A | 1.48 ± 0.03 A | 5.42 ± 0.11 A |
| Urea+nitrapyrin | 1.64 ± 0.04 b | 1.70 ± 0.07 B | 1.31 ± 0.06 B | 4.65 ± 0.06 B |
Data were means ± SD (n = 3); Different lowercase and uppercase letters in same column mean significant differences at the level of 0.05 and 0.01, respectively according to LSD test.
At the similar rate of N application, the rate of N2O emissions mitigated by nitrapyrin is distinctly affected by different crop ecosystems. For instance, Xiong et al. [24] reported that the N2O emissions decreased by 8.8, 21.0 and 24.3% with nitrapyrin mixed with fertilizer N at the corresponding rate of 200, 300 and 400 kg N ha-1 in vegetable system, and a reduction rate of 50% was found by Sun et al. [25] in rice field treated with 180 and 240 kg N ha-1. This difference also relates to the supply of water and fertilization. Although the reduction rate of N2O emission by nitrapyrin in our study was lower compared with some other reports [13–16, 26], the differences in N2O emission between urea+nitrapyrin and urea treatment were still statistically significant (Table 2). In addition, the relatively low rate of reducing N2O in this study might be associated with the lower N fertilizer rate (225 kg N ha-1) as the typical rate of N commonly used by local cotton farmers in northwest of China was above 300 kg N ha-1. It is speculative that the effect of nitrapyrin in reducing N2O emission could be considerably higher in the condition of higher N input.
In this study, the fertilizer was applied completely during the main growing season of cotton plants (June, July and August), as a consequence, higher N2O emissions occurred in these months than in other growing phase. Therefore, controlling N2O release during the fertilizer season will contribute to the reduction of annual N2O emissions, especially when other conditions (e.g. initial soil physical and chemical properties, water input, environment and agronomic management) are identical; annual emission characteristic and reducing rate of N2O can be deduced indirectly through analyzing the seasonal cumulative N2O emission.
Soil ammonium and nitrate dynamics
Soil N2O emission is yielded by both nitrification and denitrification processes, therefore, it may be closely related to soil NH4+-N and NO3--N concentrations. Due to the strong nitrification usually taking place in aerobic condition, the nitrate form of mineral N is quite predominant in calcareous soils. As a result, denitrification would be prompted by the large amounts of NO3- accumulated in soil, especially in the high soil moisture condition. For a given soil, whether nitrification or denitrification contributed more to N2O emission may be closely related to soil NH4+-N and NO3--N concentrations when the initial physic-chemical factors of the tested soil are almost identical.
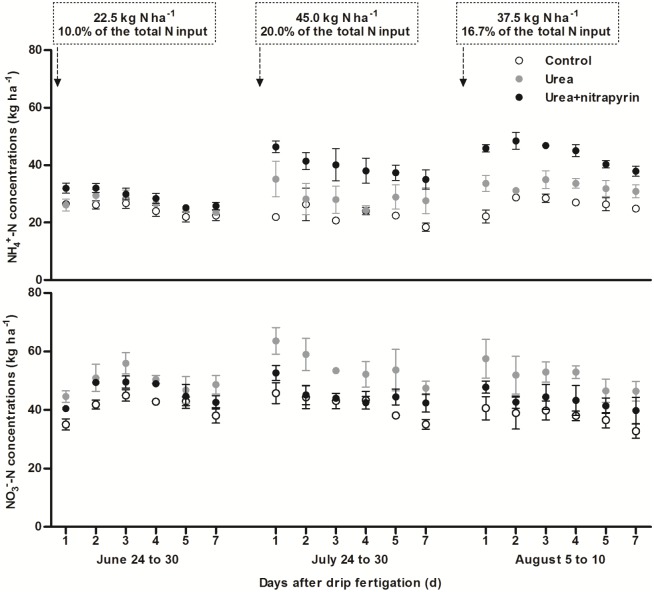
In this experiment, soil NH4+-N and NO3--N concentrations were significantly greater in both urea and urea+nitripyrin treatments than in the control (Fig 3). It was found that soil NH4+-N concentrations were consistently greater in the urea+nitrapyrin treatment than in the urea treatment while the reverse was true for the NO3--N concentrations (Fig 3). For example, the average soil NH4+-N concentrations in seven days after fertigation were 8.3% (P<0.05), 38.2% (P<0.01) and 34.8% (P<0.01) greater in the urea+nitrapyrin treatment than in the urea treatment in June, July and August, respectively, while soil NO3--N concentrations were 7.3% (P<0.05), 17.7% (P<0.01) and 15.8% (P<0.01) less in the urea+nitrapyrin treatment than in the urea treatment in June, July and August, respectively. During the main growing season, the average soil NH4+-N concentration was 28.0% greater and soil NO3--N concentration was 13.8% less in the urea+nitrapyrin treatment than in the urea treatment. These results indicate that nitrapyrin addition significantly inhibited soil nitrification, and shifted the soil inorganic nitrogen form from nitrate to ammonium. Earlier reports showed that soil NO3--N concentration was 23% less and N2O emissions were 26 to 49% less when nitrification inhibitors (i.e., DCD and DMPP) were added to ammonium sulfate-fertilized soil [8], while soil NH4+-N concentration was increased by 41% and NO3--N concentration was decreased by 39% in NH4+ low soils after nitrapyrin was added [26]. Our findings were less than the above reports probably due to the differences in the ecological systems, N applied rate or NI types used, etc. Overall, nitrapyrin addition significantly inhibited nitrification, and kept the soil NO3- concentration lower, consequently decreasing the concentration of substrate used for denitrification under high-frequency-irrigation condition in this study.
Fig 3. Dynamics of soil NH4+-N and NO3--N concentrations in the 0–15 cm depth soil layer.
Error bars represent standard deviation.
Driving factors affecting N2O emission
Many factors (e.g. soil temperature, WFPS, soil NH4+-N and soil NO3--N) influence soil N2O emission [27–28]. In this study, the factors driving N2O emissions are shown in Table 3. Interestingly, significant positive Pearson correlations were noted of soil N2O emissions with soil NO3--N concentrations (r = 0.628, P<0.001, n = 162), WFPS (r = 0.288, P<0.001, n = 162) and NH4+-N concentrations (r = 0.196, P<0.05, n = 162), but no significant correlation was found between soil N2O emission and soil temperature at the 5 cm depth. The effect of these factors on N2O emission decreased in the order of NO3--N > WFPS >NH4+-N >soil temperature. This indicates that soil NO3--N and WFPS are the key factors affecting N2O emission although NH4+-N concentrations were also significantly correlated with N2O fluxes as previously reported in a rape field [29].
Table 3. Pearson correlation and path analysis between N2O fluxes and soil NH4+-N concentrations, NO3--N concentrations, water-filled pore space (WFPS), and soil temperature at the 5 cm depth (T5cm).
| Independent variables | Pearson correlation coefficients | Direct path coefficients | Indirect path coefficients | ||||
|---|---|---|---|---|---|---|---|
| NH4+-N | NO3--N | WFPS | T5cm | SUM | |||
| NH4+-N | 0.196* | 0.139 | – | 0.081 | 0.013 | -0.036 | 0.057 |
| NO3--N | 0.628*** | 0.563 | 0.020 | – | 0.040 | 0.006 | 0.066 |
| WFPS | 0.288*** | 0.162 | 0.011 | 0.139 | – | -0.023 | 0.126 |
| Soil temperature | 0.108 | 0.144 | -0.035 | 0.024 | -0.026 | – | 0.037 |
* and ***denote significance at the 0.05 and 0.001 probability levels-2-tailed, respectively (n = 162).
We can further determine the direct and indirect effects of the above factors on N2O emissions through path analysis (Table 3). In this study, soil NH4+-N concentration, soil NO3--N concentration, WFPS, and soil temperature at the surface layer (0–5 cm in depth) directly impacted N2O emission, especially soil NO3--N. Although the indirect effects of these factors on N2O were much weaker than the direct effects, it was still found that WFPS also regulated N2O emission through affecting NO3--N. From the result of the Pearson and path analysis, N2O emission was largely co-regulated by both soil NO3--N concentrations and WFPS under this film-mulched drip-fertigated condition. In addition, it was more difficult to obtain a significant correlation between N2O and soil temperature probably due to low soil temperature (<25°C) [30] and relatively narrow range of soil temperature (20–24°C) in this test.
N2O can be produced via both nitrification and denitrification when soil WFPS is at an intermediate levels (i.e. 30% -70%) [4–5, 31]. It was discovered by Abalos et al. [27] that frequent irrigation (once per week) could maintain soil WFPS in the upper soil layer at a higher level than did conventional flood irrigation, which significantly stimulated denitrification. As a result, N2O emission was greater with frequent drip irrigation than with conventional irrigation. In our study, frequent rewetting with drip irrigation maintained soil WFPS between 31.2% and 67.0% (Fig 2), suggesting that both nitrification and denitrification could contribute to N2O emission. Combining with the strong relationship between N2O and soil NO3--N in Pearson correlation and path analysis, we could preliminarily speculate that more N2O was produced via denitrification in this plastic-film-mulched drip-fertigated system. However, more precise future work is needed to determine whether denitrification dominates nitrification during frequent rewetting of soil due to drip-fertigation in plastic-film-mulched calcareous soil.
The effect of nitrapyrin on the EF of soil N2O, N uptake, yield and NUE
A wide range of N2O emission factors (0.01–3.7%) is shown in different countries and regions [32–35] due to the differences in soil, environment, temperature, crops and management practices. In a rice-winter wheat rotation system in southeast China, the fertilizer-induced emission factor for N2O averaged 1.02% during the rice season, 1.65% during the wheat season, and 1.25% during the whole annual cycle [36]. Scheer et al. [37] reported an average N2O emission factor of 1.48% in an irrigated cotton field in Uzbekistan (the arid deserts of Aral Sea Basin). Liu et al. [38–39] also showed that direct N2O emission factors in a cotton field varied between 0.9% and 2.2%. However, information is still lacking on soil N2O emission under plastic-film-mulched drip-fertigated condition. Similar to the previous results in China [36, 38–40], the EF of N2O in this study was 1.05% in the urea treatment during the main growing season of cotton plants, compared to 0.71% when nitrapyrin was added to the urea solution (Table 4); nitrapyrin addition decreased the EF of N2O by 32.4%. In view of the fertilizer N being applied completely in three months (the main growing seasons), it can be speculated that the annual EF of N2O would be higher than the seasonal EF based on the continued effectiveness of fertilizer N, and the difference between the seasonal and annual EF would not be too much on account of lower N input (225 kg N ha-1) in this experiment.
Table 4. Yield, N uptake, emission factor (EF) of N2O and nitrogen use efficiency (NUE).
| Treatments | Lint yield (t ha-1) |
Aboveground N uptake (kg ha-1) |
EF of N2O (%) |
NUE (%) |
|---|---|---|---|---|
| Control | 1.91 ± 0.10 b | 236.92 ± 5.02 b | - | - |
| Urea | 2.48 ± 0.19 a | 356.95 ± 12.39 a | 1.05 ± 0.04 B | 53.35 ± 3.28 b |
| Urea+nitrapyrin | 2.58 ± 0.18 a | 369.79 ± 8.68 a | 0.71 ± 0.03 A | 59.05 ± 1.63 a |
Data were means ± SD (n = 3); Different lowercase and uppercase letters in same column mean significant differences at the level of 0.05 and 0.01, respectively according to LSD test.
Compared with urea alone, the yield and nitrogen uptake in the urea+nitrapyrin treatment were increased by 4.1 and 3.6% (P>0.05), respectively. The yield in this study was lower than reported earlier [13, 41–42]. Our findings were more similar to the report by Crawford and Chalk [43] who found that there was no significant difference in yield between fertilizer N plus nitrapyrin and N applied only. Under the tested condition of drip-irrigated plot with input of 225 kg N ha-1, the relatively high NUE (>50%) was gained, and more importantly, nitrapyrin addition increased NUE by 10.7% (P<0.05). Zhang et al. [44] observed that nitrapyrin raised NUE by 12.6% (P<0.05) in an intensively managed vegetable cropping system, and a similar finding was reported by Sun et al. [25] in a rice field. Clearly, these discrepancies were probably attributed to the differences indifferent crops tested as cotton crop tested in this study is less responsive to the enhanced ammonium nutrition caused by nitrificantion inhibitors, than wheat, corn or rice used in previous studies [13, 41–42].
Conclusions
In plastic-film-mulched and high frequency drip-fertigated system, repeated use of the nitrapyrin significantly inhibited nitrification, shifted the ratio of soil NH4+ to NO3-, decreased N2O emission by 14.3% and EF of N2O by 32.4%, increased NUE by 10.7% in the calcareous cotton field amended with urea at the rate of 225 kg N ha-1. NO3- was the key factor impacting on N2O emissions in this system; nitrapyrin addition decreased the concentration of denitrifying substrate (NO3-) through inhibiting nitrification process and also reduced N2O emission from denitrification process. Taken together, the repeated application of nitrapyrin through drip fertigation is an efficient approach to reducing N losses and promoting nitrogen use efficiency in plastic-film-mulched drip-fertigated calcareous soil in arid areas.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Our work was supported by three resources of funding; they were: The Natural Science Foundation of China (NSFC) entitled “The response of N2O emission and soil nitrification-denireification key processing microbial molecular ecological mechanism on nitrapyrin in drip irrigation cropland” (approved no. 41461064), P. R. China; The National Key Basic Research Support Foundation of China (NKBRSF) entitled “Soil Microbial Community Structure in Paddy-upland Crop Rotation System in Relation to Efficient Nitrogen Use in Paddy Field” (approved no. 2015CB150502); The 12th Five-Year Key Programs for Science and Technology Development of China entitled “Study of Key Technologies for Alleviating Obstacle Factors and Improving Productivity of Low-yield Cropland” (approved no. 2012BAD05B06).
References
- 1.Ravishankara AR, Daniel JS, Portman RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 2009; 326: 123–125. 10.1126/science.1176985 [DOI] [PubMed] [Google Scholar]
- 2.IPCC, Climate change 2007: the physical science basis In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt K, et al. (Eds.). Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom; New York, NY, USA, 2007; 996. [Google Scholar]
- 3.Zou JW, Lu YY, Huang Y. Estimates of synthetic fertilizer N-induced direct nitrous oxide emission from Chinese croplands during 1980–2000. Environ. Pollut. 2010; 158 (2): 631–635. 10.1016/j.envpol.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 4.Pihlatie M, Syväsalo E, Simojoki A, Esala M, Regina K. Contribution of nitrification and denitrification to N2O production in peat, clay and loamy sand soils under different soil moisture conditions. Nutr. Cycl. Agroecosyst. 2004; 70:135–141. [Google Scholar]
- 5.Bateman EJ, Baggs EM. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Bio. Fertil. Soil 2005; 41: 379–388. [Google Scholar]
- 6.Scheer C, Wassmann R, Butterbach-Bahl K, Lamers JPA, Martius C. The relationship between N2O, NO, and N2 fluxes from fertilized and irrigated dryland soils of the Aral Sea Basin, Uzbekistan. Plant Soil 2009; 314: 273–283. [Google Scholar]
- 7.Vilain G, Garnier J, Decuq C, Lugnot M. Nitrous oxide production from soil experiments: denitrification prevails over nitrification. Nutr. Cycl. Agroecosyst. 2014; 98(2):1–18. [Google Scholar]
- 8.Weiske A, Benckiser G, Herbert T, Ottow JCG. Influence of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol. Fertil. Soils. 2001; 34: 109–117. [Google Scholar]
- 9.Merino P, Mene´ndez S, Pinto M, Gonza´lez-Murua C, Estavillo JM. 3, 4-Dimethylpyrazole phosphate reduces nitrous oxide emissions from grassland after slurry application. Soil Use Manage. 2005; 21: 53–57. [Google Scholar]
- 10.Zaman M, Saggar S, Blennerhassett JD, Singh J. Effect of urease and nitrification inhibitors on N transformation gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol. Biochem. 2009; 41: 1270–1280. [Google Scholar]
- 11.Di HJ, Cameron KC. How does the application of different nitrification inhibitors affect nitrous oxide emissions and nitrate leaching from cow urine in grazed pastures? Soil Use Manage. 2012; 28: 54–61. [Google Scholar]
- 12.Freney JR, Chen DL, Mosier AR, Rochester IJ, Constable GA, Chalk PM. Use of nitrification inhibitors to increase fertilizer nitrogen recovery and lint yield in irrigated cotton. Fertil. Res.1993; 34: 37–44. [Google Scholar]
- 13.Wolt JD. A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the Midwestern USA. Nutr. Cycl. Agroecosyst. 2004; 69: 23–41. [Google Scholar]
- 14.Aulakh MS, Rennie DA, Paul EA. Acetylene and N-Serve effects upon N2O emissions from NH4 and NO3 treated soils under aerobic and anaerobic conditions. Soil Bio. Biochem. 1984; 16: 351–356. [Google Scholar]
- 15.Bronson KF, Mosier AR, Bishnoi SR. Nitrous oxide emissions in irrigated corn as affected by nitrification inhibitors. Soil Sci. Soc. Am. J. 1992; 56: 161–165. [Google Scholar]
- 16.Parkin TB, Hatfield JL. Influence of nitrapyrin on N2O losses from soil receiving fall-applied anhydrous ammonia. Agric. Ecosyst. Environ. 2010; 136: 81–86. [Google Scholar]
- 17.Liu T, Liang YC, Chu GX, Ma D, Liu Q, Wang J. Effect comparison of three different types of nitrification inhibitors (DCD, DMPP and nitrapyrin) in calcareous soils. Soils 2011; 43(5): 758–762. [Google Scholar]
- 18.Lu RK. Analytical methods of soil agrichemistry. Beijing: China Agricultural Science and Technology Press, 2000. [Google Scholar]
- 19.Zheng XH, Wang MX, Wang YS, Shen RX, Gou J, Li J, et al. Impacts of soil moisture on nitrous oxide emission from croplands: a case study on the rice-based agro-ecosystem in Southeast China. Chemosphere Global Change Sci. 2000; 2: 204–227. [Google Scholar]
- 20.Majumdar D, Pathak H, Kumar S, Jain MC. Nitrous oxide emission from a sandy loam Inceptisol under irrigated wheat in India as influenced by different nitrification inhibitors. Agric. Ecosyst. Environ. 2002; 91: 283–293. [Google Scholar]
- 21.Bhatia A, Sasmal S, Jain N, Pathak H, Kumar R, Singh A. Mitigating nitrous oxide emission from soil under conventional and no-tillage in wheat using nitrification inhibitors. Agric. Ecosyst. Environ. 2010; 136: 247–253. [Google Scholar]
- 22.Duxbury JM, Bouldin DR, Tate RL. Emission of nitrous oxide from soils. Nature 1982; 298: 462–464. [Google Scholar]
- 23.Maljanen M, Liikanen A, Silvola J, Martikainen PJ. Nitrous oxide emission from boreal organic soil under different land-use. Soil Biol. Biochem. 2003; 35: 689–700. [Google Scholar]
- 24.Xiong W, Xia YQ, Zhou W, Yan XY. Relationship between nitrogen application rate and nitrous oxide emission and effect of nitrification inhibitor in vegetable farming system. Acta Pedologica Sinica. 2013; 50: 743–751. [Google Scholar]
- 25.Sun HJ, Zhang HL, Powlson D, Min J, Shi WM. Rice production, nitrous oxide emission and ammonia volatilization as impacted by the nitrification inhibitor2-chloro-6-(trichloromethyl)-pyridine. Field Crop Res. 2015; 173: 1–7. [Google Scholar]
- 26.López NI, Austin AT, Sala OE, Méndez BS. Controls on nitrification in a water-limited ecosystem: Experimental inhibition of ammonia-oxidizing bacteria in the Patagonian steppe. Soil Biol. Biochem. 2003; 35(12): 1609–1613. [Google Scholar]
- 27.Davidson EA, Swank WT. Environmental parameters regulating gaseous nitrogen losses from two forested ecosystems via nitrification and denitrification. Appl. Environ. Microbiol. 1986; 52: 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abalos D, Sanchez-Martin L, Garcia-Torres L, Groenigen JW, Vallejo A. Management of irrigation frequency and nitrogen fertilization to mitigate GHG and NO emissions from drip-fertigated crops. Sci. Total. Environ. 2014; 490: 880–888. 10.1016/j.scitotenv.2014.05.065 [DOI] [PubMed] [Google Scholar]
- 29.Lin S, Iqbal J, Hu R, Wu J, Zhao J, Ruan L, et al. Nitrous oxide emissions from rape field as affected by nitrogen fertilizer management: A case study in Central China. Atmos. Environ. 2011; 45: 1775–1779. [Google Scholar]
- 30.Maljanen M, Martikkala M, Koponen HT, Virkajärvi P, Martikainen PJ. Fluxes of nitrous oxide and nitric oxide from experimental excreta patches in boreal agricultural soil. Soil Biol. Biochem. 2007; 39: 914–920. [Google Scholar]
- 31.Skiba U, Smith KA, Fowler D. Nitrification and denitrification as sources of nitric oxide and nitrous oxide in a sandy loam soil. Soil Biol. Biochem. 1993; 25: 1527–1536. [Google Scholar]
- 32.Brown L, Brow SA, Jarvis SC, Syed B, Goulding KWT, Phillips VR. An inventory of nitrous oxide emissions from agriculture in the UK using the IPCC methodology: emission estimate, uncertainty and sensitivity analysis. Atmos. Environ. 2001; 35: 1439–1449. [Google Scholar]
- 33.Velthof GL, Kuikman PJ, Oenema O. Nitrous oxide emission from soils amended with crop residues. Nutr. Cycl. Agroecosyst. 2002; 62: 249–261. [Google Scholar]
- 34.Ding WX, Cai Y, Cai ZC, Yagi K, Zheng XH. Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain. Sci. Total Environ. 2007; 373: 501–511. 10.1016/j.scitotenv.2006.12.026 [DOI] [PubMed] [Google Scholar]
- 35.Merino P, Artetxe A, Castellón A, Menéndez S, Aizpurua A, Estavillo JM. Warming potential of N2O emissions from rapeseed crop in Northern Spain. Soil Till. Res. 2012; 123: 29–34. [Google Scholar]
- 36.Zou JW, Huang Y, Lu YY, Zheng XH, Wang YS. Atmospheric Direct emission factor for N2O from rice–winter wheat rotation systems in southeast China. Atmos. Environ. 2005; 39: 4755–4765. [Google Scholar]
- 37.Scheer C, Wassmann R, Kienzler K, Ibragimov N, Eschanov R. Nitrous oxide emissions from fertilized, irrigated cotton (Gossypium hirsutum L.) in the Aral Sea Basin, Uzbekistan: Influence of nitrogen applications and irrigation practices. Soil Biol. Biochem. 2008; 40: 290–301. [Google Scholar]
- 38.Liu CY, Zheng XH, Zhou ZX, Han SH, Wang YH, Wang K, et al. Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China. Plant Soil.2010; 332:123–134. [Google Scholar]
- 39.Liu CY, Yao ZH, Wang K, Zheng XH. Three-year measurements of nitrous oxide emissions from cotton and wheat-maize rotational cropping systems. Atmos. Environ. 2014; 6: 201–208. [Google Scholar]
- 40.Zhang Q, Ju XT, Zhang FS. Re-estimation of direct nitrous oxide emission from agricultural soils of China via revised IPCC 2006 guideline method. Chin J Eco-Agric. 2010; 18(1): 7–13. [Google Scholar]
- 41.Swezey ANL, Turner GO. Crop experiments on the effect of 2-chloro-6-(trichloromethyl)-pyridine for the control of nitrification of ammonium and urea fertilizer. J Agron Crop Sci. 1962; 54: 532–535. [Google Scholar]
- 42.Huber DM, Warren HL, Nelson DW. Nitrification inhibitors: new tools for food production. Bioscience.1977; 27(8): 523–529. [Google Scholar]
- 43.Crawford DM, Chalk PM. Sources of N uptake by wheat (Triticum aestivum L.) and N transformations in soil treated with a nitrification inhibitor (nitrapyrin). Plant Soil 1993; 149(1): 59–72. [Google Scholar]
- 44.Zhang M, Fan CH, Li QL, Li B, Zhu YY, Xiong ZQ. A 2-yr field assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015. 201: 43–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.