Abstract
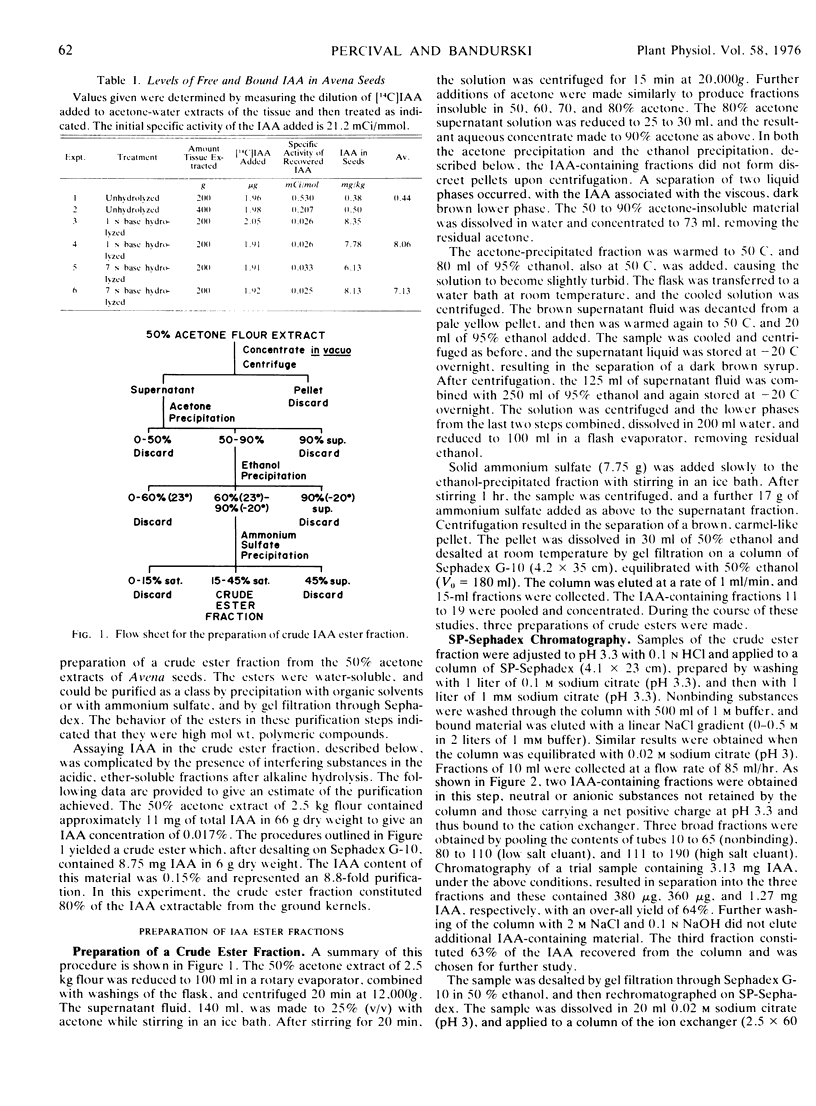
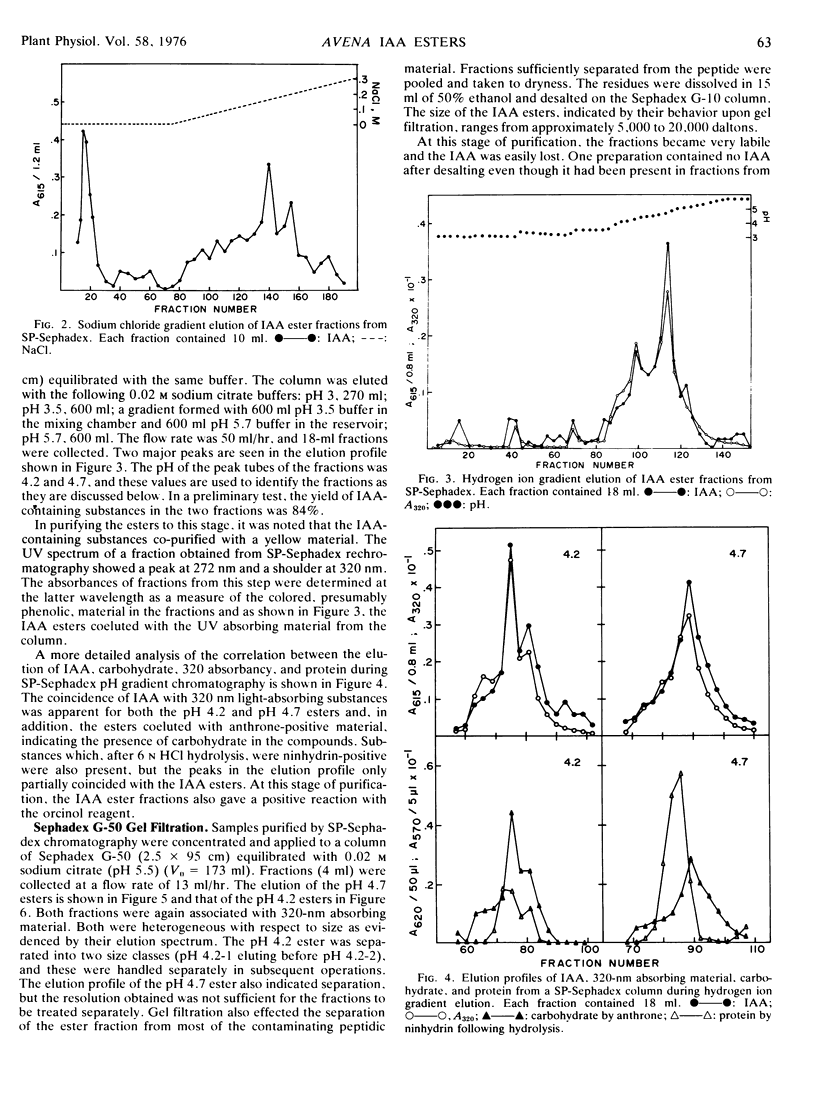
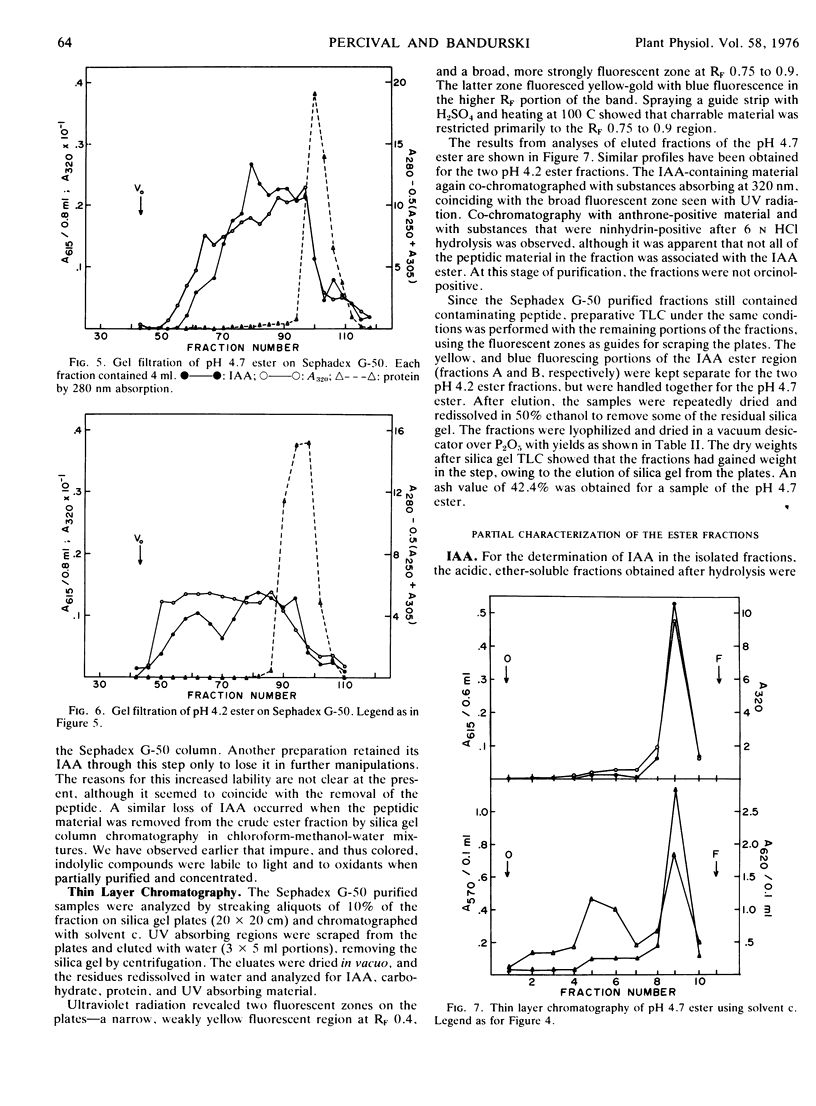
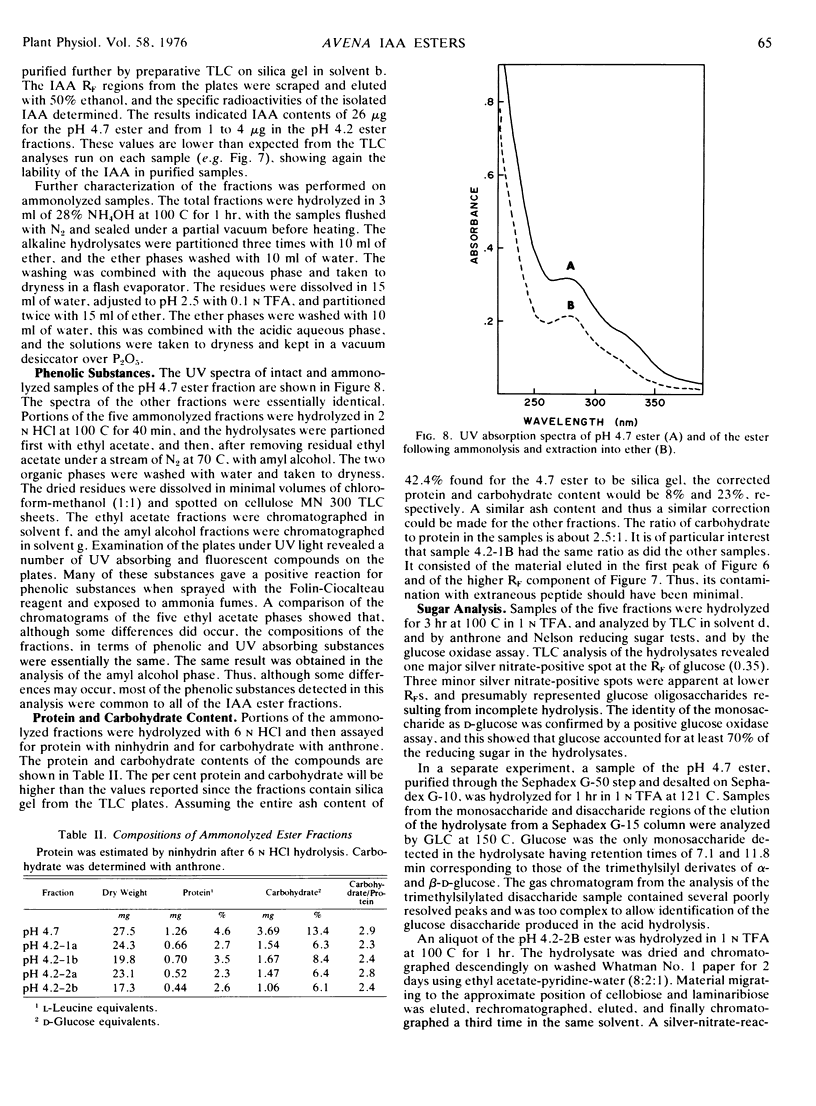
The present studies showed that about 80% of the indole-3-acetic acid extractable from Avena kernels by aqueous acetone was esterified to polymers precipitable by ammonium sulfate and ethanol or acetone. The polymers were positively charged, being adsorbed to cation exchange columns at a pH of 3, or below, and eluted at a pH greater than 4. The polymers were heterogeneous with respect to size, about 5,000 to 20,000 daltons, and charge, exhibiting apparent pKa values of 4.2 and 4.7. The polymer fractions contained esterified IAA, anthrone-reactive material that liberated glucose upon acid hydrolysis, phenolic compounds, and peptidic material with a high proportion of hydrophobic amino acids. Since the esterified IAA was unstable, establishing polymer purity was not possible, and the designation IAA-glucoprotein fraction was adopted.
Dehusked Avena kernels contained 8 mg/kg total IAA of which 5.5% was free and 94.5% esterified. IAA bound through a peptidic linkage was present, but in only trace amounts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandurski R. S., Schulze A. Concentrations of Indole-3-acetic Acid and Its Esters in Avena and Zea. Plant Physiol. 1974 Sep;54(3):257–262. doi: 10.1104/pp.54.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmann A. Identification of 2-O (indole-3-acetyl)-D-glucopyranose, 4-O-(indole-3-acetyl)-D-glucopyranose and 6-O-(indole-3-acetyl)-D-glucopyranose from kernels of Zea mays by gas-liquid chromatography-mass spectrometry. Carbohydr Res. 1974 May;34(1):99–114. doi: 10.1016/s0008-6215(00)80374-2. [DOI] [PubMed] [Google Scholar]
- Fincher G. B., Sawyer W. H., Stone B. A. Chemical and physical properties of an arabinogalactan-peptide from wheat endosperm. Biochem J. 1974 Jun;139(3):535–545. doi: 10.1042/bj1390535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Nicholls P. B., Bandurski R. S. A partial characterization of indoleacetylinositols from ZEA mays. Biochem Biophys Res Commun. 1965 Sep 8;20(5):641–646. doi: 10.1016/0006-291x(65)90448-1. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Ueda M., Bandurski R. S. A Quantitative Estimation of Alkali-labile Indole-3-Acetic Acid Compounds in Dormant and Germinating Maize Kernels. Plant Physiol. 1969 Aug;44(8):1175–1181. doi: 10.1104/pp.44.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDSCHMIDT-LEITZ E., ZWISLER O. UBER DIE PROTEINE DES HAFERS (XI. UBER SAMENPROTEINE) Hoppe Seylers Z Physiol Chem. 1963;332:216–224. doi: 10.1515/bchm2.1963.332.1.216. [DOI] [PubMed] [Google Scholar]
- ZENK M. H. I-(Indole-3-acetyl)-beta-D-glucose, a new compound in the metabolism of indole-3-acetic acid in plants. Nature. 1961 Jul 29;191:493–494. doi: 10.1038/191493a0. [DOI] [PubMed] [Google Scholar]


