Abstract
We recently demonstrated that AKT activation plays a role in prostate cancer progression and inhibits the pro-apoptotic function of FOXO3a and Par-4. AKT inhibition and Par-4 induction suppressed prostate cancer progression in preclinical models. Here, we investigate the chemopreventive effect of the phytonutrient Withaferin A (WA) on AKT-driven prostate tumorigenesis in a Pten conditional knockout (Pten-KO) mouse model of prostate cancer. Oral WA treatment was carried out at two different doses (3 and 5 mg/kg) and compared to vehicle over 45 weeks. Oral administration of WA for 45 weeks effectively inhibited primary tumor growth in comparison to vehicle controls. Pathological analysis showed the complete absence of metastatic lesions in organs from WA-treated mice, whereas discrete metastasis to the lungs was observed in control tumors. Immunohistochemical analysis revealed the down-regulation of pAKT expression and epithelial-to-mesenchymal transition markers, such as β-catenin and N-cadherin, in WA-treated tumors in comparison to controls. This result corroborates our previous findings from both cell culture and xenograft models of prostate cancer. Our findings demonstrate that the daily administration of a phytonutrient that targets AKT activation provides a safe and effective treatment for prostate cancer in a mouse model with strong potential for translation to human disease.
Introduction
Activated protein kinase B/AKT plays a central role in regulating downstream signaling pathways that control various cellular processes, such as proliferation, survival, cell cycle progression and epithelial–mesenchymal transition (EMT) [1], [2], [3]. Aberrant activation of AKT leads to tumorigenesis, drug resistance and metastasis. Therefore, AKT has become an important therapeutic target for various types of cancer, including prostate cancer (CaP) [4], [5], [6]. Despite advances in early detection and an improved understanding of the molecular mechanism of CaP, the mortality associated with CaP in the US remains high [7]. Genetically engineered mouse models (GEMMs) have been generated to recapitulate many of the key aspects of prostate tumor development [8], [9]. Conditional knockout GEMMs, in which tumor-associated genes are selectively inactivated in the cancer-bearing tissue of interest using Cre-LoxP recombination, are important tools for gaining mechanistic insights into cancer and for evaluating the efficacy of cancer therapeutics in clinically relevant models [10]. These GEMMs include a conditional phosphatase and tensin homologue (PTEN) tumor suppressor gene-deficient GEMM of the prostate, which is actively being studied as a context-specific model for evaluating the chemoprevention of CaP [4], [5].
The inactivation of the PTEN gene is one of the most frequently detected genetic alterations in CaP [3], [11], [12], [13], [14]. This inactivation often activates the oncogenic function of AKT in CaP [15], [16], [17]. Clinically, the inactivation of PTEN is associated with prostate tumor growth patterns, such as invasive ductal carcinoma (IDC), that are predictive of high-grade invasive and aggressive disease [18], [19], [20], [21]. PTEN loss is also observed in 45% of high-grade prostatic intraepithelial neoplasias (HG-PIN), which are a precursor lesion of adenocarcinoma, and in 70% of advanced CaP cases [22]. Therefore, the inhibition of AKT survival signaling and its downstream effectors represents a potential therapeutic strategy for the treatment of CaP. A number of small molecule inhibitors of AKT are being developed and tested in clinical trials. Orally bioavailable synthetic alkyl-lysophospholipid agents, such as edelfosine, miltefosine and perifosine, were shown to inhibit AKT signaling pathways in epithelial carcinoma [23]. Similarly, a number of pan-AKT inhibitors (AZD5363, GSK2110183 and GDC-0068) have been shown to inhibit AKT signaling, resulting in growth inhibition in preclinical models [24], [25], [26]. Very recently, the Ziergelbauer group demonstrated that the small molecule inhibitor BAY1125976 effectively suppresses AKT1 and AKT2 activation, resulting in tumor growth inhibition in a panel of breast cancer cell lines [27]. These studies highlight the importance of AKT as a therapeutic target in many cancer types, including CaP.
Naturally occurring phytonutrients with chemopreventive properties are under investigation as dietary supplements to reduce the incidence of CaP development and retard the progression of CaP beyond typically asymptomatic benign prostate hyperplasia (BPH) [28]. Previous studies demonstrated the chemopreventive action of a number of natural products with pharmacological activity against numerous targets that drive key CaP initiation and maintenance pathways [29], [30], [31], [32], [33], [34]. Furthermore, some evidence indicates that natural products may inhibit tumor recurrence by targeting key modulators of cancer stem cell renewal [35]. Clinically, chemoprevention may be desirable, as the utility of PSA screening as a diagnostic marker for CaP has been challenged and may be a contributing factor to overtreatment of the disease [36], [37]. Hence, chemoprevention approaches that use natural products to prevent CaP may offer a safer alternative to more aggressive strategies for inhibiting cancer progression and reducing mortality by suppressing prosurvival signaling.
Withaferin A (WA) is a small molecule phytonutrient derived from the herbal plant Withania somnifera that is used extensively in Asian and African traditional medicine to treat various ailments [38]. Recent studies in our laboratory demonstrated the therapeutic efficacy of WA against CaP in mouse models of the disease [39], [40], [41]. In the present study, our objective was to evaluate the efficacy of WA chemoprevention in the Pten conditional knockout mouse Pten-loxp/loxp:PB-Cre4+ (Pten-KO) model, since AKT serves as the primary target for PTEN-mediated signaling. By using a Pten-KO in which AKT signaling is activated constitutively, downstream processes that mimic tumor development and the progression of key stages of human CaP can be used to demonstrate the chemopreventive action of WA.
Material and Methods
Chemicals and Antibodies
For the in vivo mouse studies, WA (>99%, HPLC) was purchased from Nucleus Biopharma (King of Prussia, PA, USA). Primary monoclonal antibodies specific for AKT, phosphor-AKT (ser473), Par-4, FOXO3a, β-Catenin, and N-cadherin were purchased from Cell Signaling (Danvers, MA, USA).
Ptenloxp/loxp:PB-Cre4 (Pten-KO) Mice
Prostate-specific Pten conditional knockout mice [(Pten-loxp/loxp:PB-Cre4+) (Pten-KO)] were procured from Jackson Laboratories (Bar Harbor, ME, USA). The study was approved by the University of Louisville Institutional Animal Care and Use Committee and the methods were carried out in accordance with the approved guidelines. All of the mice were between 5 and 6 weeks of age when received. The mice were divided into three groups: (i) a control group (n = 14), which was subjected to oral gavage with sesame seed oil, (ii) a second group, which was subjected to oral gavage with WA (3 mg/kg group (n = 18), and (iii) a final group, which received WA at a dose of 5 mg/kg (n = 18) over the course of 45 weeks. Mice were euthanized periodically by CO2 exsanguination after 10, 20, 30, 40 and 45 weeks of oral treatment. The mice were dissected, and all major organs (prostate, bladder, seminal vesicles liver, lungs, kidneys, heart, and spleen) were collected and examined for gross pathological abnormalities. The body weights of all mice were recorded weekly. The genitourinary organs were weighed at the time of euthanasia, and the tissue was stored at - 80 °C for RNA, and immunohistochemical analyses.
Histology and Immunohistochemistry
For histopathological examination, prostate tumors and organ tissues were sectioned and processed for hematoxylin and eosin (H&E) staining, as described previously [41]. All stained slides were examined by a board-certified pathologist. For immunohistochemical analysis, the prostate tissue was fixed in 10% buffered formalin and processed as described previously [41]. Expression of specific proteins in tissue samples was visualized using specific antibodies that were obtained from Cell Signaling (Danvers, MA, USA) and targeted AKT, phosphor-AKT (ser473), Par-4, FOXO3a, β-Catenin, and N-cadherin.
Statistical Analysis
All reported values are expressed as the mean ± standard deviation (SD). To calculate the statistical significance of differences between the treatment and control groups, an unpaired Student's t-test was used at a statistical significance of P < .05. All statistical calculations were performed using the Graph Pad Prism Software (La Jolla, CA, USA).
Results
Withaferin-a Inhibits Prostate Tumor Growth and Metastasis in Pten-KO Mice
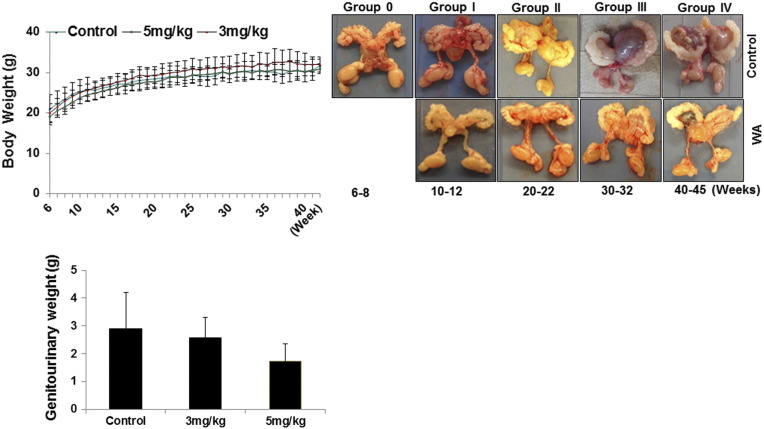
Dietary administration of WA to the mice was initiated at 5–6 weeks of age. H&E sections of prostate tissue from mice that were euthanized at the onset of dosing exhibited epithelial hyperplastic lesions that were characterized by an increase in glandular tissue in comparison with age-matched wild-type C57BL/6 control mice (n = 3, data not shown). The development of CaP was followed over the course of 45 weeks of WA treatment by euthanizing mice after 10, 20, 30, 40 and 45 weeks of treatment. The body weights were measured weekly for all mice, and no significant changes in body weight were observed between the control and treatment groups (Figure 1A). The genitourinary organs of euthanized mice were resected and weighed, and all tumors were examined. Gross pathological examination revealed well-formed prostate tumors after 10–15 weeks in the control mice. In addition, no significant differences in metastasis or toxicity caused by WA treatment were found in any organs. Daily dietary WA administration for 10–15 weeks resulted in a significant (P = .0199) dose-dependent reduction of the genitourinary apparatus weight and the prostate weight (~2.58 ± 0.71 mg and ~1.73 ± 0.71 mg for the 3 mg/kg and 5 mg/kg doses, respectively) in comparison to the control group (2.89 ± 1.31) (Figure 1B). WA inhibited tumor growth in a dose-dependent manner from the inception of detectable tumors (10 weeks) to the study endpoint (Figure 1C).
Figure 1.
A, Dose effect and tolerance of PTEN-KO mice to oral dietary WA treatment. Mice were fed with different doses of WA (vehicle only, 3 mg/kg, and 5 mg/kg), and the mean body weight was recorded weekly over 45 weeks. The error bars represent the standard deviation of the reported average at each time point. B, Representative genitourinary apparatuses of PTEN-KO mice that received vehicle (top) or 5 mg/kg of WA were excised at various time points from the start of treatment (6–8 weeks) to study termination (40–45 weeks). C, Mean weight of the genitourinary apparatus of PTEN-KO mice by treatment group. The error bars show the standard deviation of the mean.
Pathological Implications of WA Treatment in Pten-KO Mice
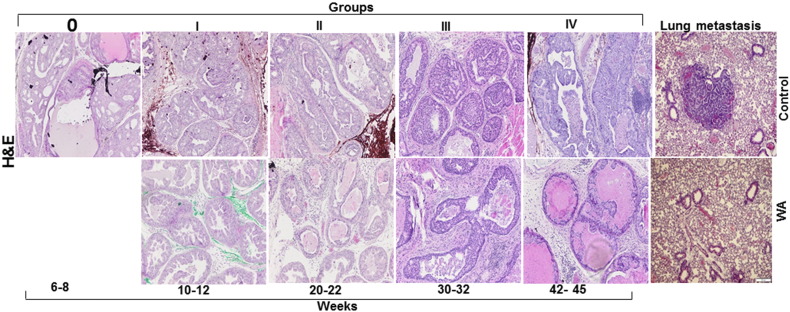
Histopathological classification and scoring of Pten-KO tumors in the treatment and control groups were performed by H&E staining. Tumor growth patterns were classified as hyperplasia, low-grade PIN, high-grade PIN, and carcinoma (high- and low-grade). Representative micrographs of H&E-stained sections of control and WA mouse prostates are shown in Figure 2. In general, microscopic examination of the prostate tissues revealed more differentiated tumors in the WA-treated group than the control group. Control mice exhibited diffuse high-grade PIN at 10–15 weeks that developed into adenocarcinoma at 20–25 weeks of age. The mice treated with 5 mg/kg of WA exhibited low-grade PIN lesions (Figure 2). At 30 weeks, WA-treated mice exhibited lower levels of high-grade PIN and invasive adenocarcinoma. In addition, WA-treated tumors showed more necrosis than the control group, suggesting that generalized anti-tumor activity may promote the destruction of tumor cells in nutrient-deprived regions. Most importantly, the WA–treated mice organs showed no signs of metastasis, whereas the control group mice showed metastatic lesions in the lungs (Figure 2).
Figure 2.
Representative micrographs of H&E-stained and sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or 5 mg/kg of WA (bottom). The tissues were excised at various time points from the start of treatment (6–8 weeks) to study termination (40–45 weeks). Representative micrographs of H&E-stained and sectioned lung tissue from PTEN-KO mice that received vehicle (top) or 5 mg/kg of WA (bottom).
WA Inhibits Tumor Development by Inhibiting AKT and Restoring Par4 Function in PTEN-KO Mice
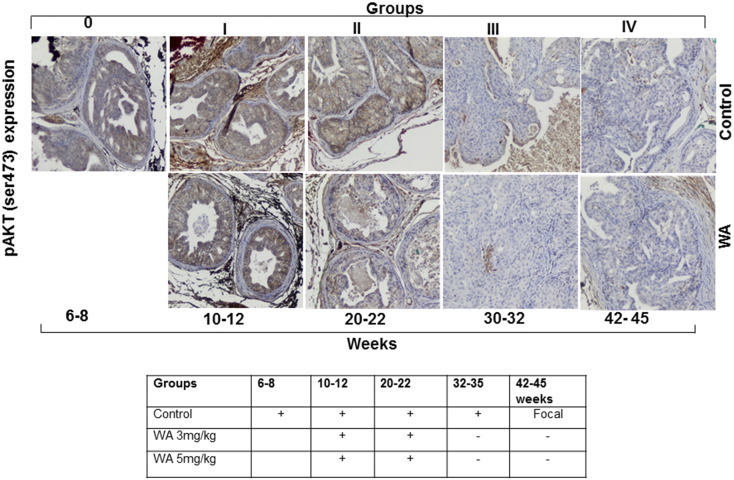
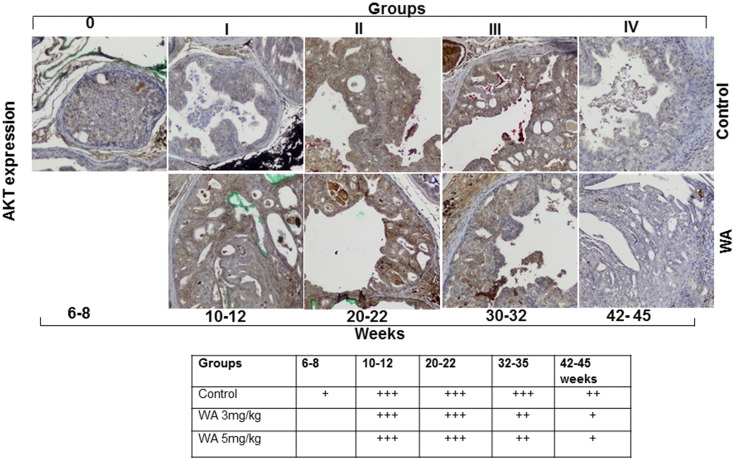
In the Pten-KO model, the conditional inactivation of Pten results in the activation of AKT signaling via AKT phosphorylation, which in turn triggers a cascade of events that are associated with tumor development. Hence, we analyzed the expression levels of pAKT(Ser473) and AKT in vehicle- and WA-treated tumors. Immunohistochemical analysis of the control group mice showed that pAKT was present after 6–8 weeks and persisted through the study endpoint at 44 weeks. Conversely, pAKT expression was reduced in mice receiving WA after only 10–12 weeks of treatment (Figure 3). Continued WA treatment resulted in a further reduction of pAKT expression until the termination of the study. Similarly, total AKT levels remained high in prostate tumors from control mice throughout the study, but decreased in WA-treated mice after 20 weeks of treatment (Figure 4).
Figure 3.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with anti-pAKT and excised at indicated time points.
Figure 4.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with anti-AKT and excised at indicated time points.
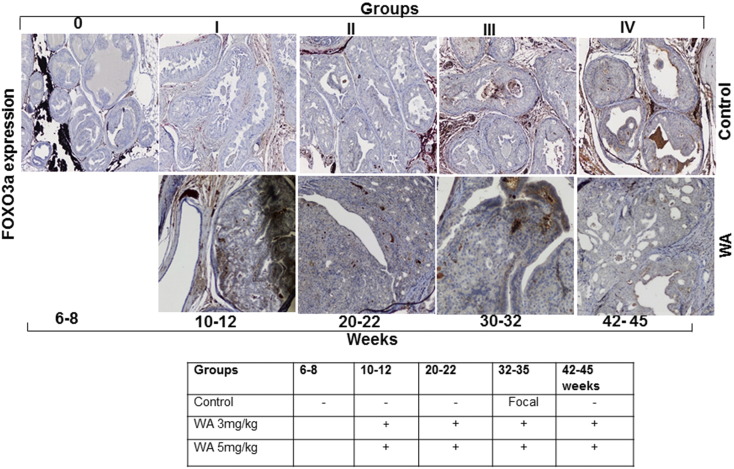
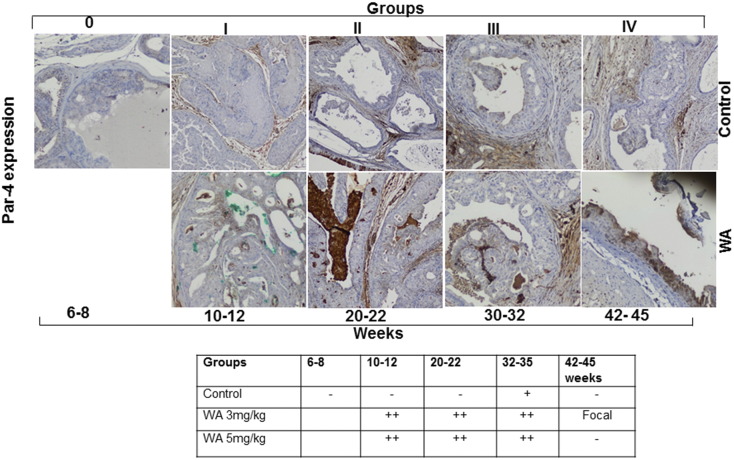
Activated AKT is known to inhibit the pro-apoptotic function of Par-4 in CaP. We recently demonstrated that the inhibition of AKT activation facilitates the FOXO3a-mediated activation of Par-4 in preclinical models of CaP (41). Hence, we probed the mechanism of tumor development inhibition in WA-treated mice by analyzing Par-4 and FOXO3a expression. WA treatment resulted in a 2- to 3-fold increase of Par-4 levels in comparison to age-matched control mice that were fed vehicle alone (Figure 6). WA treatment resulted in nascent Par-4 expression at 10 weeks that persisted until 45 weeks, whereas very low levels of Par-4 were detected in control mice at the 25–35-week time point, suggesting that Par-4 was inhibited by activated AKT signaling. Immunostaining of FOXO3a revealed higher expression levels in the nuclear and perinuclear regions of WA-treated mouse prostate tumor sections than in matched controls (Figure 5). Taken together, the immunohistochemistry expression profiles observed in PTEN-KO mice suggest that WA treatment suppresses tumor development by reactivating FOXO3a/Par-4 function through the down-regulation of AKT activation.
Figure 6.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with anti-Par-4 and excised at indicated time points.
Figure 5.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with anti-FOXO3A and excised at indicated time points.
WA Inhibits AKT-Mediated EMT in Prostate Tumors
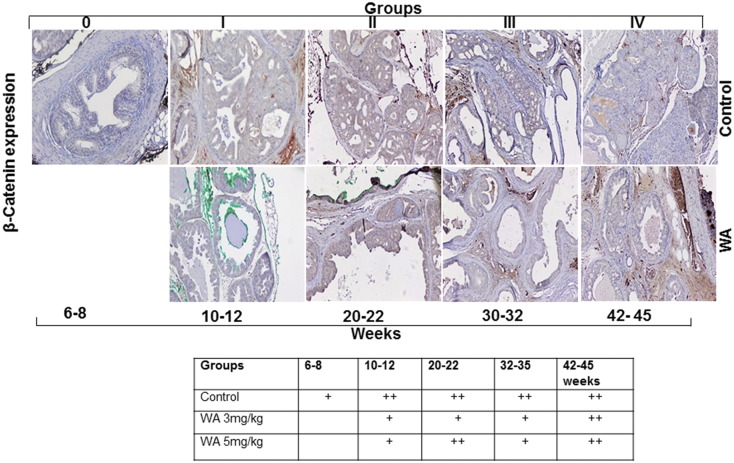
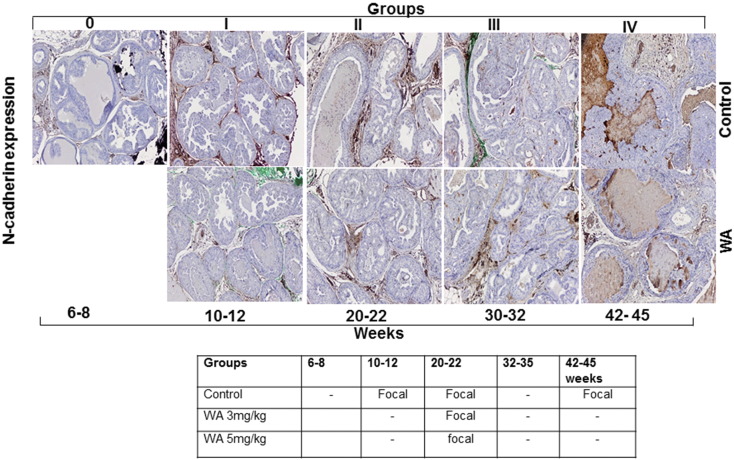
Prostate tissue sections were immunostained for β-catenin and N-cadherin, which serve as markers of stromal involvement associated with the AKT-mediated activation of EMT as a precursor to prostate tumor metastasis. The nuclear localization and expression of β-catenin in the stroma were reduced at 30 weeks in WA-treated mice in comparison to matched controls, which showed higher expression in the nucleus (Figure 7). N-cadherin functions in the trans-endothelial migration of CaP cells. We observed that no significant difference in N-cadherin expression in both control and WA-treated mice at all stages of tumor development (Figure 8). Correlating our findings concerning activated AKT expression profiles with the EMT markers supports the conclusion that WA exerts AKT pathway inhibition. IHC analysis of control tumors revealed higher expression of the mesenchymal marker β-Catenin. WA treatment resulted in a moderate reduction of β-Catenin expression. Similar findings were noted in N-cadherin immunostained slides.
Figure 7.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with β-catenin and excised at indicated time points.
Figure 8.
Representative immunohistochemical micrographs of sectioned prostate tissues from PTEN-KO mice that received vehicle (top) or WA (bottom). The tissues were stained with anti-N-Cadherin and excised at indicated time points.
Discussion
In this study, we evaluated the efficacy of WA as a chemopreventive agent against CaP in a prostate-specific Pten-KO pre-clinical mouse model (Pten-loxp/loxp:PB-Cre4+). The Pten-KO model was chosen as a surrogate to parallel the prostate specific biallelic loss of the Pten tumor suppressor gene that is associated with carcinogenesis and the progression of prostate cancer in humans. Despite the genetic basis of this approach, the translational value of Pten-KO cannot be assumed and had to be validated in terms of its ability to reliably recapitulate the key phases of human prostate cancer progression that are responsive to our chemopreventive approach. Through this process, our GEM can help validate the cellular pathways and targets of WA and inform the selection of biomarkers that could be used prospectively in clinical trials evaluating the performance of WA.
Our histological analysis of prostate tumor morphology in the Pten-KO identified a growth pattern characterized by progression from hyperplasia, through PIN, subsequent invasive ductal carcinoma (IDC), and adenocarcinoma to an undifferentiated carcinoma phenotype. Epithelial hyperplasia in the Pten-KO mice was focal, with small areas of nuclear atypia and cribiform changes that often characterize murine prostate tumors. In the absence of the chemopreventive intervention, CaP progressed from early-phase HG-PIN to invasive adenocarcinoma and metastatic dissemination of the disease due to Pten loss in the prostate epithelium of Pten-KO mice. A recent characterization of the PSA-Cre/Pten-LoxP/Loxp prostate GEMM by Korsten et al. suggested that two distinct tumor development phenotypes represented by adenocarcinoma/intraductal carcinoma and carcinosarcoma are involved [42]. Each phenotype is characterized by a distinct evolutionary pattern and expression markers that define its characteristics. In our study, perhaps due to the tumor growth cutoff (7–8 mo), we did not observe the late onset carcinosarcoma tumor phenotype. Furthermore, the Pten-KO carcinosarcomas described in the Korsten study exhibited high expression of the mesenchyme/stroma markers Snail and Fibronectin in late-stage CaP (>10 mo mice), and expression of these markers was associated with prostate tumor metastasis.
In the Pten-KO GEMM, the conditional inactivation of Pten results in constitutive activation of PI3K-AKT signaling via the phosphorylation of AKT (pAKT). pAKT triggers a cascade of events, which modulate processes associated with tumor development and progression, such as cell survival, proliferation, the cell cycle, the inhibition of apoptosis, angiogenesis, migration and invasion, leading to EMT and metastasis. AKT serves as the primary target for PTEN-mediated signaling. In this study, we first determined whether AKT is phosphorylated in the prostate tissue of this Pten-KO model.
Our evaluation of the effect of WA on CaP development and progression in Pten-KO sheds light on the role of AKT signaling in metastatic processes, such as EMT, as Pten deletion and mutations are frequently observed in metastatic prostate tumors. Our results demonstrate that dietary intake of WA delayed HG-PIN formation and abrogated the progression of Pten-deficient tumors to adenocarcinoma. Metastasis to the lungs developed in the untreated Pten-KO mice but not in WA-treated mice. Importantly, at the tested doses of WA, chemopreventive activity was observed without any toxicity to the mice or organs, as confirmed by histopathological examination of the typical metastatic sites for prostate tumor cells, including the bladder, liver, lungs, and lymph nodes.
Ultimately, the utility of Pten-KO lies in its translational value for human CaP. Anatomical, physiological and dietary differences between mice and humans can influence tumor development. Structurally, the mouse prostate is a multilobe gland, whereas the human prostate is a single-lobe gland, raising the possibility of differences in tumor growth. In addition, tumor-initiating cells can vary in mice and humans, and it is important that murine prostate malignancy originates within the epithelial cells of the prostate in order to progress to PIN as the precursor lesion to adenocarcinoma. Histological analysis of tumor development in our Pten-KO mice confirmed early PIN development in the prostate epithelium that progressed to invasive adenocarcinoma and metastatic disease, thereby validating our model.
We previously reported on the chemopreventive effects of WA treatment in several mouse models of CaP, including DU-145 ectopic xenografts in nude mice [43] and prostate carcinogenesis in TRAMP mice [41]. In the TRAMP model, AKT and AR are expressed constitutively in the mouse prostate relative to non-transgenic mice. TRAMP mice spontaneously develop autochthonous prostate tumors at puberty and closely simulate the pathogenic features of human CaP [44]. The oral administration of WA in TRAMP mice was effective in reducing the tumor burden in comparison to vehicle-treated mice. At the molecular level, WA inhibited AKT activation and promoted FOXO3a/Par-4-induced cell death [41]. We showed that the pro-apoptotic Par-4 protein is a critical downstream target of the PI3K/AKT signaling pathway whose activity is restored by WA treatment. Par-4 activation was correlated with an anticancer effect against CRPC cells. In cell culture models of CRPC (AR null cell lines), Par-4 expression was correlated with favorable outcomes in pre-clinical functional assays, including reduced proliferation, increased apoptosis and the suppression of cell cycle progression. Our results of WA treatment in the Pten-KO mice corroborated our findings in the TRAMP of CaP. As observed with the TRAMP model, the Pten-KO demonstrated the existence of a correlation between Par-4 down-regulation and the inhibitory effects of WA on AKT phosphorylation. Thus, the chemopreventive activity of WA has been demonstrated in several independent models of CaP with alterations in the PI3K/AKT pathway. In the context of the clinic, these findings suggest that CaP patients harboring tumors with mutations that activate the AKT signaling cascade may benefit most from dietary WA treatment.
The prostate-specific conditional Pten-KO mouse model is also a useful model for studying the mechanism of resistance to androgen ablation therapy, as the tumor initiating oncogenic event is not androgen-dependent. AKT has also been implicated in late-stage dysregulation of androgen receptor (AR) signaling during the progression of CaP to a castration-resistant phenotype [43], [45], [46]. As most human CaP respond to androgen deprivation, the tumor should be androgen-dependent and respond to castration. Although we did not investigate the role of androgen ablation in the current study, future studies may examine the impact of combined WA treatment and androgen withdrawal in Pten-KO mice to determine whether androgen deprivation in combination with WA treatment can further reduce tumor burden in this mouse model of CaP.
The dietary phytonutrient WA exhibited potent chemopreventive activity in the treatment of a mouse prostate cancer model harboring Pten deletions. A delay of prostate tumor progression was demonstrated to occur via the inhibition of the PI3K/AKT pathway by WA. The AKT-regulated proapoptotic proteins Par-4 and FOXO3A were up-regulated in Pten-KO mice treated with WA. Our findings suggest that WA may have clinical therapeutic benefits for prostate cancer patients harboring AKT-activating mutations, such as PTEN deletion.
Authors' Contributions
JM, SS, TD performed animal experiments such as dosing, measuring bodyweight, tumor/organ dissection and contributed in drafting the manuscript and performing the statistical analysis. CB carried out scanning the slides, AH & MA read all pathology slides. MA and CD: conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Financial Support
This work was supported by the R01CA140605 and R01CA138797 (to C. D).
Footnotes
Conflicts of interest: Authors declare that they have no conflict of interest.
Financial support: This work was supported by R01CA140605 and R01CA138797 (to C. D.)
References
- 1.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 2.Liu ZC, Wang HS, Zhang G, Liu H, Chen XH, Zhang F, Chen DY, Cai SH, Du J. AKT/GSK-3beta regulates stability and transcription of snail which is crucial for bFGF-induced epithelial-mesenchymal transition of prostate cancer cells. Biochim Biophys Acta. 2014;1840:3096–3105. doi: 10.1016/j.bbagen.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Ruscetti M, Dadashian EL, Guo W, Quach B, Mulholland DJ, Park JW, Tran LM, Kobayashi N, Bianchi-Frias D, Xing Y. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene. 2016;35:3781–3795. doi: 10.1038/onc.2015.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad I, Sansom OJ, Leung HY. Advances in mouse models of prostate cancer. Expert Rev Mol Med. 2008;10:e16. doi: 10.1017/S1462399408000689. [DOI] [PubMed] [Google Scholar]
- 5.Koike H, Nozawa M, De Velasco MA, Kura Y, Ando N, Fukushima E, Yamamoto Y, Hatanaka Y, Yoshikawa K, Nishio K. Conditional PTEN-deficient mice as a prostate cancer chemoprevention model. Asian Pac J Cancer Prev. 2015;16:1827–1831. doi: 10.7314/apjcp.2015.16.5.1827. [DOI] [PubMed] [Google Scholar]
- 6.Abolfazl Avan RN, Giovannetti Elisa, Peters Godefridus J. Role of Akt signaling in resistance to DNA-targeted therapy. World J Clin Oncol. 2016;7:352–369. doi: 10.5306/wjco.v7.i5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 8.Grabowska MM, DeGraff DJ, Yu X, Jin RJ, Chen Z, Borowsky AD, Matusik RJ. Mouse models of prostate cancer: picking the best model for the question. Cancer Metastasis Rev. 2014;33:377–397. doi: 10.1007/s10555-013-9487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisotto M, Metzger D. Genetically engineered mouse models of prostate cancer. Mol Oncol. 2013;7:190–205. doi: 10.1016/j.molonc.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaible L. Genetically Modified Animal Models. In: Conn PM, Animal Models for the Study of Human Disease, editors. The best American short stories of the century. 2013. pp. 853–855. [Google Scholar]
- 11.Fallahabadi ZR, Noori Daloii MR, Mahdian R, Behjati F, Shokrgozar MA, Abolhasani M, Asgari M, Shahrokh H. Frequency of PTEN alterations, TMPRSS2-ERG fusion and their association in prostate cancer. Gene. 2016;575:755–760. doi: 10.1016/j.gene.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 12.Ugalde-Olano A, Egia A, Fernandez-Ruiz S, Loizaga-Iriarte A, Zuniga-Garcia P, Garcia S, Royo F, Lacasa-Viscasillas I, Castro E, Cortazar AR. Methodological aspects of the molecular and histological study of prostate cancer: focus on PTEN. Methods. 2015;77-78:25–30. doi: 10.1016/j.ymeth.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 14.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 15.Costa HA, Leitner MG, Sos ML, Mavrantoni A, Rychkova A, Johnson JR, Newton BW, Yee MC, De La Vega FM, Ford JM. Discovery and functional characterization of a neomorphic PTEN mutation. Proc Natl Acad Sci U S A. 2015;112:13976–13981. doi: 10.1073/pnas.1422504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Los M, Maddika S, Erb B, Schulze-Osthoff K. Switching Akt: from survival signaling to deadly response. Bioessays. 2009;31:492–495. doi: 10.1002/bies.200900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vignarajan S, Xie C, Yao M, Sun Y, Simanainen U, Sved P, Liu T, Dong Q. Loss of PTEN stabilizes the lipid modifying enzyme cytosolic phospholipase A(2)alpha via AKT in prostate cancer cells. Oncotarget. 2014;5:6289–6299. doi: 10.18632/oncotarget.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotan TL, Gumuskaya B, Rahimi H, Hicks JL, Iwata T, Robinson BD, Epstein JI, De Marzo AM. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol. 2013;26:587–603. doi: 10.1038/modpathol.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy SJ, Karnes RJ, Kosari F, Castellar BE, Kipp BR, Johnson SH, Terra S, Harris FR, Halling GC, Klein JL. Integrated analysis of the genomic instability of PTEN in clinically insignificant and significant prostate cancer. Mod Pathol. 2016;29:143–156. doi: 10.1038/modpathol.2015.136. [DOI] [PubMed] [Google Scholar]
- 20.Phin S, Moore MW, Cotter PD. Genomic Rearrangements of PTEN in Prostate Cancer. Front Oncol. 2013;3:240. doi: 10.3389/fonc.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Guo X, Wang J, Jiang C, Bosland MC, Lu J, Deng Y. Methylseleninic Acid Superactivates p53-Senescence Cancer Progression Barrier in Prostate Lesions of Pten-Knockout Mouse. Cancer Prev Res (Phila) 2016;9:35–42. doi: 10.1158/1940-6207.CAPR-15-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs. 2003;14:167–173. doi: 10.1097/00001813-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Dumble M, Crouthamel MC, Zhang SY, Schaber M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA, Seefeld MA. Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS One. 2014;9:e100880. doi: 10.1371/journal.pone.0100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 27.Politz O, Siegel F, Barfacker L, Bomer U, Hagebarth A, Scott WJ, Michels M, Ince S, Neuhaus R, Meyer K. BAY 1125976, a selective allosteric AKT1/2 inhibitor, exhibits high efficacy on AKT signaling-dependent tumor growth in mouse models. Int J Cancer. 2017;140:449–459. doi: 10.1002/ijc.30457. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa G. Nutrition and benign prostatic hyperplasia. Curr Opin Urol. 2013;23:38–41. doi: 10.1097/MOU.0b013e32835abd05. [DOI] [PubMed] [Google Scholar]
- 29.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132–1139. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Dhar S, Kumar A, Zhang L, Rimando AM, Lage JM, Lewin JR, Atfi A, Zhang X, Levenson AS. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget. 2016;7:18469–18484. doi: 10.18632/oncotarget.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MY, Shin IS, Kyoung H, Seo CS, Son JK, Shin HK. alpha-Spinasterol from Melandrium firmum attenuates benign prostatic hyperplasia in a rat model. Mol Med Rep. 2014;9:2362–2366. doi: 10.3892/mmr.2014.2081. [DOI] [PubMed] [Google Scholar]
- 32.Safe S, Kasiappan R. Natural Products as Mechanism-based Anticancer Agents: Sp Transcription Factors as Targets. Phytother Res. 2016;30:1723–1732. doi: 10.1002/ptr.5669. [DOI] [PubMed] [Google Scholar]
- 33.Wang R, Kobayashi Y, Lin Y, Rauwald HW, Fang L, Qiao H, Kuchta K. A phytosterol enriched refined extract of Brassica campestris L. pollen significantly improves benign prostatic hyperplasia (BPH) in a rat model as compared to the classical TCM pollen preparation Qianlie Kang Pule'an Tablets. Phytomedicine. 2015;22:145–152. doi: 10.1016/j.phymed.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Romigh T, He X, Orloff MS, Silverman RH, Heston WD, Eng C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum Mol Genet. 2010;19:4319–4329. doi: 10.1093/hmg/ddq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moselhy J, Srinivasan S, Ankem MK, Damodaran C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015;35:5773–5788. [PMC free article] [PubMed] [Google Scholar]
- 36.Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr Opin Endocrinol Diabetes Obes. 2013;20:204–209. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 37.Lin K, Lipsitz R, Miller T, Janakiraman S, Force USPST. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:192–199. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 38.Palliyaguru DL, Singh SV, Kensler TW. Withania somnifera: From prevention to treatment of cancer. Mol Nutr Food Res. 2016;60:1342–1353. doi: 10.1002/mnfr.201500756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy RV, Suman S, Das TP, Luevano JE, Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76:1909–1915. doi: 10.1021/np400441f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 41.Suman S, Das TP, Moselhy J, Pal D, Kolluru V, Alatassi H, Ankem MK, Damodaran C. Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget. 2016;7:53751–53761. doi: 10.18632/oncotarget.10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korsten H, Ziel-van der Made AC, van Weerden WM, van der Kwast T, Trapman J, Van Duijn PW. Characterization of Heterogeneous Prostate Tumors in Targeted Pten Knockout Mice. PLoS One. 2016;11:e0147500. doi: 10.1371/journal.pone.0147500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das TP, Suman S, Alatassi H, Ankem MK, Damodaran C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016;7:e2111. doi: 10.1038/cddis.2015.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu H, Zou Z, Pan Z, Zhang T, Deng N, Chen G, Wang Z. IL-7/IL-7 receptor axis stimulates prostate cancer cell invasion and migration via AKT/NF-kappaB pathway. Int Immunopharmacol. 2016;40:203–210. doi: 10.1016/j.intimp.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Johnson D, Luong R, Sun Z. Crosstalking between androgen and PI3K/AKT signaling pathways in prostate cancer cells. J Biol Chem. 2015;290:2759–2768. doi: 10.1074/jbc.M114.607846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahajan K, Mahajan NP. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J Cell Physiol. 2010;224:327–333. doi: 10.1002/jcp.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]