Abstract
Colorectal cancer (CRC) is one of the most common malignances in the gut. Liver is the most common metastasis site of CRC. This study focuses on the primary CRC and its liver metastasis, aiming to discover several liver metastasis related genes and provide therapeutic candidates. We compared gene expression patterns among the groups of normal colorectal mucosa, primary tumor and the liver metastasis using a CRC gene expression dataset. 84 genes were found to be upregulated in both primary tumor and liver metastases. Function enrichment analysis indicated that these genes are enriched in pathways such as chemotaxis, coagulation and lipid metabolism which are crucial in multi-step cancer metastasis. Gene network analysis identified several important hub genes that may be involved in carcinogenesis and liver metastasis. Then we used a validation dataset containing 562 CRC samples with detailed clinical information, to screen prognostic biomarkers for overall survival (OS) and relapse free survival (RFS). Finally, overexpression of THBS2 (thrombospondin 2), INHBB (inhibin, beta B) and BGN (biglycan) were proved to be correlated with poor OS and RFS. In conclusion, this study indicated that chemotaxis, coagulation and lipid metabolism might play critical roles in the processes of carcinogenesis and liver metastasis. THBS2, INHBB and BGN are prognostic markers and potential therapeutic targets for CRC.
Keywords: colorectal cancer, prognostic marker, liver metastasis
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignances in the digest system [1–3]. Frequently, distal metastasis affects clinical outcomes. Colorectal cancer liver metastasis (CRCLM) is the most common metastasis pattern in CRC [4]. It is possible to have a radical surgery on advanced colorectal cancer accompanied with liver metastasis in certain cases [4, 5]. It is also probable to cure thoroughly by radical surgery, radio-chemotherapy, and molecular targeted therapy [6–9]. Unfortunately, such patients only accounts for a very small proportion while most CRCLM patients have poor outcomes. Therefore, two questions need to be addressed. Firstly, which subgroup of CRC patients will progress to liver metastasis? Secondly, which subgroup of patients with CRCLM will have poorer prognosis? Investigations on novel prognostic biomarkers and therapeutic targets are needed to improve the outcomes of CRCLM patients.
RESULTS
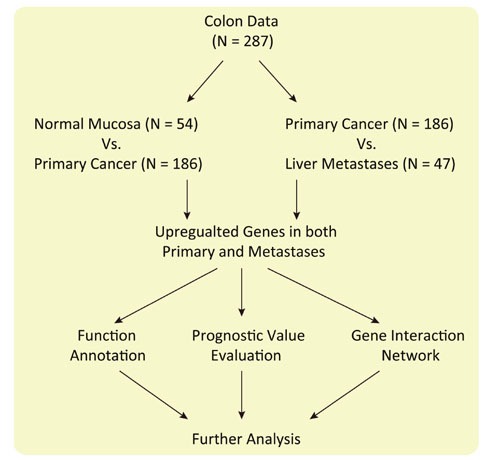
Flow chart of this study
This study focuses on the carcinogenesis and liver metastasis of CRC (Figure 1). Firstly, we searched Gene Expression Omnibus (GEO) for choosing appropriate dataset for further analysis (GSE68468). Then we compared the gene expression pattern between normal colon mucosa and primary tumors; primary tumor and liver metastasis, respectively. Genes upregulated in both primary tumors and liver metastasis were selected for function enrichment analysis, gene-gene interaction analysis and prognostic power evaluation.
Figure 1. Flow chart of this study.
Identification of upregulated genes in both primary tumor and liver metastasis
The genes expression patterns of 54 normal colon mucosa (N), 186 primary tumors (PT) and 54 liver metastases (LM) were compared to discover upregulated genes in both PT and LM. 100 probes representing 84 genes were found to be upregulated in both PT and LM (Figure 2 and Table 1). Heat map visualization of 84 upregulated genes in N, PT and LM samples was demonstrated in Figure 3. Function enrichment of these 84 upregulated genes indicated that chemotaxis, coagulation and lipid metabolism etc. were critical signaling pathways (Figure 4). Gene-gene interaction network was constructed (Figure 5) and hub genes were listed in Table 2 (degree ≥ 15).
Figure 2. Heat map of 84 upregulated genes in both PT and LM.
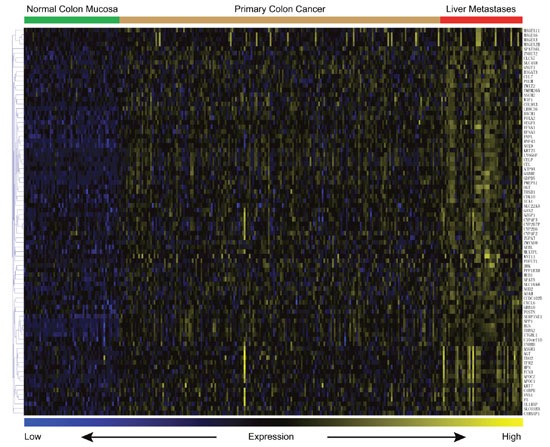
Table 1. 84 genes upregulated in both primary tumor and liver metastasis.
| Gene Symbol | Gene Title |
|---|---|
| EFNA1 | ephrin A1 |
| SERPINE1 | serpin family E member 1 |
| AGT | angiotensinogen |
| SOX9 | SRY-box 9 |
| THBS2 | thrombospondin 2 |
| B3GAT3 | beta-1,3-glucuronyltransferase 3 |
| CDK10 | cyclin dependent kinase 10 |
| MED1 | mediator complex subunit 1 |
| CUL7 | cullin 7 |
| APOC1 | apolipoprotein C1 |
| EVPL | envoplakin |
| GABRE | gamma-aminobutyric acid type A receptor epsilon subunit |
| PPP1R3D | protein phosphatase 1 regulatory subunit 3D |
| APOC2 | apolipoprotein C2 |
| WIF1 | WNT inhibitory factor 1 |
| F5 | coagulation factor V |
| COL9A3 | collagen type IX alpha 3 chain |
| HPN | hepsin |
| INHBB | inhibin beta B subunit |
| SLC22A3 | solute carrier family 22 member 3 |
| ITGBL1 | integrin subunit beta like 1 |
| DACH1 | dachshund family transcription factor 1 |
| AOAH | acyloxyacyl hydrolase |
| VNN1 | vanin 1 |
| GAS2 | growth arrest specific 2 |
| FCN3 | ficolin 3 |
| CEL | carboxyl ester lipase |
| TDO2 | tryptophan 2,3-dioxygenase |
| CXCL6 | C-X-C motif chemokine ligand 6 |
| SLCO1B3 | solute carrier organic anion transporter family member 1B3 |
| CYP4F2 | cytochrome P450 family 4 subfamily F member 2 |
| CYP4F3 | cytochrome P450 family 4 subfamily F member 3 |
| WNT11 | Wnt family member 11 |
| SSUH2 | ssu-2 homolog (C. elegans) |
| ASGR1 | asialoglycoprotein receptor 1 |
| CYP2B7P | cytochrome P450 family 2 subfamily B member 7, pseudogene |
| CYP2B6 | cytochrome P450 family 2 subfamily B member 6 |
| SLC16A6 | solute carrier family 16 member 6 |
| SLC4A8 | solute carrier family 4 member 8 |
| ZMYND8 | zinc finger MYND-type containing 8 |
| GNGT1 | G protein subunit gamma transducin 1 |
| CELP | carboxyl ester lipase pseudogene |
| LY6G6F | lymphocyte antigen 6 complex, locus G6F |
| C4BPB | complement component 4 binding protein beta |
| KRT7 | keratin 7 |
| C10orf10 | chromosome 10 open reading frame 10 |
| CLCN7 | chloride voltage-gated channel 7 |
| AZGP1 | alpha-2-glycoprotein 1, zinc-binding |
| SPP1 | secreted phosphoprotein 1 |
| MAGEA3 | MAGE family member A3 |
| FOXA2 | forkhead box A2 |
| EFNA3 | ephrin A3 |
| TFR2 | transferrin receptor 2 |
| IL1RAP | interleukin 1 receptor accessory protein |
| POFUT1 | protein O-fucosyltransferase 1 |
| MAGEA11 | MAGE family member A11 |
| VEGFA | vascular endothelial growth factor A |
| OGT | O-linked N-acetylglucosamine (GlcNAc) transferase |
| GDPD5 | glycerophosphodiester phosphodiesterase domain containing 5 |
| TMEM265 | transmembrane protein 265 |
| BGN | biglycan |
| ICA1 | islet cell autoantigen 1 |
| MAGEA2B | MAGE family member A2B |
| MAGEA6 | MAGE family member A6 |
| JRK | Jrk helix-turn-helix protein |
| POSTN | periostin |
| NFAT5 | nuclear factor of activated T-cells 5 |
| GRB10 | growth factor receptor bound protein 10 |
| ATP9A | ATPase phospholipid transporting 9A (putative) |
| NEBL | nebulette |
| PMEPA1 | prostate transmembrane protein, androgen induced 1 |
| RNF43 | ring finger protein 43 |
| KRT23 | keratin 23 |
| ZNHIT2 | zinc finger HIT-type containing 2 |
| THSD1 | thrombospondin type 1 domain containing 1 |
| LRRC36 | leucine rich repeat containing 36 |
| NOD2 | nucleotide binding oligomerization domain containing 2 |
| SPATA6L | spermatogenesis associated 6 like |
| CCDC102B | coiled-coil domain containing 102B |
| CAMSAP1 | calmodulin regulated spectrin associated protein 1 |
| MLXIPL | MLX interacting protein like |
| ZMIZ2 | zinc finger MIZ-type containing 2 |
| ZGPAT | zinc finger CCCH-type and G-patch domain containing |
| POLM | DNA polymerase mu |
Figure 3. Venn diagram of differentially expressed genes in PT vs. N and PT vs. LM.
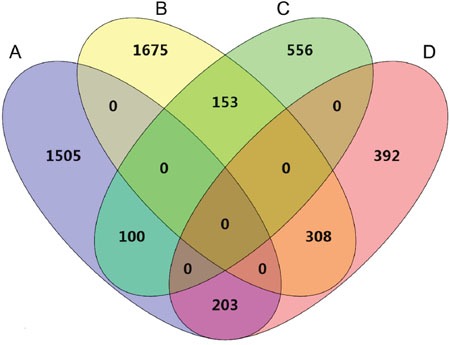
(A) genes upregulated in PT compared to N. (B) genes down regulated in PT compared to N. (C) genes upregulated in LM compared to PT. (D) genes down regulated in LM compared to PT.
Figure 4. Function enrichment of 84 genes upregulated in both PT and LM.
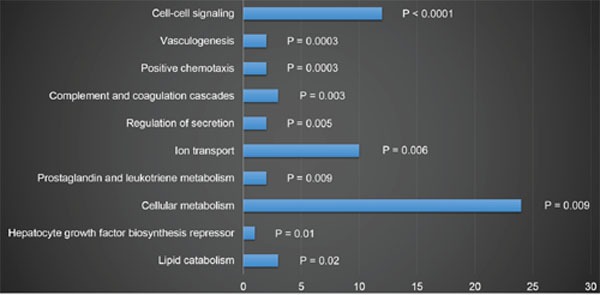
Figure 5. Gene-gene interaction network of 84 genes upregulated in both PT and LM.
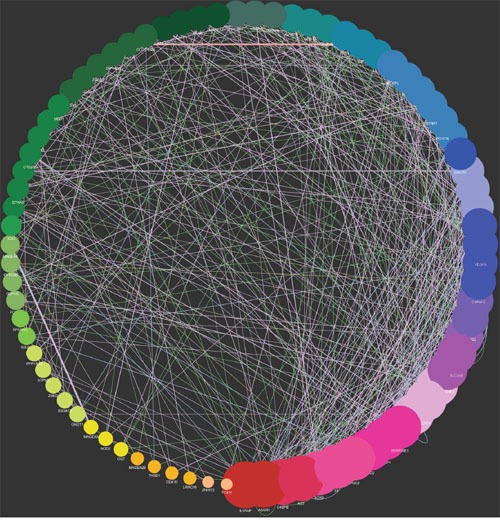
Table 2. Network analysis of 84 upregulated genes in both PT and LM (Degree≥15).
| Gene Symbol | Degree | Average Shortest Path Length | Clustering Coefficient |
|---|---|---|---|
| ASGR1 | 28 | 1.74358974 | 0.29347826 |
| IL1RAP | 28 | 1.80769231 | 0.22529644 |
| AGT | 27 | 1.79487179 | 0.36758893 |
| C4BPB | 27 | 1.75641026 | 0.24675325 |
| SOX9 | 25 | 1.80769231 | 0.21212121 |
| APOC2 | 25 | 1.83333333 | 0.4 |
| F5 | 25 | 1.82051282 | 0.34632035 |
| TDO2 | 23 | 1.87179487 | 0.33986928 |
| AZGP1 | 23 | 1.82051282 | 0.28063241 |
| SERPINE1 | 23 | 1.80769231 | 0.27619048 |
| INHBB | 22 | 1.84615385 | 0.19883041 |
| SPP1 | 22 | 1.88461538 | 0.24836601 |
| CXCL6 | 22 | 1.87179487 | 0.27368421 |
| SLC4A8 | 21 | 1.82051282 | 0.22222222 |
| CYP4F2 | 21 | 1.91025641 | 0.19852941 |
| HPN | 20 | 1.82051282 | 0.31052632 |
| SLCO1B3 | 19 | 1.88461538 | 0.35833333 |
| THBS2 | 19 | 1.8974359 | 0.25735294 |
| VEGFA | 18 | 1.8974359 | 0.19852941 |
| CYP4F3 | 18 | 1.97435897 | 0.32967033 |
| APOC1 | 18 | 1.93589744 | 0.34065934 |
| GAS2 | 18 | 1.92307692 | 0.2952381 |
| TFR2 | 17 | 1.98717949 | 0.59090909 |
| VNN1 | 17 | 1.8974359 | 0.34166667 |
| KRT23 | 17 | 2 | 0.30769231 |
| DACH1 | 16 | 1.94871795 | 0.175 |
| FCN3 | 15 | 1.93589744 | 0.24761905 |
| POSTN | 15 | 2.02564103 | 0.23076923 |
| EFNA1 | 15 | 1.97435897 | 0.37179487 |
| COL9A3 | 15 | 1.93589744 | 0.28571429 |
| BGN | 15 | 1.93589744 | 0.26666667 |
| MLXIPL | 15 | 1.94871795 | 0.48351648 |
Evaluation the prognostic power of 84 upregulated genes
A dataset containing 562 samples was utilized to evaluate the prognostic power of 84 upregulated genes. The expression of each gene was defined as high expression and low expression by quartile division at the cutoff of 25%, 50% and 75%. Then we analyzed the relationship between survival and gene expression at different cutoff level. Survival analysis results indicated that four hub genes, namely thrombospondin 2 (THBS2), inhibin beta B (INHBB), biglycan (BGN), and serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 (SERPIN1) were associated with both OS and RFS (Figure 6, cut-off value: upper quartiles of gene expression, p<0.05). Since the association between SERPIN1 and CRC metastasis has been reported [10, 11], we chose THBS2, INHBB and BGN for further study.
Figure 6. THBS2, INHBB, BGN, and SERPIN1 were correlated with poor OS and RFS.
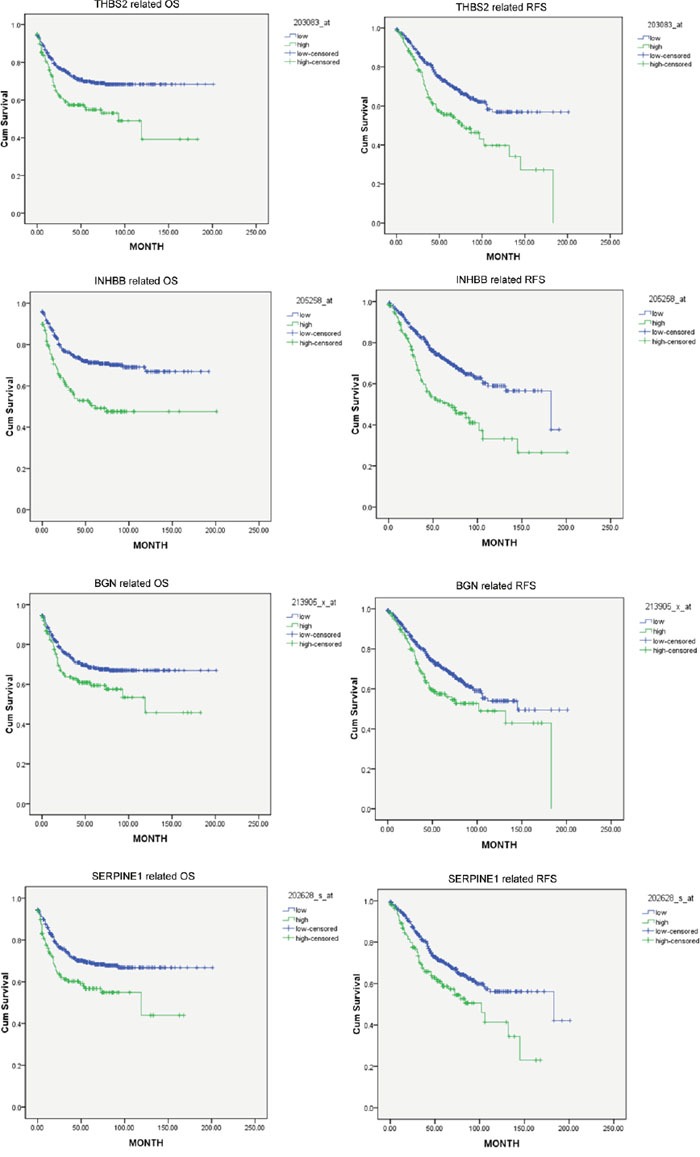
Cut-off value was selected as the upper quartiles of each gene ranked by expression values.
The prognostic power of three hub genes in different tumor stages
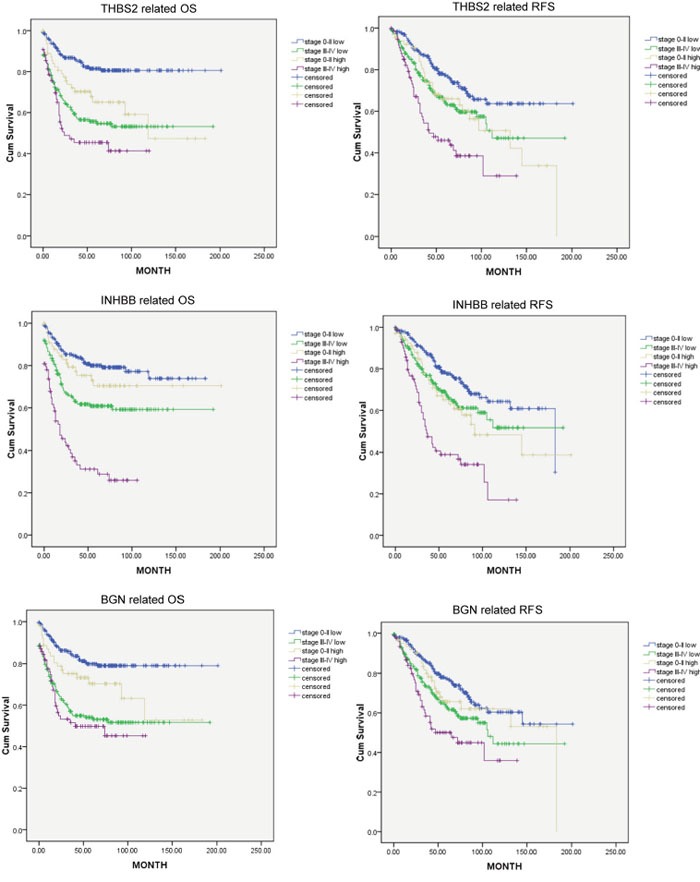
Patients were divided into groups of stage 0-II and stage III-IV. Then the prognostic power of each hub genes were tested on these two groups separately. Figure 7 indicated that prognostic power of THBS2 and INHBB were independent from tumor staging (cut-off value: upper quartiles, P<0.05). However, BGN was not an independent prognostic marker (cut-off value: upper quartiles, P>0.05).
Figure 7. Kaplan-Meier plot analyses of THBS2, INHBB and BGN (cut-off value of high and low group: upper quartiles) in stage 0-II and stage III-IV.
Combination of three hub genes for predicting OS and RFS
According to the expression level of three hub genes (cut-off value: upper quartiles), samples are divided into four groups: triple high expression, double high expression, double low expression and triple low expression, respectively (TH, DH, DL and TL). TL patients have the best survival for OS and RFS while the survival of TH patients are the worst (p<0.5). There was no significant difference between DL and DH groups (Figure 8).
Figure 8. THBS2, INHBB and BGN were classified into four groups (TL, DL, DP and TH; cut-off value of high and low group: upper quartiles).
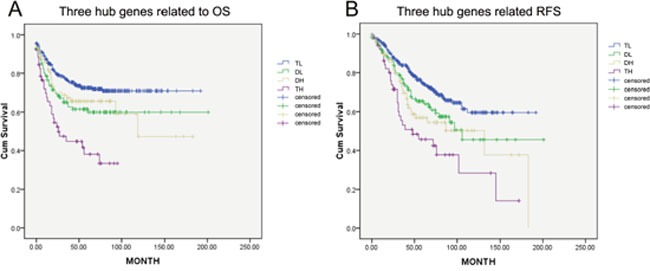
OS and RFS were significantly different between these groups (p<0.05). TN has the best survival while TH the worst.
DISCUSSION
Colorectal cancer with liver metastasis (CRCLM) is the research hotspot in recent years [12]. As is known, liver is the most common metastasis site of colorectal cancer, because of dissemination via the portal venous system. About 10% CRCLM can be resected by surgery, however, only 25%-50% of them could be free of recurrence after treatments [13]. Cheng D et al. reported that MicroRNA -20a-5p, as an intermediator, may promote the ability of invasion and metastasis in colorectal cancer by suppressing Smad4 [14]. Mismatch repair deficiency status, such as frequent PIK3CA mutation, was also seemed as a cause to carcinogenesis in colorectal cancer [15, 16]. Moreover, microsatellite instability in colorectal cancer was also very important. For example, methylation of ITF2 plays a role in modulating WNT signaling in CRC [17]. However, key gene regulators related to carcinogenesis and metastasis of CRC are still unclear. Therefore, discovering novel genes associated with CRCLM and distinguishing new prognostic biomarkers are critical for managing patients with CRCLM.
In this study, 84 genes were found to be associated with the carcinogenesis and liver metastasis of CRC. Chemotaxis, coagulation and lipid metabolism are key signaling pathways involved in carcinogenesis and liver metastasis. By performed gene-gene interaction network analysis, 32 hub genes were discovered. Kaplan-Meier analysis was performed for each gene separately, and SERPINE1, THBS2, INHBB and BGN were found to be associated with poorer OS and RFS.
Metastasis to distant organs is a multistage process: local invasion, intravasation, survival in the circulation, extravasation and colonization [18]. The activation of the coagulation system and platelets have a critical role in the progression of cancer [19]. By forming cell aggregates that protect tumour cells from immune surveillance or by collaborating during extravasation, tumour cell-platelet interactions could also enable dissemination [20–23]. Chemotaxis and vasculogenesis in cancer are crucial for increasing vascular permeability which could improve extravasation and lead to metastasis eventually [19]. In the present study, we showed that chemotaxis and coagulation processes etc. are key characteristics CRC progression and are potential therapeutic targets.
SERPINE1 encodes a member of the serine proteinase inhibitor superfamily which is the principal inhibitor of tissue plasminogen activator (tPA) and urokinase (uPA), and hence is a inhibitor of fibrinolysis. It reacts directly to integrin, uPA-uPAR and ECM, causing to invade and migrate to surrounding and distance [10, 24]. Meanwhile, SERPINE1 has been considered as biomarker related to poorer prognosis in CRC [11]. Since there are few reports on THBS2, INHBB and BGN in CRC, especially in CRCLM, we chose these three genes for further analysis.
THBS2, a member of thrombospondin family, is a disulfide-linked homotrimeric glycoprotein that mediates cell-to-cell and cell-to-matrix interactions, and hence modulates the cell adhesion and migration. Initially, THBS2 was thought to be related to heart failure [25]. Subsequently, it’s also found linked to poorer prognosis in oral cavity squamous cell carcinoma [26]. Interestingly, in many other carcinomas, THBS2 was found downregulated and was correlated with poorer prognosis in tumors such as gastric cancer [27, 28], prostate cancer [29] and breast cancer [30]. In the present study, we found that THBS2 was overexpressed in both primary colon tumor and liver metastases. Moreover, its expression was negatively correlated with OS and RFS.
INHBB is the subunit of inhibin, which regulates gonadal stromal cell proliferation negatively and has tumor-suppressor activity. It seems as an independent prognostic parameter in uterine non-endometrioid cancer, and a low expression demonstrates a significant better cause-specific survival [31]. Investigating the effects of INHBB gene knockdown on the development of mouse granulosa cells in vitro, Mohamed et al. found INHBB has the function of inhibiting apoptosis in mouse granulosa cell [32]. However, the role of INHBB in gastrointestinal cancer, especially in CRC, has not been thoroughly studied from now on. Our analysis confirmed the overexpression of INHBB in CRCLM and its association with poorer OS and RFS, which may result from its function of inhibiting apoptosis.
BGN gene encodes a member of the small leucine-rich proteglycan family of proteins. The encoded protein may contribute to atherosclerosis and aortic valve stenosis in human patients. It seems to enhance the ability of migration and invasion in endometrial cancer [33]. BGN also promotes tumor invasion and metastasis of gastric cancer both in vitro and in vivo [34]. Activated FAK signaling pathway, which regulates cell adhesion and motility by relaying ECM signals from integrin to the intracellular compartment, leads to tumor invasion and metastasis [35]. However, there are few reports on BGN in CRC. In the present study, BGN was found overexpression in CRCLM, and it was correlated with poorer OS and RFS. The underlying molecular mechanism was not clear at present, but FAK signaling pathway may take an important role in this process.
The correlation among three hub genes and survival were also explored in respective to TNM stage. THBS2 and INHBB were independent prognostic biomarker for OS and RFS in both stage 0-II and stage III-IV, while the prognostic value of BGN was associated with TNM staging. Nevertheless, synchronous high expression of these three hub genes indicates the worst clinical outcome, while synchronous low level indicates the best, emphasizing their contributions to poor prognosis.
In summary, several critical signaling pathways such as chemotaxis, coagulation and lipid metabolism may have critical roles in the processes of CRC carcinogenesis and liver metastasis. THBS2, INHBB and BGN are prognostic markers and potential therapeutic targets for CRC. Validation of large cohorts and wet lab experiments are still needed before achieving any clinical significance.
MATERIALS AND METHODS
Ethics statement
The databases used in our study are available online. Anyone is permitted to use all the data in the website of ArrayExpress (http://www.ebi.ac.uk/arrayexpress/), which contains more than 65 thousands of experiments and about 2 millions of assays. It also supports for a search facility to get what type of array and the related information we want. Gene expression datasets were downloaded from Gene Expression Omnibus (GEO) website (www.ncbi.nlm.nih.gov/geo/), which can be linked in the experiments searched out in ArrayExpress. GEO is a public functional genomics data and open for everyone. It contains more than 4300 datasets, 77000 series and 2000000 samples. It provides us some useful tools, such as GEO2R, to get the information we needs. Since all the data are publicly available, The Research Ethics Committee of Zhejiang Provincial People’s Hospital therefore waived the requirement for ethical approval.
Selection of databases
We searched in the ArrayExpress with the condition of colorectal adenocarcinoma and liver metastasis, filtered by organism of Homo sapiens, experiment type of array assay and RNA assay. As a result, we searched out 29 experiments that satisfied the conditions. According to the total sample capacity and the volume of colorectal mucosa, primary tumor and liver metastasis respectively, we chose GSE68468 as the database looking for upregulated genes both in primary CRC and in liver metastasis. Then we re-searched the ArrayExpress with the condition of colorectal adenocarcinoma, filtered by organism of Homo sapiens, experiment type of array assay and RNA assay. As a result, there are 479 experiments satisfied. According to the data-integrity and sample quantity, we focused on the dataset of GSE40967 on the platform of GPL570, which contains the follow-up of OS and RFS in 562 samples testing the gene expression of primary tumor.
Genomic analyses
GEO dataset [36] GSE68468 [37], which containing 54 normal colon mucosa, 186 primary tumors and 47 liver metastases, was reanalyzed for discovering gene associated with both colon carcinogenesis and liver metastasis. While GSE40967 [38] was used for validating the prognostic value of these genes in 562 patients with colon cancer. Differentially expressed genes were computed using Limma package in R environment (version 3.2.1, R Foundation for Statistical Computing [http://www.r-project.org/]). Venn diagram was plotted by Venny online software (Oliveros, J.C. [http://bioinfogp.cnb.csic.es/tools/venny/index.html]). MeV was employed for Heat map and clustering analyses [39]. Function enrichment was performed using GATHER [40], while gene-gene interaction network was constructed by utilizing GeneMANIA plugin [41].
Statistical analyses
Differentially expressed genes were selected by the following criteria: P < 0.05, Log 2 Transformed Fold Change > 0.6 or < -0.6. Kaplan-meier plot analysis was performed using SPSS software (IBM, version 21.0) and log-rank test was employed to evaluate statistical significance. All the data were analyzed using standard statistical tests. Significance was defined as P value less than 0.05.
Acknowledgments
We thank Key Laboratory of Gastroenterology of Zhejiang Province for helpful suggestion and guidance. This study was supported by Medical science Foundation of National Health and Family planning commission (No. WKJ-ZJ-1716). Natural Science Foundation of Zhejiang Province (No. LY17H160065). Research Foundation of Zhejiang Provincial Administration of Traditional Chinese Medicine (No. 2016ZB018).
Footnotes
CONFLICTS OF INTEREST
The authors declared that no competing interests exist.
REFERENCES
- 1.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M, Imamura Y, Willett WC, Rosner BA, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. The New England journal of medicine. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Vol. 49. Oxford, England: 1990. 2013. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer; pp. 1374–1403. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Lancet. Vol. 375. London, England: 2010. Colorectal cancer; pp. 1030–1047. [DOI] [PubMed] [Google Scholar]
- 5.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clinical epidemiology. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: The evolution of determining prognosis. World journal of gastrointestinal oncology. 2013;5:207–221. doi: 10.4251/wjgo.v5.i12.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, Adinin R, Overman MJ, Valero V, Wen S, Lieu C, Yan S, Tran HT, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. Journal of clinical oncology. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyerhardt JA, Li L, Sanoff HK, Carpenter Wt, Schrag D. Effectiveness of bevacizumab with first-line combination chemotherapy for Medicare patients with stage IV colorectal cancer. Journal of clinical oncology. 2012;30:608–615. doi: 10.1200/JCO.2011.38.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas Llimpe FL, Di Fabio F, Ercolani G, Giampalma E, Cappelli A, Serra C, Castellucci P, D’Errico A, Golfieri R, Pinna AD, Pinto C. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: results of the PROMETEO-01 study. British journal of cancer. 2014;111:667–673. doi: 10.1038/bjc.2014.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavon MA, Arroyo-Solera I, Tellez-Gabriel M, Leon X, Viros D, Lopez M, Gallardo A, Cespedes MV, Casanova I, Lopez-Pousa A, Mangues MA, Quer M, Barnadas A, et al. Enhanced cell migration and apoptosis resistance may underlie the association between high SERPINE1 expression and poor outcome in head and neck carcinoma patients. Oncotarget. 2015;6:29016–29033. doi: 10.18632/oncotarget.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzoccoli G, Pazienza V, Panza A, Valvano MR, Benegiamo G, Vinciguerra M, Andriulli A, Piepoli A. ARNTL2 and SERPINE1: potential biomarkers for tumor aggressiveness in colorectal cancer. Journal of cancer research and clinical oncology. 2012;138:501–511. doi: 10.1007/s00432-011-1126-6. [DOI] [PubMed] [Google Scholar]
- 12.Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer treatment reviews. 2015;41:729–741. doi: 10.1016/j.ctrv.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Jung SW, Kim DS, Yu YD, Han JH, Suh SO. Risk factors for cancer recurrence or death within 6 months after liver resection in patients with colorectal cancer liver metastasis. Annals of surgical treatment and research. 2016;90:257–264. doi: 10.4174/astr.2016.90.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng D, Zhao S, Tang H, Zhang D, Sun H, Yu F, Jiang W, Yue B, Wang J, Zhang M, Yu Y, Liu X, Sun X, et al. MicroRNA-20a-5p promotes colorectal cancer invasion and metastasis by downregulating Smad4. Oncotarget. 2016;7:45199–45213. doi: 10.18632/oncotarget.9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen SA, Turner EH, Beightol MB, Jacobson A, Gooley TA, Salipante SJ, Haraldsdottir S, Smith C, Scroggins S, Tait JF, Grady WM, Lin EH, Cohn DE, et al. Frequent PIK3CA Mutations in Colorectal and Endometrial Tumors With 2 or More Somatic Mutations in Mismatch Repair Genes. Gastroenterology. 2016;151:440–447.e441. doi: 10.1053/j.gastro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JH, Powell AG, Roxburgh CS, Horgan PG, McMillan DC, Edwards J. Mismatch repair status in patients with primary operable colorectal cancer: associations with the local and systemic tumour environment. British journal of cancer. 2016;114:562–570. doi: 10.1038/bjc.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savio AJ, Daftary D, Dicks E, Buchanan DD, Parfrey PS, Young JP, Weisenberger D, Green RC, Gallinger S, McLaughlin JR, Knight JA, Bapat B. Promoter methylation of ITF2, but not APC, is associated with microsatellite instability in two populations of colorectal cancer patients. BMC cancer. 2016;16:113. doi: 10.1186/s12885-016-2149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 19.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature reviews Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpatkin S, Pearlstein E. Role of platelets in tumor cell metastases. Annals of internal medicine. 1981;95:636–641. doi: 10.7326/0003-4819-95-5-636. [DOI] [PubMed] [Google Scholar]
- 21.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer research. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 22.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, Muschel RJ. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer research. 2004;64:8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 23.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, Ware J. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sang Y, Chen MY, Luo D, Zhang RH, Wang L, Li M, Luo R, Qian CN, Shao JY, Zeng YX, Kang T. TEL2 suppresses metastasis by down-regulating SERPINE1 in nasopharyngeal carcinoma. Oncotarget. 2015;6:29240–29253. doi: 10.18632/oncotarget.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanatani S, Izumiya Y, Takashio S, Kimura Y, Araki S, Rokutanda T, Tsujita K, Yamamoto E, Tanaka T, Yamamuro M, Kojima S, Tayama S, Kaikita K, et al. Circulating thrombospondin-2 reflects disease severity and predicts outcome of heart failure with reduced ejection fraction. Circulation journal. 2014;78:903–910. doi: 10.1253/circj.cj-13-1221. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CW, Yu JS, Peng PH, Liu SC, Chang YS, Chang KP, Wu CC. Secretome profiling of primary cells reveals that THBS2 is a salivary biomarker of oral cavity squamous cell carcinoma. Journal of proteome research. 2014;13:4796–4807. doi: 10.1021/pr500038k. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Shin J, Park KH, Jeung HC, Rha SY, Noh SH, Yang WI, Chung HC. Molecular basis of the differences between normal and tumor tissues of gastric cancer. Biochimica et biophysica acta. 2007;1772:1033–1040. doi: 10.1016/j.bbadis.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Sun R, Wu J, Chen Y, Lu M, Zhang S, Lu D, Li Y. Down regulation of Thrombospondin2 predicts poor prognosis in patients with gastric cancer. Molecular cancer. 2014;13:225. doi: 10.1186/1476-4598-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavin S, Yeh CR, Da J, Yu S, Miyamoto H, Messing EM, Guancial E, Yeh S. Estrogen receptor alpha in cancer-associated fibroblasts suppresses prostate cancer invasion via modulation of thrombospondin 2 and matrix metalloproteinase 3. Carcinogenesis. 2014;35:1301–1309. doi: 10.1093/carcin/bgt488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch M, Hussein F, Woeste A, Grundker C, Frontzek K, Emons G, Hawighorst T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast cancer research and treatment. 2011;128:337–346. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mylonas I. European journal of cancer. Vol. 46. Oxford, England: 1990. 2010. Inhibin-alpha, -betaA and -betaB subunits in uterine non-endometrioid carcinomas: prognostic significance and clinical implications; pp. 2485–2493. [DOI] [PubMed] [Google Scholar]
- 32.M’Baye M, Hua G, Khan HA, Yang L. RNAi-mediated knockdown of INHBB increases apoptosis and inhibits steroidogenesis in mouse granulosa cells. The Journal of reproduction and development. 2015;61:391–397. doi: 10.1262/jrd.2014-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Wang X, Zhang Y, Che X, Liu Z, Zhang L, Qiu C, Lv Q, Jiang J. Biglycan enhances the ability of migration and invasion in endometrial cancer. Archives of gynecology and obstetrics. 2016;293:429–438. doi: 10.1007/s00404-015-3844-5. [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Duan YT, Li JF, Su LP, Yan M, Zhu ZG, Liu BY, Yang QM. Biglycan enhances gastric cancer invasion by activating FAK signaling pathway. Oncotarget. 2014;5:1885–1896. doi: 10.18632/oncotarget.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer metastasis reviews. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 36.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic acids research. 2013;41:D991–995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheffer M, Bacolod MD, Zuk O, Giardina SF, Pincas H, Barany F, Paty PB, Gerald WL, Notterman DA, Domany E. Association of survival and disease progression with chromosomal instability: a genomic exploration of colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7131–7136. doi: 10.1073/pnas.0902232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D, Ayadi M, Kirzin S, Chazal M, Flejou JF, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS medicine. 2013;10:e1001453. doi: 10.1371/journal.pmed.1001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 40.Chang JT, Nevins JR. GATHER: a systems approach to interpreting genomic signatures. Bioinformatics (Oxford, England) 2006;22:2926–2933. doi: 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]
- 41.Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics (Oxford, England) 2010;26:2927–2928. doi: 10.1093/bioinformatics/btq562. [DOI] [PMC free article] [PubMed] [Google Scholar]


