Abstract
Previous research in cultural psychology shows that cultures vary in the social orientation of independence and interdependence. To date, however, little is known about how people may acquire such global patterns of cultural behavior or cultural norms. Nor is it clear what genetic mechanisms may underlie the acquisition of cultural norms. Here, we draw on recent evidence for certain genetic variability in the susceptibility to environmental influences and propose a norm sensitivity hypothesis, which holds that people acquire culture, and rules of cultural behaviors, through reinforcement-mediated social learning processes. One corollary of the hypothesis is that the degree of cultural acquisition should be influenced by polymorphic variants of genes involved in dopaminergic neural pathways, which have been widely implicated in reinforcement learning. We reviewed initial evidence for this prediction and discussed challenges and directions for future research.
Introduction
The last two decades of research in cultural psychology shows that cultures vary in social orientations of the self as independent or interdependent [1–3]. Western cultures, especially European American cultures, value the independence of the self from others. In contrast, Eastern cultures, particularly Asian cultures, value the interdependence of the self with others. The social orientation dimension of independence versus interdependence has systematic influences on cognition [4–6], emotion [7–9], and motivation [10–12]. So far, however, it remains unclear what mechanisms might underlie the acquisition of the culture-typical social orientations of independence and interdependence, particularly, both explicit and implicit rules governing these orientations [13], although some initial evidence suggests that culture-typical behavioral characteristics emerge after 6 or 7 years of age and begin to be more pronounced over the course of adolescence [14–16].
Here, we explore a novel perspective on the acquisition of explicit and implicit rules of social behavior, or cultural norms, by drawing on recent advancements in social genomics – a new field of research investigating ways in which genetic and epigenetic processes are dynamically linked to socio-cultural processes to constitute various phenotypes including health and other psycho-social outcomes [3, 17–19]. Evidence suggests that individuals are genetically variable in terms of their sensitivity to environmental influences [17, 18, 20]. Extending this work, we propose a norm sensitivity hypothesis [21], which holds that people are genetically variable in their sensitivity to global patterns of cultural behaviors or social norms. Here, we will review initial evidence for it and discuss challenges and directions of research in this area.
Mutual Influences between Culture and Genes
Recent research in population genetics suggests that over the past 10,000 years of human history, numerous polymorphic genetic changes have been positively selected, even though this time period is much briefer than might be expected, and the rate of positive selection appears to have accelerated [22–24]. This seems likely to be related to the massive increase in human population and exposure to new environments (including domesticated animals and plants) and resulting diversity in both infectious diseases and available nutrition. This is consistent with ideas in evolutionary biology and biological anthropology that genetic evolution and cultural evolution have proceeded in tandem, e.g. theories of dual inheritance [25] or gene-culture co-evolution [25–28]. Initial empirical support for this theory has focused on effects of herding and milk production on emergence of genetic mutations that support the digestion of lactose – the milk sugar [29], leading to rapid incorporation of these mutations and supporting the growth of dairying culture.
One intriguing recent proposal is that some genetic variants may lend themselves to plasticity of behavior [18]; that is, carriers of certain alleles could be differentially susceptible to environmental influences depending on certain genetic variants [18, 30, 31]. Such individuals might be more susceptible to early childhood adversity or maltreatment. Indeed, early life traumas increase the risk of depression and posttraumatic stress disorder later in life, but particularly in carriers of specific alleles in the serotonin transporter gene (5-HTTLPR) [30], a glucocorticocoid receptor chaperone gene (FKBP5) [32], and the beta2 adrenergic receptor gene (ADRB2) [33].
Extending this literature, Kim and colleagues argued that culture is one element of one’s eco-social environment that encourages certain behaviors and inhibits others. It would seem to follow that genetic alleles that increase behavioral plasticity might also amplify cultural differences in behavior [17]. For example, it is normative to seek emotional or social support at times of distress in European American cultural contexts, but not in Korean cultural contexts; Kim and colleagues found cultural differences tended to be larger for carriers of one allele (G) of the oxytocin receptor gene (OXTR) polymorphism rs53576, previously linked to increased socioemotional sensitivity [34].
So far work has focused on isolated behavioral traits such as social support [35] and emotion suppression [36], leaving open the question of whether genetic polymorphisms might modulate each individual’s readiness or preparedness to acquire global phenotypic traits such as norms and behavioral patterns of independence and interdependence. Although social learning has long been argued to be central in maintaining long-lasting cultural traditions [37, 38] (See also the Tomasello article in this Special Issue), rarely has this line of reasoning considered genetic factors that foster social learning.
The Norm Sensitivity Hypothesis
Reinforcement-Mediated Social Learning and Dopaminergic (DA) System Genes
The norm sensitivity hypothesis suggests that acquisition of global behavioral patterns and norms of culture, such as independence and interdependence, is influenced by learning processes such as reinforcement-mediated social learning. This type of learning is based on a set of mechanisms that enable the organism to select behavioral options that maximize anticipated rewards [39]. These mechanisms include discerning behavioral patterns, selection of one’s behaviors, and tracking reinforcements accorded on the behaviors [40, 41]. Major components of reinforcement-mediated social learning (e.g., social rule learning and reinforcement tracking) involve dopamine-mediated brain substrates (e.g., frontal cortex and striatal reward processing area [42, 43]. By highlighting the role of rewards in social learning, we hypothesize that cultural and social learning is not merely cognitive, but also inherently motivational. We may therefore anticipate that cultural acquisition would be facilitated by gene variants that increase the efficiency of central dopaminergic pathways.
To illustrate, children in any society must infer the rules governing their “street” by trial and error. The emerging cognitive representation of others’ response patterns constitutes the perceived norm for the community. Individuals respond to such norms by formulating their own responses, which may in turn be reinforced either positively (i.e., complimented and praised) or negatively (i.e., punished and ignored). This social mechanism is universal, although cultures vary in terms of how tight or loose in application of social norms [44]. The individuals must track reinforcement history to assess validity of inferred social norms. Resulting behaviors tend to be consistent with group norms, some aspects of which are culture-specific (e.g., independent vs. interdependence) and others more universal (e.g., within-group cooperation and altruistic behavior); although culture-unique socio-ecological conditions such as mobility and strength of within-group ties are likely to influence the extent of such behaviors [45].
Our theoretical framework, illustrated in Figure 1, explains contemporary cultural variations in terms of large-scale ecological considerations. Anatomically modern humans evolved in Africa approximately 200,000 years ago [46], spread out of Africa approximately 50,000 years ago, and started farming and herding approximately 10,000 years ago. One factor that initially differentiated Eastern vs. Western regions of the Eurasian continent is the type of crops available and successfully domesticated (e.g., wheat vs. rice) [47]. This differentiation might have set a strong constraint on divergent paths of cultural evolution in the two broadly demarcated regions of the continent.
Figure 1.
The norm sensitivity hypothesis is based on an observation that since initiation of sedentary forms of living, people have organized their groups by certain patterns and norms of social behavior that are afforded and constrained by the forms of subsistence (e.g., farming different crops such as rice and wheat and herding different animals). Acquisition of these cultural patterns and norms is hypothesized to have been facilitated by certain polymorphic variants of dopaminergic (DA) system genes, including those of the dopamine D4 receptor gene (DRD4), that increase the dopamine signaling efficiency.
As a result of sedentary forms of living afforded by newly-emerged subsistence systems, human groups became increasingly large and started to incorporate non-kin members. We may assume social norms were utilized to breed much-needed within-group cooperation [48, 49]. Dopaminergic system genes may therefore have played an instrumental role in facilitating the norm-based system of cooperation – the system we call culture. Given that human groups expanded in size over the last 10,000 years since the inception of sedentary living, the evolution of norm sensitivity must have been critical over this recent evolutionary past [50, 51]. While complex traits influencing social learning are likely to be highly polygenic, involving variations in multiple genes within the dopaminergic as well as other systems, it is plausible that individual mutations directly influencing dopaminergic functioning could have effects on norm sensitivity.
Evidence for the Norm Sensitivity Hypothesis
Dopamine D4 Receptor Gene (DRD4)
One candidate in the context of gene-culture co-evolution is the dopamine D4 receptor gene (DRD4). Exon 3 of DRD4 has a variable number tandem repeat (VNTR) polymorphism (2–11 repeats), with 2, 4, and 7 repeat alleles (2R, 4R, and 7R) the most frequent. Receptors coded by 7R alleles show less in vitro dopamine functioning and poorer response to agonists than 4R alleles [52, 53], whereas the 2R allele is intermediate. Physiologically, diminished dopamine inhibitory feedback in 7R and 2R alleles carriers [24] is thought to lead to relatively higher physiological dopamine signaling capacity relative to 4R carriers [54].
Haplotype linkage disequilibrium (LD) patterns suggest the DRD4 7R allele was likely derived from the ancestal 4R allele 40,000–50,000 years ago, when humans started to expand their territory [24] . The 2R allele is more recent, supposedly appearing ~10,000 years ago, when humans started herding and farming. Herding and farming, as well as the kind of crops farmed, all have systematic influences on cultural patterns of behavior [47, 55, 56]. Moreover, the 7R and 2R alleles may be under selection pressures associated with migration [57, 58]. The 7R allelic frequency increases as a function of migratory distance humans spread over the globe (see Figure 2), suggesting DRD4 variants linked to altered dopamine signaling capacity could have co-evolved with cultural forms of human adaptation. Specifically, the population-level frequency of 7R and 2R alleles of DRD4 might have increased over the last 10,000–50,000 years as different groups underwent a series of challenges to survive in “frontier-like” social and ecological conditions fraught with a variety of life-threats [59]. A recent simulation suggests that such social and ecological conditions conduce to the emergence of strong social norms for cooperation and coordination within an ingroup [60].
Figure 2.
Migratory distance out of Africa as a function of the prevalence of the 7R/2R allele of DRD4. Taken from Matthews & Butler, 2011, American Journal of Physical Anthropology, 145, 382–389.
DRD4 and Environmental Sensitivity
Previous work reported associations between the 7R allele of DRD4 and certain behavioral traits including novelty seeking [61], heavy drinking [61], and financial risk taking [62] although these associations are not always replicable [63]. Other evidence indicates that 7R allele carriers are sometimes relatively better socialized, with superior attention control [31] and greater prosocial orientations [20, 64]. The seemingly conflicting pattern above could reflect environmental sensitivity of the DRD4 7R/2R alleles [18, 20]. That is, under adverse environmental conditions (e.g., neighborhoods dominated by gangs), the 7R/2R alleles may be associated with more negative outcomes (e.g., impulsive, antisocial behaviors). The norm sensitivity hypothesis suggests that the behaviors that are considered less desirable or even explicitly anti-social may be rewarded and thus fostered in such conditions. In contrast, under desirable environmental conditions, these alleles may be associated with more advantageous outcomes because such environments enforce norms fostering desirable behaviors. In support of this analysis, developmental work shows that children with these alleles are influenced more by the quality of caregiving [65–68]. Consistent with the norm sensitivity hypothesis, children with the 7R/2R alleles of DRD4 might more readily infer informal behavioral norms from their caregivers.
DRD4 x Culture Interaction
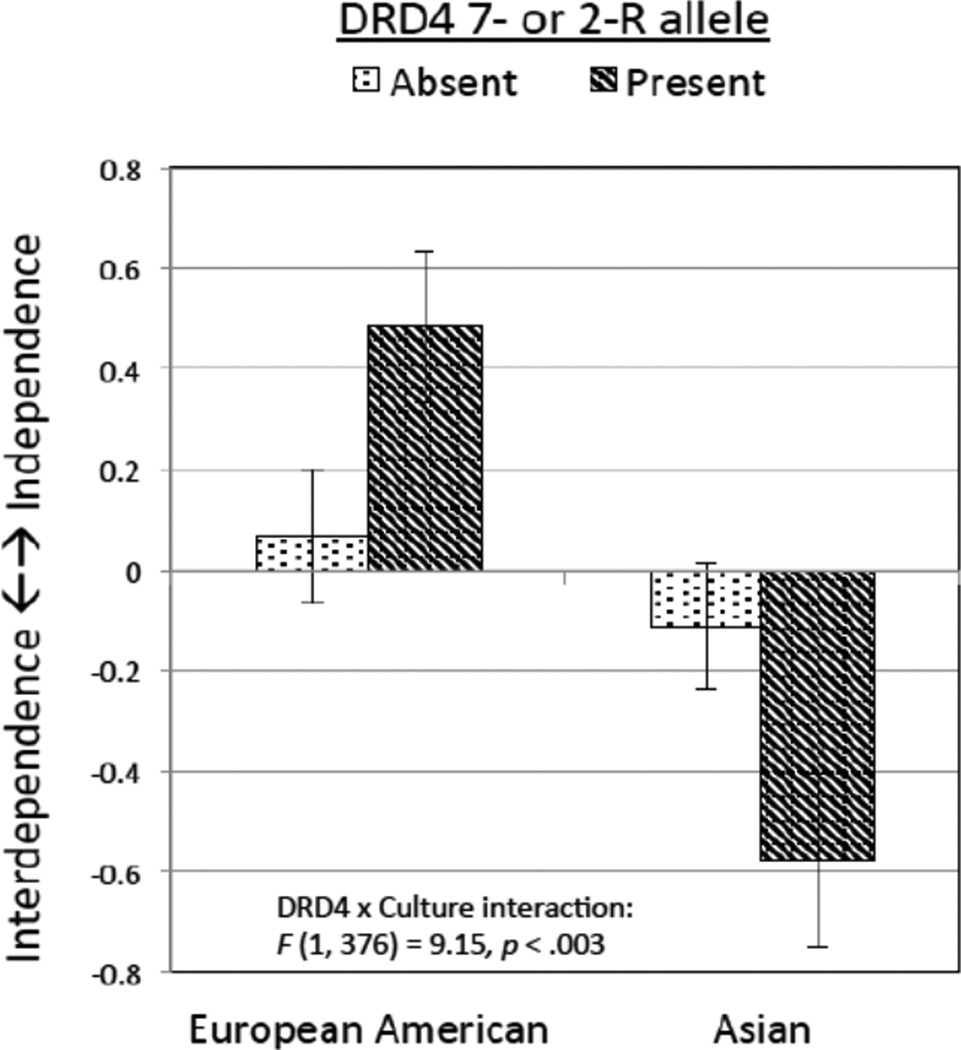
Culture is an environment that is constituted by beliefs, values, and human behaviors derived from them [69]. On the basis of the norm sensitivity hypothesis, we may anticipate that higher dopamine signaling variants of DRD4 (7R and 2R) will also moderate cultural differences in global behavioral patterns of independence and interdependence. In our recent work [21], 194 European Americans and 204 Asians completed several scales assessing independence (e.g., independent self-construal, self-efficacy) or interdependence (e.g., interdependent self-construal, holistic cognitive style). As summarized in Figure 3, the predicted DRD4 x culture interaction was significant. Overall, Asians tended to be more interdependent or less independent than European Americans. Importantly, whereas this cultural difference was sizable for the 7/2R allele carriers of DRD4, it was negligible for the 7/2R allele non-carriers. Within each cultural group, the 7/2R allele carriers showed a greater extent of the social orientations typical for their cultures. This finding resonates with an earlier study showing that when the prevalent norms are made salient through induction of accountability, individuals from individualistic and collectivistic societies behave in diametrically opposite ways in a social negotiation task. Whereas individualists become more competitive, collectivists become more cooperative [70].
Figure 3.
Composite measure of independent versus interdependent orientation (independent factor score – interdependent factor score) as a function of culture and DRD4 VNTR polymorphisms. Taken from Kitayama et al., 2014, Psychological Science, 25, 1169–1177.
If carriers of high dopaminergic signaling variants of DRD4 acquire and internalize social norms, they may show more pronounced effects of priming of such norms. Consistent with this hypothesis, a recent study demonstrates that priming of religious ideas increases pro-social behaviors only among the carriers of these gene variants [20]. Consistent with the norm sensitivity hypothesis, this result might show that carriers of the high dopamine signaling variant of DRD4 acquire religious ideas (which encourage altruism) more deeply. Also consistent is a recent review indicating that these gene variants are associated with greater altruism – a behavior that is positively sanctioned in all societies [64].
Challenges and Future Directions
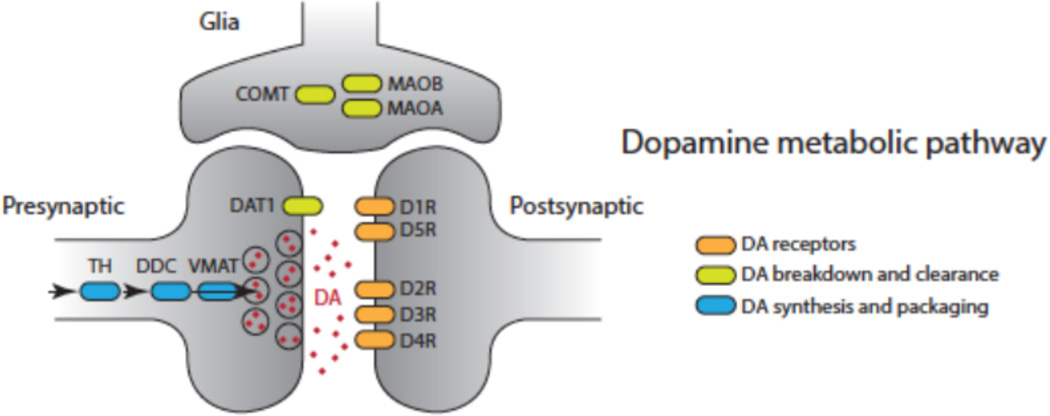
There are important challenges and directions for future work. Future work should examine the function of genes within biological pathways and their interactions. In the context of dopamine, for example, this would include at a minimum dopamine receptors (DRD1 through DRD5), those involved in dopamine synthesis (TH, DDC, VMAT), update (DAT), and clearance (COMT, MAOA, and MAOB). These genes are assumed to work in highly interactive fashion [71, 72] to influence social cognition and behavior [73]. One important research agenda is to combine information from multiple genes for phenotypic traits including norm sensitivity [71, 74, 75].
As compared to complex personality traits such as extraversion and neuroticism and disease categories such as anxiety disorder and schizophrenia, the dimension of norm sensitivity is relatively unitary. Thus, it can be seen as a component process that participates in more complex behaviors such as group cooperation and competition as well as psychological states such as personality and mental illnesses. It may be seen as an endophenotype, or intermediate trait, that links the operation of genes to actual behaviors [76]. Nevertheless, as should be clear from our discussion, the norm sensitivity itself could be divided into various component processes (e.g., norm induction, reward sensitivity, and reward tracking), each of which could be influenced by different genes implicated in dopamine signaling. These processes may also be influenced by myriad background mutations that might produce certain perturbations and biases in genetic signaling [77]. A challenge for future work is the inherently polygenic nature of psychological parameters of social behavior, including norm sensitivity.
It is equally important to assess norm sensitivity directly. One promising approach may be to use economic games and test the degrees to which participants learn response patterns of other participants (rule induction) as well as changing their own behaviors accordingly (reinforcement tracking) [71]. Initial evidence shows that certain dopaminergic system genes are systematically related to relevant parameters (rule induction and reward tracking) estimated from such a behavioral experiment [71].
There may be other mechanisms of gene x culture interaction. As noted above, oxytocin (associated with enhanced sensitivity to certain socially relevant cues [78]) is likely to moderate certain cultural differences [35, 79]. For another example, serotonin innervates cortico-limbic systems of emotion processing. Serotonergic genetic variants may then amplify culture-typical emotional response patterns [80, 81] as well as other processes that are linked to them [82, 83].
To conclude, although at its infancy, the social genomic analysis of cultural acquisition suggests some new avenues of research [3, 17, 19]. We argue that some people may be genetically more sensitive to cultural norms than others. The norms sensitivity hypothesis sheds light on a genetic source of within-culture individual differences by examining biologically based reinforcement-mediated social learning mechanisms that are likely influenced by dopaminergic system genes.
Figure 4.
Dopaminergic (DA) pathway genes. Adapted from Set et al., 2014, Proceedings of National Academy of Sciences, 111, 9615–9620.
Acknowledgments
Writing of this paper was supported by a National Science Foundation grant (SES 1325881) and an Airforce grant (FA955-01-41-0020) to SK and a National Institute of Health grant (RO1 MH098023) to MH.
References
- 1.Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98:224–253. [Google Scholar]
- 2.Masuda T, Nisbett RE. Attending holistically versus analytically: Comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology. 2001;81:922–934. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- 3.Kitayama S, Uskul AK. Culture, Mind, and the Brain: Current Evidence and Future Directions. Annual Review of Psychology. 2011;62:419–449. doi: 10.1146/annurev-psych-120709-145357. [DOI] [PubMed] [Google Scholar]
- 4.Kitayama S, Duffy S, Kawamura T, Larsen JT. Perceiving an object and its context in different cultures A cultural look at new look [Internet] Psychological Science. 2003;14:201–206. doi: 10.1111/1467-9280.02432. [DOI] [PubMed] [Google Scholar]
- 5.Masuda T, Nisbett RE. Attending holistically versus analytically: Comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology. 2001;81:922–934. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- 6.Hedden T, Ketay S, Aron A, Markus HR, Gabrieli JDE. Cultural Influences on Neural Substrates of Attentional Control. Psychological Science. 2008;19:12–17. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- 7.Uchida Y, Kitayama S. Happiness and unhappiness in east and west: Themes and variations. Emotion. 2009;9:441–456. doi: 10.1037/a0015634. [DOI] [PubMed] [Google Scholar]
- 8.Murata A, Moser JS, Kitayama S. Culture shapes electrocortical responses during emotion suppression. Social Cognitive and Affective Neuroscience. 2013;8:595–601. doi: 10.1093/scan/nss036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai JL. Ideal affect: Cultural causes and behavioral consequences. Perspectives on Psychological Science. 2007;2:242–259. doi: 10.1111/j.1745-6916.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- 10.Kitayama S, Tompson S. A Biosocial Model of Affective Decision Making: Implications for Dissonance, Motivation, and Culture. In: Zanna MP, Olson J, editors. Advances in Experimental Social Psychology. Elsevier; 2015. [Google Scholar]
- 11.Kitayama S, Park J. Error-related brain activity reveals self-centric motivation: Culture matters. Journal of Experimental Psychology: General. 2014;143:62–70. doi: 10.1037/a0031696. [DOI] [PubMed] [Google Scholar]
- 12.Heine SJ, Lehman DR, Markus HR, Kitayama S. Is there a universal need for positive self-regard? Psychological Review. 1999;106:766–794. doi: 10.1037/0033-295X.106.4.766. [DOI] [PubMed] [Google Scholar]
- 13.Morris MW, Hong Y-Y, Chiu C-Y, Liu Z. Normology: Integrating insights about social norms to understand cultural dynamics. Organizational Behavior and Human Decision Processes. 2015;129:1–13. [Google Scholar]
- 14.Greenfield PM, Keller H, Fuligni A, Maynard A. Cultural Pathways Through Universal Development. Annual Review of Psychology. 2003;54:461–490. doi: 10.1146/annurev.psych.54.101601.145221. [DOI] [PubMed] [Google Scholar]
- 15.Rothbaum F, Pott M, Azuma H, Miyake K. The Development of Close Relationships in Japan and the United States: Paths of Symbiotic Harmony and Generative Tension. Child Development. 2000;71:1121–1142. doi: 10.1111/1467-8624.00214. [DOI] [PubMed] [Google Scholar]
- 16.Keller H. Cultures of infancy. Lawrence Erlbaum Associates Publishers; 2007. [Google Scholar]
- 17.Kim HS, Sasaki JY. Cultural Neuroscience: Biology of the Mind in Cultural Contexts. Annual Review of Psychology. 2014;65:487–514. doi: 10.1146/annurev-psych-010213-115040. [DOI] [PubMed] [Google Scholar]
- 18.Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 19.Cole SW. Social regulation of human gene expression [Internet] Current Directions in Psychological Science. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki JY, Kim HS, Mojaverian T, Kelley LD, Park IY, Janušonis S. Religion priming differentially increases prosocial behavior among variants of the dopamine D4 receptor (DRD4) gene. Social Cognitive and Affective Neuroscience. 2013;8:209–215. doi: 10.1093/scan/nsr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitayama S, King A, Yoon C, Tompson S, Huff S, Liberzon I. The Dopamine D4 Receptor Gene (DRD4) Moderates Cultural Difference in Independent Versus Interdependent Social Orientation. Psychological Science. 2014;25:1169–1177. doi: 10.1177/0956797614528338. [DOI] [PubMed] [Google Scholar]
- 22.Hawks J, Wang ET, Cochran GM, Harpending HC, Moyzis RK. Recent acceleration of human adaptive evolution. Proceedings of the National Academy of Sciences. 2007;104:20753–20758. doi: 10.1073/pnas.0707650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y-C, Chi H-C, Grady DL, Morishima A, Kidd JR, Kidd KK, Flodman P, Spence MA, Schuck S, Swanson JM, et al. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of National Academy of Sciences. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang E, Ding YC, Flodman P, Kidd D, Kidd KK, Grady DL, Ryder MA, Spence MA, Swanson JM, Moyzis RK. The Genetic Architecture of Selection at the Human Dopamine Receptor D4 ( DRD4) Gene Locus. The American Journal of Human Genetics. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richerson PJ, Boyd R. Not By Genes Alone: How Culture Transformed Human Evolution. The University of Chicago Press; 2004. [Google Scholar]
- 26.Feldman MW, Laland KN. Gene-culture coevolutionary theory. Trends in Ecology & Evolution. 1996;11:453–457. doi: 10.1016/0169-5347(96)10052-5. [DOI] [PubMed] [Google Scholar]
- 27.Laland KN, Brown G. Sense and Nonsense: Evolutionary Perspectives on Human Behaviour. Oxford University Press; 2011. [Google Scholar]
- 28.Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proceedings of the Royal Society B: Biological Sciences. 2010;277:529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS, Powell K, Mortensen HM, Hirbo JB, Osman M, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2006;39:31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, et al. Influence of life stress on deprression: Moderation by polymorphism in the 5-HTT gene. Science. 2003;2003:288tw–288. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 31.Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Develop. Psychopathol. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- 32.Binder EB, Bradley RG, Liu W, Epstein MP. Association of FKBP5 Polymorphisms and Childhood Abuse With Risk of Posttraumatic Stress Disorder Symptoms in Adults. The Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberzon I, King AP, Ressler KJ, Almli LM. Interaction of the ADRB2 Gene Polymorphism With Childhood Trauma in Predicting Adult Symptoms of Posttraumatic Stress Disorder. The Journal of the American Medical Association. 2014;71:1174–1182. doi: 10.1001/jamapsychiatry.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Sciences. 2010;107:15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HS, Sherman DK, Mojaverian T, Sasaki JY, Park J, Suh EM, Taylor SE. Gene-Culture Interaction: Oxytocin Receptor Polymorphism (OXTR) and Emotion Regulation. Social Psychological and Personality Science. 2011;2:665–672. [Google Scholar]
- 37.Mathew S, Perreault C. Behavioural variation in 172 small-scale societies indicates that social learning is the main mode of human adaptation. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20150061. doi: 10.1098/rspb.2015.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasello M. A Natural History of Human Thinking. Harvard University Press; 2014. [Google Scholar]
- 39.Sutton RS, Barto AG. Reinforcement Learning. MIT Press; 1998. [Google Scholar]
- 40.Frank MJ, Claus ED. Anatomy of a decision: Striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychological Review. 2006;113:300–326. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 41.Kitayama S, Tompson S. Advances in Experimental Social Psychology. Elsevier; 2015. A Biosocial Model of Affective Decision Making. In; pp. 71–137. [Google Scholar]
- 42.Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 43.Glimcher PW. Understanding dopamine and reinforcement learning: The dopamine reward prediction error hypothesis. Proceedings of the National Academy of Sciences. 2011;108:15647–15654. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelfand MJ, Raver JL, Nishii L, Leslie LM, Lun J, Lim BC, Duan L, Almaliach A, Ang S, Arnadottir J, et al. Differences Between Tight and Loose Cultures: A 33-Nation Study. Science. 2011;332:1100–1104. doi: 10.1126/science.1197754. [DOI] [PubMed] [Google Scholar]
- 45.Roos P, Gelfand M, Nau D, Carr R. High strength-of-ties and low mobility enable the evolution of third-party punishment. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20132661. doi: 10.1098/rspb.2013.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cann RL, Stoneking M, Wilson AC. Mitochondrial DNA and human evolution. Nature. 1987;325:31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- 47.Talhelm T, Zhang X, Oishi S, Shimin C, Duan D, Lan X, Kitayama S. Large-scale psychological differences within China explained by rice versus wheat agriculture. Science. 2014;344:603–608. doi: 10.1126/science.1246850. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann B, Thoni C, Gachter S. Antisocial Punishment Across Societies. Science. 2008;319:1362–1367. doi: 10.1126/science.1153808. [DOI] [PubMed] [Google Scholar]
- 49.Henrich J, McElreath R, Barr A, Ensminger J, Barrett C, Bolyanatz A, Cardenas JC, Gurven M, Gwako E, Henrich N. Costly punishment across human societies. Science. 2006;312:1767–1770. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- 50.Nowak MA, Tarnita CE, Wilson EO. The evolution of eusociality [Internet] Nature. 2010;466:1057–1062. doi: 10.1038/nature09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyd R, Richerson PJ. Culture and the evolution of human cooperation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3281–3288. doi: 10.1098/rstb.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. Modulation of Intracellular Cyclic AMP Levels by Different Human Dopamine D4 Receptor Variants. Journal of Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 53.Seeger G, Schloss P, Schmidt MH. Marker gene polymorphisms in hyperkinetic disorder – predictors of clinical response to treatment with methylphenidate? Neuroscience & Biobehavioral Reviews. 2001;313:45–48. doi: 10.1016/s0304-3940(01)02253-4. [DOI] [PubMed] [Google Scholar]
- 54.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus Genetic Profile for Dopamine Signaling Predicts Ventral Striatum Reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uskul AK, Kitayama S, Nisbett RE. Ecocultural basis of cognition: Farmers and fishermen are more holistic than herders. Proceedings of the National Academy of Sciences. 2008;105:8552–8556. doi: 10.1073/pnas.0803874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edgerton RB. The individual in cultural adaptation: a study of four East African peoples. University of California Press; 1971. [Google Scholar]
- 57.Chen C, Burton M, Greenberger E, Dmitrieva J. Population Migration and the Variation of Dopamine D4 Receptor (DRD4) Allele Frequencies Around the Globe. Evolution and Human Behavior. 1999;20:309–324. [Google Scholar]
- 58.Matthews LJ, Butler PM. Novelty-seeking DRD4 polymorphisms are associated with human migration distance out-of-Africa after controlling for neutral population gene structure. Am. J. Phys. Anthropol. 2011;145:382–389. doi: 10.1002/ajpa.21507. [DOI] [PubMed] [Google Scholar]
- 59.Kitayama S, Conway LG, III, Pietromonaco PR, Park H. Ethos of Independence Across Regions in the United States. American Psychologist. 2010;65:559–574. doi: 10.1037/a0020277. [DOI] [PubMed] [Google Scholar]
- 60.Roos P, Gelfand M, Nau D, Lun J. Societal threat and cultural variation in the strength of social norms: An evolutionary basis. Organizational Behavior and Human Decision Processes. 2015;127:14–23. [Google Scholar]
- 61.Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty Seeking Involved in Mediating the Association Between the Dopamine D4 Receptor Gene Exon III Polymorphism and Heavy Drinking in Male Adolescents: Results from a High-Risk Community Sample [Internet] Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 62.Dreber A, Apicella CL, Eisenberg DTA, Garcia JR, Zamore RS, Lum JK, Campbell B. The 7R polymorphism in the dopamine receptor D4 gene (DRD4) is associated with financial risk taking in men. Evolution and Human Behavior. 2009;30:85–92. [Google Scholar]
- 63.Munafò MR, Yalcin B, Willis-Owen SA, Flint J. Association of the Dopamine D4 Receptor (DRD4) Gene and Approach-Related Personality Traits: Meta-Analysis and New Data. Biological Psychiatry. 2008;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y, Chew SH, Ebstein RP. The role of D4 receptor gene exon III polymorphisms in shaping human altruism and prosocial behavior. Frontiers in Human Neuroscience. 2013;7:1–7. doi: 10.3389/fnhum.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikitopoulos J, Zohsel K, Blomeyer D, Buchman AF, Schmid B, Jennen-Steinmetz C, Becker K, Schmidt MH, Esser G, Brandeis D, et al. Are infants differentially sensitive to parenting? Early maternal care, DRD4 genotype and externalizing behavior during adolescence. Journal of psychiatric Research. 2014;59:53–59. doi: 10.1016/j.jpsychires.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a metaanalysis. Develop. Psychopathol. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- 67.Berry D, McCartney K, Petrill S. Gene–environment interaction between DRD4 7-repeat VNTR and early child-care experiences predicts self-regulation abilities in prekindergarten. Developmental Psychology. 2014;56:373–391. doi: 10.1002/dev.21105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belsky J, Pluess M. Genetic Moderation of Early Child-Care Effects on Social Functioning Across Childhood: A Developmental Analysis. Child Development. 2013;84:1209–1225. doi: 10.1111/cdev.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitayama S. Culture and basic psychological processes--Toward a system view of culture: Comment on Oyserman et al. (2002) Psychological Bulletin. 2002;128:89–96. doi: 10.1037/0033-2909.128.1.89. [DOI] [PubMed] [Google Scholar]
- 70.Gelfand MJ, Realo A. Individualism-collectivism and accountability in intergroup negotiations. Journal of Applied Psychology. 1999;84:721–736. [Google Scholar]
- 71.Set E, Saez I, Zhu L, Houser DE, Myung N, Zhong S, Ebstein RP, Chew SH, Hsu M. Dissociable contribution of prefrontal and striatal dopaminergic genes to learning in economic games. Proceedings of the National Academy of Sciences. 2014;111:9615–9620. doi: 10.1073/pnas.1316259111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saez I, Set E, Hsu M. From genes to behavior: placing cognitive models in the context of biological pathways. Frontiers in Neuroscience. 2014 doi: 10.3389/fnins.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yacubian J, Buchel C. The genetic basis of individual differences in reward processing and the link to addictive behavior and social cognition. Neuroscience. 2009;164:55–71. doi: 10.1016/j.neuroscience.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Bogdan R, Hyde LW, Hariri AR. A neurogenetics approach to understanding individual differences in brain, behavior, and risk for psychopathology. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.35. [DOI] [PubMed] [Google Scholar]
- 75.Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene--environment interactions [Internet] Trends in Cognitive Sciences. 2011;15:417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: Which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404. doi: 10.1017/S0140525X06009095. [DOI] [PubMed] [Google Scholar]
- 78.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Kim HS, Sherman DK, Mojaverian T, Sasaki JY, Park J, Suh EM, Taylor SE. Gene-Culture Interaction: Oxytocin Receptor Polymorphism (OXTR) and Emotion Regulation. Social Psychological and Personality Science. 2011;2:665–672. [Google Scholar]
- 80.Lee BT, Ham BJ. Serotonergic genes and amygdala activity in response to negative affective facial stimuli in Korean women [Internet] Genes, Brain and Behavior. 2008;7:899–905. doi: 10.1111/j.1601-183X.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 81.Long H, Liu B, Hou B, Wang C, Li J, Qin W, Wang D, Zhou Y, Kendrick KM, Yu C, et al. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neurosci. Bull. 2013;29:4–15. doi: 10.1007/s12264-013-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma Y, Li B, Wang C, Shi Z, Sun Y, Sheng F, Zhang Y, Zhang W, Rao Y, Han S. 5-sHTTLPR Polymorphism Modulates Neural Mechanisms of Negative Self-Reflection. Cerebral Cortex. 2014;24:2421–2429. doi: 10.1093/cercor/bht099. [DOI] [PubMed] [Google Scholar]
- 83.Kim HS, Sherman DK, Taylor SE, Sasaki JY, Chu TQ, Ryu C, Suh EM, Xu J. Culture, serotonin receptor polymorphism and locus of attention [Internet] Social Cognitive and Affective Neuroscience. 2010;5:212–218. doi: 10.1093/scan/nsp040. [DOI] [PMC free article] [PubMed] [Google Scholar]