Abstract
The purpose of the current study was to examine the time course of changes in neuromuscular responses from the vastus medialis (VM) during low versus high intensity dynamic constant external resistance (DCER) leg extension muscle actions to failure. Thirteen men performed DCER leg extensions to failure at 30% and 70% 1-repetition maximum (1-RM) as well as 1-RM measurements pretest and posttest. Electromyogaphy and mechanomyographic signals were measured from the VM. There were no differences in neuromuscular responses pretest versus posttest 1-RM. There were time-dependent differences between the 30% and 70% 1-RM protocols. The initial phase of the 30% 1-RM protocol exhibited increases in electromyographic-amplitude and mechanomyographic amplitude, but no changes at 70% 1-RM. The middle phases indicated decreases in mechanomyographic amplitude at 30% 1-RM, but increases in mechanomyographic amplitude at 70% 1-RM. The 70% 1-RM protocol had earlier decrease in mechanomyographic frequency than 30% 1-RM. Both protocols in the final phases exhibited increases in electromyographic amplitude and mechanomyogrpahic-amplitude, but decreases in electromyographic frequency and mechanomyographic frequency. Low and high intensity DCER leg extensions to failure have time-dependent differences in neuromuscular responses during the process of fatigue which suggested that motor unit activation strategies may by influenced by the intensity of a fatiguing workbout. Thus, examining the time course of changes in neuromuscular responses during a fatiguing workbout allowed for the identification of the time-points associated with the onset of fatigue.
Keywords: EMG, MMG, resistance training, fatigue
INTRODUCTION
Fatigue is a process that occurs over time which can be reflected by a decrease in maximal force production and may be influenced by the intensity of the workbout. Identifying the presence of fatigue has become common place, however, identifying the time-point that fatigue began can be difficult. Thus, examining the time course of change in neuromuscular responses may allow for the identification of the onset of fatigue in a muscle. Surface electromyography (EMG) and mechanomyography (MMG) have been used to examine neuromuscular responses during fatiguing workbouts (7–9, 32). It has been suggested that a fatigue-induced increase in the amplitude (root mean square; RMS) of the EMG signal reflects greater muscle activation (4), while a decrease in the frequency (mean power frequency; MPF) content reflects a slowing of motor unit action potential conduction velocity (MUAP CV) (4). The MMG signal, however, has been described as the mechanical counterpart of the motor unit electrical activity as measured by EMG and quantifies the low-frequency oscillations of activated skeletal muscle fibers (26). Under some conditions, the amplitude (root mean square; RMS) of the MMG signal reflects motor unit recruitment (26) and the frequency (mean power frequency; MPF) content is qualitatively related to the global motor unit firing rate of unfused, activated motor units (26). Thus, a fatigue-induced increase in MMG amplitude may indicate greater motor unit recruitment, while a decrease in MMG frequency is associated with a decrease in firing rate (6, 25). Typically (4, 9, 18, 31) during fatiguing submaximal workbouts there are fatigue-induced increases in EMG amplitude and MMG amplitude, but decreases in EMG frequency and MMG frequency. It has been suggested (19, 20, 31), however, that the intensity of a fatiguing workbout may uniquely influence the magnitude and direction of changes in neuromuscular parameters. Thus, examining the magnitude and time-dependent changes in neuromuscular parameters at different intensities may provide a better understanding of the process of fatigue and allow for comparisons between studies which performed fatiguing muscle actions at different intensities.
The majority of research examining fatigue of the quadriceps during dynamic muscle actions have focused on the vastus lateralis (VL). The vastus medialis (VM), however, has a unique role during leg extension muscle actions which result in different neuromuscular responses throughout a fatiguing workbout compared to the VL (8, 21, 31). In addition, the VM is a major contributor to leg extension muscle actions (8, 21) and has different fatigue characteristics than the VL (16). Thus, identifying the time course of changes in neuromuscular responses from the VM may provide additional information regarding the process of fatigue during submaximal leg extension muscle actions. No previous studies, however, have examined the effects of low versus high intensity dynamic constant external resistance (DCER) leg extension muscle actions on the time course of changes in neuromuscular parameters during the process of fatigue. Therefore, the purpose of the current study was to examine the time course of changes in neuromuscular responses from the VM during low versus high intensity DCER leg extension muscle actions to failure. Based on previous studies (8, 20, 31), we hypothesized that there would be greater fatigue-induced increases in EMG RMS and MMG RMS, as well as greater decreases in EMG MPF and MMG MPF during the high compared to low intensity DCER leg extension muscle actions to failure. In addition, we hypothesized that the high intensity workbout would result in earlier changes in the neuromuscular parameters compared to the low intensity workbout.
METHODS
Participants
Thirteen men (mean ± SD age 20.1 ± 1.8 yr; body mass 79.0 ± 6.2 kg; height 1.73 ± .05 m) volunteered to participate in this study. The subjects ranged between 19 to 26 years of age, were free from any musculoskeletal injuries or neuromuscular disorders, and stated that they performed recreational resistance training for at least 6 months prior to the study. This study was approved by the University of Nebraska – Lincoln Institutional Review Board; and all subjects signed a written informed consent and completed a health history questionnaire prior to participation.
Protocol
The pretest unilateral concentric (CON)-only one repetition maximum (1-RM) tests were performed using the dominant leg (based on kicking preference) and in accordance with the National Strength and Conditioning Association’s guidelines (3). The subjects performed a warm-up set of 5 to 10 repetitions at approximately 50% 1-RM, and 3 to 5 repetitions at approximately 75% 1-RM. The subjects then performed a series of single repetitions to determine the unilateral CON-only 1-RM within 1.13 kg. The unilateral CON-only 1-RM was defined as the greatest amount of weight that was moved through the full range of motion during the DCER leg extension (free weight, plate-loaded, leg extensions). After completion of the pretest CON-only 1-RM subjects were given 3-min of rest prior to performing the fatiguing protocol. The posttest unilateral CON-only 1-RM tests were performed immediately following the 30% 1-RM and 70% 1-RM protocols. Weight was added until the greatest amount of weight that could successfully be move through the full range of motion was determined (± 1.13 kg). This usually required 2 to 3 trials for the pretest 1-RM, and 1 trial for the posttest 1-RM.
The 30% 1-RM and 70% 1-RM protocols were performed on different days, randomly ordered and separated by at least 48 hrs. During the 30% 1-RM and 70% 1-RM protocols the subjects performed unilateral CON-only DCER leg extensions to failure with the dominant leg. Failure was defined as the inability to extend the leg to full extension during the CON phase of the leg extension or the inability to complete the CON phase of the leg extension within 1.5 seconds. During each repetition an investigator lowered the lever arm at the end of each CON phase of the leg extension to the starting position to eliminate the eccentric (ECC) phase of the muscle action. All testing was performed on a Hammer Strength Iso-Lateral Leg Extension machine (LifeFitness, Rosemont, IL).
A bipolar electrode arrangement (Ag/AgCl, AccuSensor, Lynn Medical) was placed on the vastus medialis (VM) of the dominant leg with an interelectrode distance of 30 mm during the unilateral CON-only 1-RM tests, 30% 1-RM protocol, and 70% 1-RM protocol. The bipolar electrode arrangement was placed 80% the distance between the anterior superior iliac spina (ASIS) and the joint space in front of the anterior border of the medial ligament and orientated at a 53° angle to approximate the pennation angle of the muscle fibers (17). A reference electrode was placed over the ASIS. The skin was dry shaven, abraded, and cleaned with isopropyl alcohol prior to placing the electrodes. The MMG signal was measured using accelerometers (EGAS-FT-10/V05, Measurement Specialties, Inc., Hampton, VA) placed between the bipolar electrode arrangements on the VM using double-sided adhesive foam tape. All electrode and accelerometer placements were performed by the primary investigator (CMS) and were marked with black permanent marker to assure the same electrode and accelerometer placements each day.
The EMG and MMG signals were zero-meaned and bandpass filtered (fourth-order Butterworth) at 10–500 Hz and 5–100 Hz, respectively (4, 5, 23, 25). The EMG RMS, EMG MPF, MMG RMS, and MMG MPF values were calculated between knee joint angles of 110° and 160° (180° being full extension) during each unilateral CON-only 1-RM test, as well as for each repetition at every 10% of the repetitions to failure during the 30% 1-RM and 70% 1-RM protocols. All signals were collected at a sampling frequency of 2000 per second. A goniometer was placed along the long axis of the femur and tibia of each subject to determine the knee joint angle throughout the range of motion. The EMG RMS, EMG MPF, MMG RMS, and MMG MPF values were normalized as a percent of the first repetition to examine the time course of changes in neuromuscular parameters during the unilateral CON-only DCER leg extensions to failure at 30% 1-RM and 70% 1-RM. Repetitions were normalized as a percentage of the total repetitions completed and if the percent to failure was between repetitions, the repetition immediately following were selected (i.e., if 10% of the time to failure was at repetition 5.5, repetition 6 was used as the 10% of the time to failure). The EMG RMS, EMG MPF, MMG RMS, and MMG MPF from the MVIC muscle actions were calculated from a 2 second time period corresponding to the middle 33% of each 6 second MVIC. All signal processing was performed using custom programs written with LabVIEW programming software (Version 15.0, National Instruments, Austin TX).
Statistical Analysis
Five separate, 2 (Protocol: 30% 1-RM protocol and 70% 1-RM protocol) by 2 (Time: pretest and posttest) repeated measures ANOVAs were performed to compare the EMG RMS, EMG MPF, MMG RMS, MMG MPF, and unilateral CON-only 1-RM strength from the pretest versus posttest measurements. Follow-up paired sampled t-tests were performed when appropriate.
The time course of changes in neuromuscular responses involved combining polynomial regression analyses with ANOVA and post-hoc Student Newman-Keuls comparisons to identify the patterns of responses and time-points at which these values became different than the initial values. Polynomial regression analyses were used to determine the patterns (linear, quadratic, or cubic) for the mean, normalized (% of initial repetition) EMG RMS, EMG MPF, MMG RMS, and MMG MPF versus repetition relationships from the 30% 1-RM and 70% 1-RM protocols. Time course of changes in normalized EMG RMS, EMG MPF, MMG RMS, and MMG MPF from the initial repetition were identified by two, one-way repeated measures ANOVA (1 × 11) with post-hoc Student Newman-Keuls tests from the 30% 1-RM and 70% 1-RM protocols. The Student Newman-Keuls test was chosen for the post-hoc analyses because it is designed to analyze the time course of changes in repeated measure variables (30). An alpha of p ≤ 0.05 was considered statistically significant for all statistical analyses (SPSS Version 22.0, Armonk, NY).
RESULTS
Table 1 shows the results for the pretest versus posttest neuromuscular responses and 1-RM strength during the 1-RM measurements for the 30% 1-RM and 70% 1-RM protocols. The 2 (Protocol: 30% 1-RM and 70% 1-RM) by 2 (Time: pretest and posttest) ANOVAs indicated no changes from pretest to posttest EMG RMS (p = 0.98), MMG RMS (p = 0.89), or MMG MPF (p = 0.87). The 2 (Protocol: 30% 1-RM and 70% 1-RM) by 2 (Time: pretest and posttest) repeated measures ANOVAs for EMG MPF indicated no significant 2-way interactions, but there was a significant (p < 0.01) main effect for time (pretest > posttest). The 2 (Protocol: 30% 1-RM and 70% 1-RM) by 2 (Time: pretest and posttest) repeated measures ANOVA for 1-RM values indicated a significant protocol by time interaction (p < 0.01). The follow-up paired sampled t-tests indicated that 1-RM strength decreased significantly from pretest to posttest for both the 30% 1-RM (p = 0.01) and 70% 1-RM protocols (p < 0.01). In addition, there was a significant (p = 0.04) difference between the 30% and 70% 1-RM posttest strength (70% 1-RM > 30% 1-RM) (Table 1).
Table 1.
Mean ± SD for the pretest and posttest electromyographic (EMG) root mean square (RMS), EMG mean power frequency (MPF), mechanomyographic (MMG) RMS, and MMG MPF during the 1 repetition maximum (1-RM) measurements from the 30% 1-RM and 70% 1-RM protocols.
| Protocol | Pretest | Posttest | |
|---|---|---|---|
| EMG RMS (μV) | 30% | 810 ± 463 | 845 ± 460 |
| 70% | 765 ± 354 | 795 ± 377 | |
| MMG RMS (m ·s2) | 30% | 0.46 ± 0.13 | 0.43 ± 0.09 |
| 70% | 0.46 ± 0.13 | 0.48 ± 0.22 | |
| EMG MPF (Hz) | 30% | 78 ± 19 | 60 ± 12* |
| 70% | 81 ± 18 | 71 ± 12* | |
| MMG MPF (Hz) | 30% | 20.7 ± 10.1 | 19.1 ± 5.8 |
| 70% | 19.8 ± 7.9 | 16.5 ± 3.7 | |
| 1-RM Strength (kg) | 30% | 46.5 ± 10.7 | 12.7 ± 3.0** |
| 70% | 45.6 ± 10.5 | 29.2 ± 6.7* |
Significantly less than Pretest,
Significantly less than Pretest and 70% Posttest
Figure 1 shows the pretest to posttest EMG RMS, EMG MPF, MMG RMS, and MMG MPF normalized to the initial MVIC for both the 30% 1-RM and 70% 1-RM protocols.
Figure 1.
Electromyographic (EMG) amplitude (root mean square; RMS), mechanomyographic (MMG) RMS, EMG mean power frequency (MPF), and MMG MPF responses pretest versus posttest 1 repetition maximum (1-RM) measurements during the 30% and 70% 1-RM protocol, normalized to pretest maximal voluntary isometric contraction (MVIC). * Significantly less than pretest value at p < 0.05
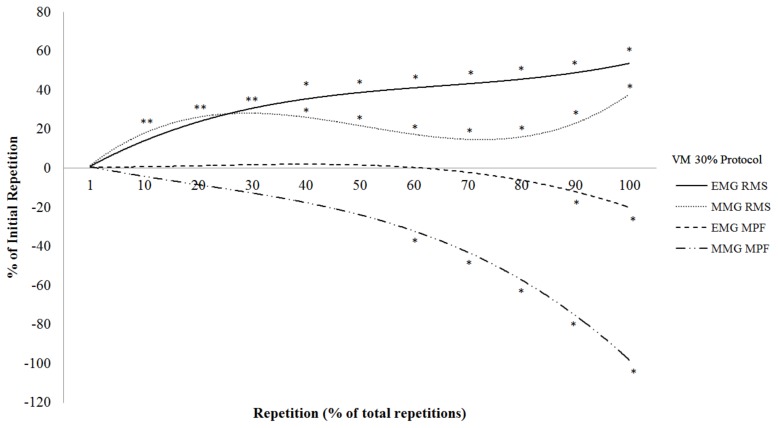
The total repetitions completed during the 30% 1-RM protocol were 55 ±19. Figure 2 shows the results of the polynomial regression analyses and one-way repeated measure ANOVAs with post-hoc Student Newman-Keuls tests for the normalized EMG RMS, EMG MPF, MMG RMS, and MMG MPF versus repetition relationships from the VM at 30% 1-RM. There were significant cubic relationships for the EMG RMS (R2 = 0.95) and MMG RMS (R2 = 0.67) versus repetitions from the VM at 30% 1-RM that were greater than the initial repetition from 10 to 100% of the total repetitions (Figure 2). There were significant negative quadratic relationships for EMG MPF (R2 = 0.97) and MMG MPF (R2 = 0.91) versus repetition from the VM at 30% 1-RM that began to decrease from the initial repetition at 90 and 60% to 100% of the total repetitions, respectively (Figure 2).
Figure 2.
The time course of changes in electromyographic (EMG) amplitude (root mean square; RMS), EMG mean power frequency (MPF), mechanomyographic (MMG) RMS, and MMG MPF during the 30% 1 repetition maximum (1-RM) protocol (normalized to the initial repetition). *Significantly different from the initial repetition at p < 0.05
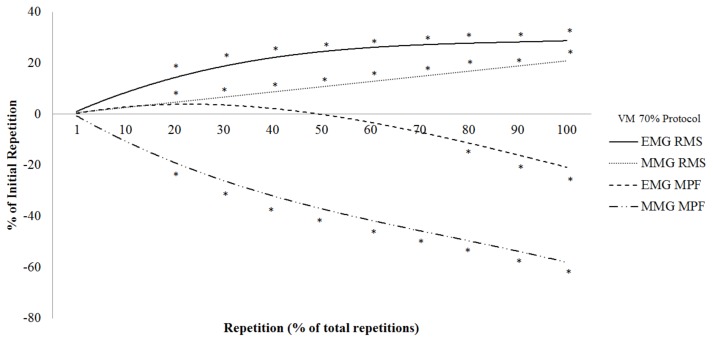
The total repetitions completed during the 70% 1-RM protocol were 15 ± 4. Figure 3 shows the results of the polynomial regression analyses and one-way repeated measures ANOVAs with post-hoc Student Newman-Keuls tests for the normalized EMG RMS, EMG MPF, MMG RMS, and MMG MPF versus repetition relationships from the VM at 70% 1-RM. There was a significant positive quadratic relationship for the EMG RMS (R2 = 0.97) versus repetition from the VM at 70% 1-RM that was greater than the initial repetition from 20 to 100% of the total repetitions (Figure 3). There was a significant positive linear relationship for the MMG RMS (R2 = 0.45) versus repetition from the VM at 70% 1-RM that was greater than the initial repetition from 20 to 100% of the total repetitions (Figure 3). There were significant negative quadratic relationships for EMG MPF (R2 = 0.96) and MMG MPF (R2 = 0.84) versus repetition from the VM at 70% 1-RM that began to decrease from the initial repetition at 80 and 20% to 100% of the total repetitions, respectively (Figure 3).
Figure 3.
The time course of changes in electromyographic (EMG) amplitude (root mean square; RMS), EMG mean power frequency (MPF), mechanomyographic (MMG) RMS, and MMG MPF during the 70% 1 repetition maximum (1-RM) protocol (normalized to the initial repetition). *Significantly different from the initial repetition at p < 0.05
DISCUSSION
In the present study, there were 76 and 36% decreases in 1-RM strength as a result of the fatiguing DCER workbout after the 30 and 70% 1-RM protocols, respectively, but no changes in EMG RMS, MMG RMS, or MMG MPF following either protocol (Figure 1). The EMG MPF, however, decreased following both protocols (Table 1). These findings were in agreement with Pincivero et al. (29) who reported no pretest versus posttest changes in EMG RMS during 1-RM measurements, but decreases in EMG MPF from the VL, VM, and RF after DCER leg extension muscle actions to failure at 50% 1-RM. These findings were also in agreement with Akima et al. (1) who reported no changes in EMG RMS from the VL, VM, and RF after DCER leg extension muscle actions to failure at 50 and 70% 1-RM. It was suggested (1, 29) that the decrease in pretest versus posttest strength, without changes in EMG RMS, were a result of excitation contraction coupling failure. Thus, these findings indicated no difference between the 30% versus 70% protocols in the neuromuscular responses from the pretest versus posttest 1-RM measurements. In addition, the current findings were in agreement with previous studies (1, 29) which suggested no changes in muscle activation (EMG RMS), but decreases in MUAP CV (EMG MPF) during the 1-RM measurements following submaximal, DCER leg extension muscle actions to failure.
The results of the present study indicated four unique phases (1 to 30, 30 to 60, 60 to 90, and 90 to 100% of the repetitions to failure) of the neuromuscular responses from the VM during the 30% 1-RM protocol (Figure 2). The unique phases were identified by the time-points that the neuromuscular responses became significantly different than the initial repetition. During the first 30% of the repetitions to failure there were increases in EMG RMS and MMG RMS, but no change in EMG MPF or MMG MPF. These findings were not consistent with those of Pincivero et al. (29) who reported an increase in EMG RMS, but decrease in EMG MPF from the VM during the first 30% of DCER leg extensions to failure at 50% 1-RM. These findings were also not consistent with those of Ebersole et al. (12) who reported increases in EMG RMS and MMG RMS, decreases in MMG MPF, and no change in EMG MPF from the VM during the first 15 of 50 maximal CON-only isokinetic muscle actions of the leg extensors. Thus, these findings (12, 28) suggested mode- (isokinetic versus DCER) and intensity-related (maximal versus submaximal) differences in neuromuscular responses during dynamic leg extension muscle actions. During the second phase (30 to 60% of the repetitions to failure) there was an increase in EMG RMS, decrease in MMG RMS, and no changes in EMG MPF or MMG MPF. The MMG RMS responses were similar to those of Ebersole et al. (12) who reported a decrease in MMG RMS from the VM during repetitions 15 to 40 of 50 maximal CON-only isokinetic leg extension muscle actions. The decrease in MMG RMS was likely a result of intramuscular fluid pressure (12, 24) having a greater effect on the MMG signal than increases in motor unit recruitment(12). During the third phase (60 to 90% of the repetitions to failure), however, there were increases in EMG RMS and MMG RMS, a decrease in MMG MPF, but no change in EMG MPF. These findings were similar to those of Ebersole et al. (12) who reported increases in EMG RMS and MMG RMS, a decrease in MMG MPF, and no change in EMG MPF from the VM during repetitions 40 to 50 of 50 maximal CON-only isokinetic muscle actions of the leg extensors. The increases in EMG RMS and MMG RMS suggested that motor unit recruitment overcame the competing influences of intramuscular fluid pressure on the MMG signal (12). In addition, the decrease in MMG MPF suggested a decrease in the global motor unit firing rate of activated motor units (9, 22). During the final phase (90 to 100% of the repetitions to failure) there were increases in EMG RMS and MMG RMS, but decreases in EMG MPF and MMG MPF. These findings were similar to those of Jenkins et al.(20) and Pincivero et al. (29) who reported increases in EMG RMS and decreases in EMG MPF from the VM during the CON phase of DCER leg extension muscle actions to failure at 30% and 50% 1-RM, respectively. The decrease in EMG MPF in the present study suggested a buildup of metabolic byproducts that slowed MUAP CV, which further supported the fatiguing nature of the workbout. Thus, the VM exhibited four unique phases (1 to 30, 30 to 60, 60 to 90, and 90 to 100%) of neuromuscular responses during CON-only DCER leg extension muscle actions to failure at 30% 1-RM.
The results of the present study indicated three unique phases (1 to 20, 20 to 80, and 80 to 100% of the repetitions to failure) of neuromuscular responses from the VM during the 70% 1-RM protocol (Figure 3). During the first 20% of the repetitions to failure there were no changes in EMG RMS, MMG RMS, EMG MPF, and MMG MPF from the VM. These findings were not in agreement with Akima et al. (1) who reported an increase in EMG RMS from the VM during the first 25% of the repetitions to failure of DCER leg extension muscle actions to failure at 70% 1-RM. The fatigue-related differences in neuromuscular responses in the current study and those of Akima et al. (1) may be explained by the differences between protocols. Specifically, the present study included only the CON phase of the leg extension muscle actions, but Akima et al. (1) included the CON and ECC phases of the leg extension muscle actions. Indicating the ECC phase following the CON phase may result in earlier fatigue-related changes in the neuromuscular responses than during CON-only DCER leg extension muscle actions. During the middle phase (20 to 80% of the repetitions to failure) there were increases in EMG RMS and MMG RMS, a decrease in MMG MPF, but no change in EMG MPF. These findings were in agreement with Akima et al. (1) who reported an increase in EMG RMS from the VM during 25 to 75% of the repetitions to failure of DCER leg extension muscle actions to failure at 70% 1-RM. The current study was not in agreement, however, with those of Croce et al. (8) who reported a decrease in EMG RMS, MMG RMS, and MMG MPF, but a plateau in EMG MPF from the VM during 15 to 75% of the repetitions to failure during maximal isokinetic muscle actions. The differences in the present study and those of Croce et al. (8) suggested mode- (isokinetic versus DCER) and intensity-related (maximal versus submaximal) differences in neuromuscular responses. Thus, the neuromuscular responses during the middle phase (20 to 80% of the repetitions to failure) suggested increases in muscle activation (EMG RMS) and motor unit recruit (MMG RMS) that were accompanied by a decrease in global motor unit firing rate (MMG MPF).
From 80 to 100% of the repetitions to failure there was a plateau in EMG RMS, an increase in MMG RMS, but decreases in EMG MPF and MMG MPF. These findings were similar to those of Akima et al. (1) and Pincivero et al. (29) who reported a plateau in EMG RMS from VM during 75 and 80 to 100% of the repetitions to failure of DCER leg extension muscle actions to failure at 70 and 50% 1-RM, respectively. Thus, the neuromuscular responses from 80 to 100% of the repetitions to failure suggested an increase in motor unit recruitment, but decreases in global motor unit firing rate and MUAP CV. Thus, during the 70% 1-RM protocol there were three unique phases (1 to 20, 20 to 80, and 80 to 100% of the repetitions to failure) of neuromuscular responses during CON-only DCER leg extension muscle actions to failure at 70% 1-RM.
During the initial phase of the 30% 1-RM protocol there were increases in EMG RMS and MMG RMS, but no changes in EMG MPF or MMG MPF. There were no changes, however, for the neuromuscular parameters during the initial phase of the 70% 1-RM protocol. These findings were similar to those of Akima et al. (1) who reported an approximate 12% increase in EMG RMS from the VM during the initial phase of DCER leg extension muscle action to failure at 50% 1-RM, but only a 3% increase at 70% 1-RM. Thus, the current findings and those of Akima et al. (1) indicated greater changes in EMG RMS and MMG RMS at low to moderate intensities (30 to 50% 1-RM) compared to high intensities (70% 1-RM) during fatiguing DCER leg extension muscle actions. It has been suggested (20, 24, 27, 31) that at lower intensities, there is a greater reserve of unrecruited motor units than at higher intensities which may account for the greater magnitude of increases in muscle activation (EMG RMS) and motor unit recruitment (MMG RMS) throughout the fatiguing workbout at 30% 1-RM than 70% 1-RM.
During the middle phases (30 to 60% of the repetitions to failure) there were decreases in MMG RMS during the 30% 1-RM protocol, but increases throughout the 70% 1-RM protocol. It has been suggested (11, 24, 31) that during fatiguing tasks, both intramuscular fluid pressure and motor unit recruitment can affect MMG RMS. Typically, fatigue-induced increases in intramuscular fluid pressure decreases MMG RMS by restricting the lateral oscillations of the active muscle fibers while motor unit recruitment increases MMG RMS (12, 24, 31). Thus, during fatiguing tasks, intramuscular fluid pressure and motor unit recruitment are competing influences on MMG RMS. Furthermore, the intensity and number of repetitions performed during a fatiguing workbout can affect intramuscular fluid pressure. Thus, the decreases in MMG RMS during the middle phases of the 30% 1-RM protocol suggested that intramuscular fluid pressure had a greater effect on the MMG signal than did increases in motor unit recruitment. The increases in MMG RMS during the 70% 1-RM protocol, however, suggested that the increases in motor unit recruitment had a greater effect than intramuscular fluid pressure on the MMG signal. In addition, MMG MPF decreased during the middle phase of the 70% 1-RM protocol, but was unchanged at 30% 1-RM (Figure 2 and 3). The decrease in MMG MPF during the 70% 1-RM protocol suggested an earlier reduction in global motor unit firing rate when compared to the 30% 1-RM protocol. It has been suggested (9, 10, 13, 22, 31) that decreases in MMG MPF and global motor unit firing rates during the process of fatigue are a result of motor unit activation strategies being employed to optimize force production and maintain the required force. Thus during the middle phases, the neuromuscular responses indicated that the increases in intramuscular fluid pressure had a greater effect on the MMG signal during the 30% 1-RM than the 70% 1-RM protocol. In addition, the decrease in MMG MPF during the 70% 1-RM protocol suggested an earlier reduction in global motor unit firing rate than during the 30% 1-RM protocol. Together these findings indicated intensity-related differences in the neuromuscular responses during fatiguing DCER workbouts which resulted in unique, time-dependent differences in the motor unit activation strategies used to maintain force production.
During the final phases (60 to 100% of the repetitions to failure) there were increases in EMG RMS and MMG RMS, but decreases in EMG MPF and MMG MPF for both the 30% 1-RM and 70% 1-RM protocols (Figure 2 and 3). These neuromuscular responses were typical of those observed in previous studies (1, 2, 20) of submaximal, fatiguing workbouts. The overall direction and magnitude of changes in these neuromuscular parameters are often used to describe differences in neuromuscular responses and make inferences regarding the motor unit activation strategies used to maintain force production. For example, in the current study, there were no differences in neuromuscular responses between protocols during the final phase of the fatiguing workbouts. There were, however, distinct differences in neuromuscular responses between the two protocols during the initial and middle phases of the fatiguing workbout. These findings indicated that although the direction of changes in neuromuscular parameters were similar during the 30% 1-RM and 70% 1-RM protocols, there were time-dependent differences between the protocols in the patterns of changes which may reflect the motor unit activation strategies used to maintain force production during the process of fatigue.
The current findings should be viewed within the limitations of the study’s methodology. The neuromuscular parameters assessed in the present study are indirect indicators of muscle activation (EMG RMS), motor unit action potential conduction velocity (EMG MPF), motor unit recruitment (MMG RMS), and global motor unit firing rate (MMG MPF). That is, the surface EMG signal is affected by a number of physiological and nonphysiological factors that are not reflective of motor unit activation strategies (14, 15). The MMG signal is also affected by many factors, including motor unit synchronization, intramuscular pressure, and muscle stiffness (24–26).
In summary, there were no differences in the neuromuscular responses during the pretest versus posttest 1-RM measurements from the 30% 1-RM and 70% 1-RM protocols. The decreases in pretest versus posttest 1-RM strength without changes in EMG RMS for both intensities suggested excitation contraction coupling failure. The time course of changes in neuromuscular parameters from the 30% 1-RM and 70% 1-RM protocols, however, indicated distinct differences in responses during the process of fatigue. During the initial phase of the 30% 1-RM protocol there were increases in EGM RMS and MMG RMS, but no changes at 70% 1-RM. The middle phases indicated a decrease in MMG RMS during the 30% 1-RM protocol, but an increase during the 70% 1-RM protocol which suggested intramuscular fluid pressure had a greater effect on the MMG RMS during the 30% 1-RM than the 70% 1-RM protocol. In addition, the 70% 1-RM protocol had an earlier reduction in MMG MPF than the 30% 1-RM protocol which suggested an earlier reduction in global motor unit firing rate during the 70% 1-RM protocol. During the final phase, there were no differences in neuromuscular responses between the 30% 1-RM and 70% 1-RM protocols. Thus, in the current study, there were no differences in the neuromuscular responses during the pretest versus posttest 1-RM measurements, but there were time-dependent differences between the 30% 1-RM and 70% 1-RM protocols in the direction and magnitude of changes in neuromuscular parameters during the process of fatigue. Therefore, the time course of changes in neuromuscular responses were sensitive to both intensity and fatigue which may allow for this methodology to be used to examine the effectiveness of training programs, supplements, and rehabilitation.
ACKNOWLEDGEMENTS
We would like to thank our subjects for their commitment and participation in this study.
REFERENCES
- 1.Akima H, Saito A. Activation of quadriceps femoris including vastus intermedius during fatiguing dynamic knee extensions. Euro J Appl Physiol. 2013;113(11):2829–2840. doi: 10.1007/s00421-013-2721-9. [DOI] [PubMed] [Google Scholar]
- 2.Akima H, Saito A. Inverse activation between the deeper vastus intermedius and superficial muscles in the quadriceps during dynamic knee extensions. Muscle Nerve. 2013;47(5):682–690. doi: 10.1002/mus.23647. [DOI] [PubMed] [Google Scholar]
- 3.Baechle TR, Earle RW, Strength N, Association C. Essentials of Strength Training and Conditioning. Human Kinetics; 2008. [Google Scholar]
- 4.Basmajian JV, De Luca C. Muscles alive: their functions revealed by electromyography. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- 5.Beck T, Housh T, Cramer J, Weir J, Johnson G, Coburn J, Malek M, Mielke M. Mechanomyographic amplitude and frequency responses during dynamic muscle actions: a comprehensive review. Biomed Eng. 2005;4:67. doi: 10.1186/1475-925X-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck T, Housh T, Johnson G, Cramer J, Weir J, Coburn J, Malek M. Does the frequency content of the surface mechanomyographic signal reflect motor unit firing rates? A brief review. J Electromyog Kinesiol. 2007;17(1):1–13. doi: 10.1016/j.jelekin.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Contessa P, De Luca C. Neural control of muscle force: indications from a simulation model. J Neurophysiol. 2013;109(6):1548–1570. doi: 10.1152/jn.00237.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croce R, Craft A, Miller J, Chamberlin K, Filipovic D. Quadriceps mechano-and electromyographic time-frequency responses during muscular contractions to volitional exhaustion. Muscle Nerve. 2015;53:452–463. doi: 10.1002/mus.24764. [DOI] [PubMed] [Google Scholar]
- 9.De Luca C, Contessa P. Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech. 2015;48(2):195–203. doi: 10.1016/j.jbiomech.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Luca C, Erim Z. Common drive of motor units in regulation of muscle force. Trend Neurosci. 1994;17(7):299–305. doi: 10.1016/0166-2236(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 11.Ebersole K, Housh T, Johnson G, Evetovich T, Smith D, Perry S. The effect of leg flexion angle on the mechanomyographic responses to isometric muscle actions. Euro J Appl Physiol. 1998;78(3):264–269. doi: 10.1007/s004210050418. [DOI] [PubMed] [Google Scholar]
- 12.Ebersole K, O’Connor K, Wier A. Mechanomyographic and electromyographic responses to repeated concentric muscle actions of the quadriceps femoris. J Electromyogr Kinesiol. 2006;16(2):149–157. doi: 10.1016/j.jelekin.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Eccles J, Eccles R, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958;142(2):275. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farina D, Merletti R, Enoka R. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96(4):1486–1495. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 15.Farina D, Merletti R, Enoka R. The extraction of neural strategies from the surface EMG: an update. J Appl Physiol. 2014;117(11):1215–1230. doi: 10.1152/japplphysiol.00162.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix C, Housh T, Zuniga J, Camic C, Mielke M, Johnson G, Schmidt R. A mechanomyographic frequency-based fatigue threshold test. J Neurosci Method. 2010;187(1):1–7. doi: 10.1016/j.jneumeth.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Hermens H, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hägg G. European recommendations for surface electromyography. Roessingh Research and Development. 1999;8(2):13–54. [Google Scholar]
- 18.Hill E, Housh T, Camic C, Smith C, Cochrane K, Jenkins N, Cramer J, Schmidt R, Johnson G. The Effects of Velocity on Electromyographic, Mechanomyographic, and Torque Responses to Repeated Eccentric Muscle Actions. J Strength Cond Res. 2016;30(6):1743–1751. doi: 10.1519/JSC.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins N, Housh T, Buckner S, Bergstrom H, Cochrane K, Smith C, Hill E, Schmidt R, Cramer J. Individual Responses for Muscle Activation, Repetitions, and Volume during Three Sets to Failure of High- (80% 1RM) versus Low-Load (30% 1RM) Forearm Flexion Resistance Exercise. Sports. 2015;3(4):269–280. [Google Scholar]
- 20.Jenkins N, Housh T, Bergstrom H, Cochrane K, Hill E, Smith C, Johnson G, Schmidt R, Cramer J. Muscle activation during three sets to failure at 80 vs. 30 % 1RM resistance exercise. Eur J Appl Physiol. 2015;115(11):2335–2347. doi: 10.1007/s00421-015-3214-9. [DOI] [PubMed] [Google Scholar]
- 21.Kouzaki M, Shinohara M, Fukunaga T. Non-uniform mechanical activity of quadriceps muscle during fatigue by repeated maximal voluntary contraction in humans. European journal of applied physiology and occupational physiology. 1999;80(1):9–15. doi: 10.1007/s004210050551. [DOI] [PubMed] [Google Scholar]
- 22.Marsden C, Meadows J, Merton P. “Muscular wisdom” that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol. 1983;39:169–211. [PubMed] [Google Scholar]
- 23.Merletti R, Parker P. Electromyography: physiology, engineering, and non-invasive applications. John Wiley & Sons; 2004. [Google Scholar]
- 24.Moalla W, Merzouk A, Costes F, Tabka Z, Ahmaidi S. Muscle oxygenation and EMG activity during isometric exercise in children. J Sports Sci. 2006;24(11):1195–201. doi: 10.1080/02640410500457893. [DOI] [PubMed] [Google Scholar]
- 25.Orizio C. Muscle sound: bases for the introduction of a mechanomyographic signal in muscle studies. Crit Rev Biomed Eng. 1993;21(3):201–243. [PubMed] [Google Scholar]
- 26.Orizio C, Gobbo M, Diemont B, Esposito F, Veicsteinas A. The surface mechanomyogram as a tool to describe the influence of fatigue on biceps brachii motor unit activation strategy. Historical basis and novel evidence. Eur J Appl Phys. 2003;90(3–4):326–336. doi: 10.1007/s00421-003-0924-1. [DOI] [PubMed] [Google Scholar]
- 27.Orizio C, Perini R, Veicsteinas A. Muscular sound and force relationship during isometric contraction in man. Eur J Appl Phys. 1989;58(5):528–533. doi: 10.1007/BF02330708. [DOI] [PubMed] [Google Scholar]
- 28.Pincivero D, Coelho A, Campy R. Contraction mode shift in quadriceps femoris muscle activation during dynamic knee extensor exercise with increasing loads. J Biomech. 2008;41(15):3127–3132. doi: 10.1016/j.jbiomech.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Pincivero D, Gandhi V, Timmons M, Coelho A. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J Biomech. 2006;39(2):246–254. doi: 10.1016/j.jbiomech.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Salkind N. Encyclopedia of research design. Sage; 2010. [Google Scholar]
- 31.Smith C. MS Thesis. University of Nebraska-Lincoln; 2016. Time Course of Changes in Neuromuscular Parameters during Fatiguing High-Load and Low-Load Concentric Dynamic Constant External Resistance Leg Extension Muscle Actions. [Google Scholar]
- 32.Smith C, Housh T, Herda T, Zuniga J, Ryan E, Camic C, Bergstrom H, Smith D, Weir J, Cramer J, Hill E, Cochrane K, Jenkins N, Schmidt R, Johnson G. Effects of the innervation zone on the time and frequency domain parameters of the surface electromyographic signal. J Electromyog Kinesiol. 2015;25(4):565–570. doi: 10.1016/j.jelekin.2015.04.014. [DOI] [PubMed] [Google Scholar]