Abstract
Aim
We aimed to evaluate impact of spironolactone (S) on cardiovascular toxicity of concomitant use of radiotherapy (RT) and trastuzumab (T).
Background
S, an aldosterone receptor antagonist, is known to ameliorate the cardiac damage. S ameliorates anthracycline -induced cardiotoxicity, there is no data regarding to effect of S on both T and radiation-induced cardiotoxicity.
Materials/Methods
Eighty rats were divided into eight groups: group (G) 1 was defined as control group. G2, G3 and G4 were RT, S and T groups respectively. G5, G6, G7 and G8 were RT + T, T + S, RT + S and RT + T + S groups respectively. Rats were sacrificed at 6th hour; 21st and 100th days after RT. Heart and thoracic aorta samples were taken for microscopical examination.
Results
Cardiac inflammation and fibrosis scores and; TGF-β expression were not significantly different within study groups at 6th hour and 21st days of RT. By 100th days of RT fibrosis scores and TGF-β expression in cardiac samples were significantly different between study groups (p values were 0.004 and 0.002 respectively). Pair-wise comparisons revealed that both cardiac fibrosis scores and TGF-β expression levels were higher in G5 when compared to G8 (p values were 0.046 and 0.028 respectively). Moreover the TGF-β expression was higher in G5 when compared to G2 (p = 0.046). We could not demonstrate any significant differences with respect to inflammation, fibrosis and TGF-β expression in thoracic aorta samples between study groups.
Conclusions
Although S had a protective effect on cardiac tissue it had no protective effect on thoracic aorta when administered with RT + T.
Keywords: Aldosterone, Cardiovascular toxicity, Radiotherapy, Spironolactone, Trastuzumab
1. Background
Fifteen percent to 25% of breast cancers express human epidermal growth factor II (HER2) amplification.1 Trastuzumab is a recombinant DNA-derived monoclonal antibody that selectively binds to the extracellular domain of the HER2 protein in breast cancer cells.2, 3 Trastuzumab therapy is important in the treatment of both early and advanced disease. Five randomized controlled trials have addressed the addition of adjuvant Trastuzumab to chemotherapy in node-positive and high-risk node-negative patients with HER2 overexpression and showed a survival advantage with Trastuzumab.4, 5, 6, 7, 8 Its use, however, results in a small to modest risk for cardiotoxicity, which is typically manifested by an asymptomatic decrease in left ventricular ejection fraction and less often by clinical heart failure.9, 10, 11 The rate of cardiac dysfunction with use of Trastuzumab varied from 1 to 27% in different arms of these trials.12
Although the heart was initially thought to be relatively radio-resistant, cardiovascular disease resulting from chest radiotherapy (RT) for therapeutic purposes is now clearly recognized. The majority of the radiation-induced cardiovascular disease has been reported in patients who previously treated with thoracic RT for Hodgkin's disease and breast cancer. Estimated relative risk of fatal cardiovascular events after RT for Hodgkin's disease and left-sided breast cancer range between 2.2 and 7.2 and 1.0 to 2.2, respectively, compared to healthy controls.13
Numerous studies of radiation induced toxicity show that endothelial cell injury is the key point in most tissues even though the endothelial cells compromise only a minor fraction of cardiac cells.14, 15, 16, 17 The sequence of endothelial injury, cell detachment, thrombosis and fibrosis result in significant tissue injury that often limits radiation oncologist in attempting to deliver curative doses to a nearby tumor. Steward and Fajardo have demonstrated that damage to the myocardium develops through three phases of injury.13, 18 The acute inflammation phase occurs about 6 h after RT and a neutrophilic infiltrate develops involving all layers of heart. The second phase also known as latent phase in which a slight progressive fibrosis begins about 2 days after exposure. However electron microscopy of the myocardial capillary endothelial cells demonstrates progressive damage leading to obstruction of the lumen and thrombi of fibrin and platelets. Though healthy endothelial cell replication in the vicinity occurred, it is generally inadequate and an inevitable ischemia leads to progressive fibrosis. Animals begin to die at approximately 70th day due to extensive fibrosis. The hallmark of this late stage is extensive fibrosis (Fig. 1, Fig. 2).
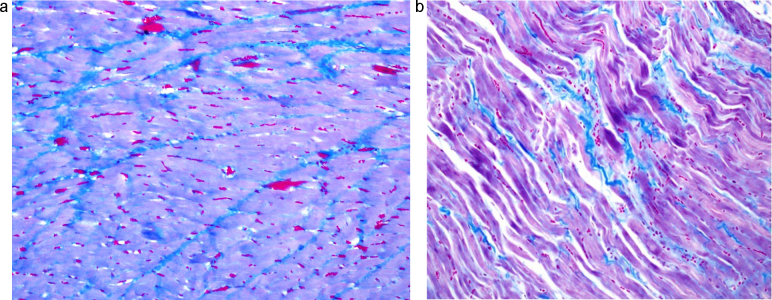
Fig. 1.
Heart sample of concomitant RT + T group (a); and RT + T + S (b) group at 100th day of RT. a: Heart sample of RT + T group at 100th day of RT. There was very extensive subendocardial fibrosis (Masson trichrome staining: ×200). b: Heart sample of RT + T + S group at 100th day of RT. There was moderate subendocardial fibrosis (Masson trichrome staining: ×200).
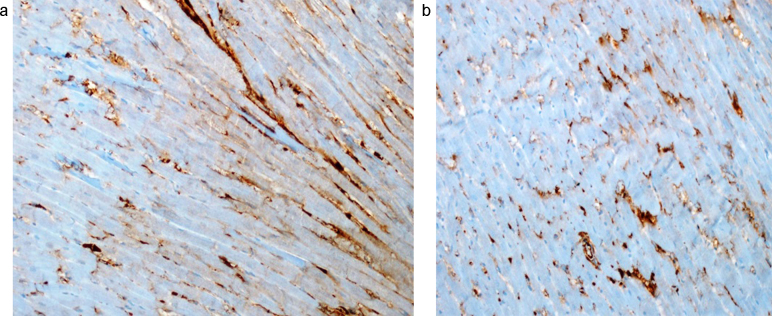
Fig. 2.
TGF-β positivity in the samples taken from the cardiac tissues of RT + T group (a); and RT + T + S group (b) at 100th days of RT (TGF-βX200). a There was severe immunohistochemical TGF-β positivity in the samples taken from the cardiac tissue at 100th days of RT + T (TGF-βX200). b There was moderate immunohistochemical TGF-β positivity in the samples taken from the cardiac tissue at 100th days of RT + T + S (TGF-βX200).
For patients with breast cancers that overexpress the HER2 receptor, both RT and Trastuzumab take place in the treatment. In the clinical practice RT and Trastuzumab may be used either concomitantly or sequentially. The risk of toxicity of combined Trastuzumab and RT on cardiovascular structures has not yet been evaluated in detail. There is conflicting data with respect to the cardiac toxicity profile of using concomitant RT and Trastuzumab. Preclinical in vitro and in vivo studies have shown that the cascade of events through the HER2 receptor is involved in tumor radiosensibility,19, 20 application of Trastuzumab concurrently with radiation thus increases the antitumor effect of radiation. There are same clinical evidences in the literature that Trastuzumab also radiosensibilizes human healthy tissues and in this way it could increase the toxicity of the treatment.21 Currently the most important question remains whether the concomitant therapy with Trastuzumab and RT increases cardiotoxicity of the treatment. In the literature, there are limited data about the safety of concomitant therapy with RT and Trastuzumab.19 In an experimental study, it was shown that when combined with high dose RT, and Trastuzumab may lead severe vascular damage.22
The use of antagonists of the mineralocorticoid receptor (MR) in the treatment of myocardial hypertrophy and heart failure has gained increasing importance in the last years. Steroids, including aldosterone, are able to interact with peptide hormone signaling. The epidermal growth factor (EGF) and its receptor (EGFR) represent one of these signals involving aldosterone. Pathophysiologically, an interaction between Aldosterone/MR and the EGFR signaling pathway was demonstrated by an up-regulation of EGF-induced arterial contraction mediated by mineralocorticoids and a reduction of cerebrovascular remodeling process after ischemia by MR inhibition concomitant decrease in EGFR-mRNA.23, 24 Additionally, the cardioprotective mechanism of Spironolactone was associated with the EGFR22 and inhibition of Aldosterone synthase was shown to ameliorate angiotensin (ang)-II induced end-organ damage, thus highlighting the significance of aldosterone and the EGFR for general pathophysiology in renocardiovascular system.25 The underlying mechanism for the genomic interactions seems to be a stimulation of the EGFR promoter by Aldosterone-bound MR, which then leads to dose-dependently enhanced EGFR proteins that can be inhibited by Spironolactone.26
2. Aim
In the current study we aimed to evaluate whether the use of spironolactone would be able to ameliorate the cardiac toxicity induced by concomitant Trastuzumab and RT. Our second aim was to determine cardiotoxicity profile of using Trastuzumab and RT concomitantly.
3. Methods and materials
3.1. Study design
This study included 80 female Wistar–Albino rats (250–300 g); the use of which was approved by the Necmettin Erbakan University Ethical Committee. Animals were housed 4 per cage in a controlled animal holding room with a 12/12-hour light/dark cycle; temperature and relative humidity were continually monitored to provide standard laboratory conditions. Rats were divided into 8 groups (G) composed of 10 animals. Group (G) 1 was defined as control group. G2, G3 and G4 were RT, Spironolactone and Trastuzumab groups respectively. G5, G6, G7 and G8 were RT + Trastuzumab, Trastuzumab + Spironolactone, RT + Spironolactone and RT + Trastuzumab + Spironolactone groups respectively (Table 1). RT was applied under general anesthesia with intraperitoneally administered 90 mg/kg ketamine hydrochloride and 10 mg/kg xylazine. A single dose of 15 Gy was applied to the mediastinum. Trastuzumab (6 mg/kg) was administered intraperitoneally and Spironolactone (80 mg/kg) was administered by oral gavage. Rats were sacrificed via cervical dislocation at 6th hour, 21st day and 100th day after RT and the heart and thoracic aorta samples were taken for microscopical examination.
Table 1.
Study groups.
| Group (G) | |
|---|---|
| G1 | Sham-irradiated control group |
| G2 | Radiotherapy control group |
| G3 | Spironolactone control group |
| G4 | Trastuzumab control group |
| G5 | Radiotherapy + Trastuzumab group |
| G6 | Spironolactone + Trastuzumab group |
| G7 | Radiotherapy + Spironolactone group |
| G8 | Radiotherapy + Trastuzumab + Spironolacton group |
3.2. Radiotherapy protocol
RT was applied under general anesthesia with intraperitoneally administered 90 mg/kg ketamine hydrochloride (Ketalar®, EWL Eczacibasi Warner Lambert Ilaç Sanayi ve Ticaret A.S., Istanbul, Turkey) and 10 mg/kg xylazine (Rompun® 2%, Bayer Kimya San. Ltd. Sti., Istanbul, Turkey). A single dose of 15 Gy that has been shown to lead cardiovascular toxicity with 6 MV photon beams was applied via a single anterior field to 2 cm depth with SAD (source-axis distance) technique.19 One cm elasto-gel bolus was used to build up the radiation dose on the heart and the thoracic aorta; and to provide contour regularity. The field size was 4 × 4 cm and included the heart and the thoracic aorta.
3.3. Trastuzumab protocol
Trastuzumab (Herceptin®; Genentech Inc, South San Francisco, CA, USA) dose which was equivalent to 6 mg/kg adult dose was calculated for each rat and injected i.p. 2 h before the RT. The rats in G4, G6 and G8 were applied 0.5 cc 0.9% NaCl i.p.
3.4. Spironolactone protocol
Spironolactone (Aldakton-A®, Ali Raif İlaç San. A.Ş., İstanbul, Turkey) dose which was equivalent to 80 mg/kg adult dose was calculated for each rat and was administered by oral gavage. Spironolactone administration was started a week before RT and continued until the animals sacrificed.
3.5. Light microscopy
The heart and thoracic aorta samples were excised and fixed in 10% formaldehyde solution and embedded in paraffin. Four μ thick sections were obtained with microtome for light microscopic examination. The slides obtained were stained with hematoxylen and eosin (H&E) to evaluate the inflammation, and with histochemical Masson Trichrome staining to identify the cardiac fibrosis. Additionally transforming growth factor-beta (TGF-β) expressions were investigated immunohistochemically. As a quantitive end point, extend of the radiation-induced fibrosis and TGF-β expressions were graded on a scale of 0 (normal heart-normal aorta) to 3 (severe). The pathologist was not aware of the treatment groups at the time of the histological examination of the specimens. After examining the whole sections for each rat, the average value was taken as the fibrosis and TGF-β expression scores and mean values of the groups were calculated.
3.6. Statistical analysis
The Statistical Package for Social Sciences (SPSS) v. 15.0 was used for statistical analyses. As the pathological scores were ordinal in nature, the differences in pathological findings between the study groups were analyzed using the Kruskal–Wallis test. When an overall statistically significant difference was observed, pairwise comparisons were performed using the Mann–Whitney U test. Bonferroni correction was used for multiple comparisons. A 5% type I error level was used for the statistical significance cutoff for overall comparisons.
4. Results
4.1. 6th hour findings
At 6th hour of RT; cardiac inflammation, cardiac fibrosis and TGF-β scores were not significantly different within the study groups (p values were 0.338, > 0.99 and >0.99 respectively). The samples taken from RT groups (G2, G5, G7 and G8) had minimal inflammation; however we did not observe any fibrosis or TGF-β expression even in RT groups.
We could not demonstrate any significant differences with respect to the inflammation, fibrosis and TGF-β expression in the thoracic aorta samples between the study groups at 6th hour of RT.
4.2. 21st day findings
At 21st day of RT; cardiac inflammation, cardiac fibrosis and TGF-β scores were not significantly different with in the study groups (P values were; 0.264, 0.761 and 0.761 respectively). The samples taken from RT groups (G2, G5, G7 and G8) showed minimal fibrosis. In parallel to this finding the TGF-β expressions of the RT groups were also minimally positive. However this positivity did not reach to the statistically significant level.
We could not demonstrate any significant differences with respect to the inflammation, fibrosis and TGF-β expression in the thoracic aorta samples between the study groups.
4.3. 100th day findings
By 100th days of RT the fibrosis scores and TGF-β expression in the cardiac samples were significantly different between the study groups (p values were 0.004 and 0.002 respectively) (Table 2, Table 3). Pair-wise comparisons revealed that both the cardiac fibrosis scores and TGF-β expression levels were higher in G5 when compared to G8 (p values were 0.046 and 0.028 respectively) (Table 4, Table 5). Moreover the TGF-β expression was higher in G5 when compared to G2 (p = 0.046).
Table 2.
Fibrosis scores of the samples taken from cardiac tissue 100 days after radiotherapy.
| Group (G) | Mean | Median | Minimum | Maximum | P |
|---|---|---|---|---|---|
| G1 | 0 | 0 | 0 | 0 | 0.004 |
| G2 | 1.50 | 1.50 | 1 | 2 | |
| G3 | 0 | 0 | 0 | 0 | |
| G4 | 0.50 | 0.50 | 0 | 1 | |
| G5 | 2.50 | 2.50 | 2 | 3 | |
| G6 | 0.50 | 1 | 0.5 | 1 | |
| G7 | 1 | 1 | 1 | 1 | |
| G8 | 1.25 | 1 | 1 | 2 |
Table 3.
TGF-β expression levels in the cardiac samples 100 days after radiotherapy.
| Group (G) | Mean | Median | Minimum | Maximum | P |
|---|---|---|---|---|---|
| G1 | 0 | 0 | 0 | 0 | 0.002 |
| G2 | 1.25 | 1 | 1 | 2 | |
| G3 | 0 | 0 | 0 | 0 | |
| G4 | 1 | 1 | 1 | 1 | |
| G5 | 2.50 | 2.50 | 2 | 3 | |
| G6 | 1 | 1 | 1 | 1 | |
| G7 | 1 | 1 | 1 | 1 | |
| G8 | 1 | 1 | 1 | 1 |
TGF-β, transforming growth factor beta.
Table 4.
Pair-wise comparison of the study groups with respect to the cardiac fibrosis scores 100 days after radiotherapy.
| Group 1 | Group 2 | P |
|---|---|---|
| G1 | G2 | 0.025 |
| G1 | G6 | 0.046 |
| G1 | G8 | 0.022 |
| G2 | G3 | 0.004 |
| G3 | G5 | 0.009 |
| G3 | G6 | 0.008 |
| G3 | G8 | 0.003 |
| G5 | G8 | 0.046 |
Table 5.
Pair-wise comparison of the study groups with respect to the TGF-β expression levels in the cardiac samples 100 days after radiotherapy.
| Group 1 | Group 2 | P |
|---|---|---|
| G1 | G2 | 0.022 |
| G1 | G4 | 0.046 |
| G1 | G6 | 0.046 |
| G1 | G8 | 0.014 |
| G2 | G3 | 0.003 |
| G2 | G5 | 0.046 |
| G3 | G4 | 0.008 |
| G3 | G5 | 0.009 |
| G3 | G6 | 0.008 |
| G3 | G8 | 0.003 |
| G5 | G8 | 0.028 |
TGF-β, transforming growth factor beta.
We could not demonstrate any significant differences with respect to the inflammation, fibrosis and TGF-β expression in the thoracic aorta samples between the study groups.
5. Discussion
Adjuvant therapies for the treatment of breast cancer including RT, chemotherapy (CT), targeted therapy, and hormonal treatment significantly decrease the risk of recurrence and increase the survival. However, cardiovascular problems including treatment complications caused by many of these treatment modalities are responsible for most common cause of non-cancer mortality in breast cancer survivors.27 HER2 receptor tyrosine kinase is known to have a critical role in cardiac development and growth, repair and survival of adult cardiomyocytes. HER2 overexpression is reported to be around 15–25% of all breast cancer patients and this overexpression was shown to decrease overall and disease free survival rates in addition to diminish the response to variety of chemotherapeutic and hormonal agents.28, 29, 30, 31, 32 Trastuzumab, a humanized monoclonal antibody that specifically targets HER2 positive breast cancer cells has been shown to increase survival rates both in patients with metastatic and early stage breast cancer.33, 34, 35, 36, 37, 38 The cardiotoxic effect of Trastuzumab especially when used with antracyclin chemotherapy has been well known, though the mechanism is not yet fully understood.39 Preclinical studies indicate that the direct blockade of the HER2 receptor on myocytes has at least partially an important impact on the cardiotoxicity of Trastuzumab.40 RT on the other hand causes multiple effects on the heart as acute, subacute and chronic pericarditis, coronary vascular disease, valvular heart disease and restrictive cardiomyopathy.41 Radiation-induced heart disease is believed to be resulted from injury to microvasculature network and subsequent ischemia.40 On the other hand Spironolactone, an Aldosterone receptor antagonist, has a cardioprotective effect. The cardioprotective mechanism of Spironolactone was associated with the EGFR42 and inhibition of Aldosterone synthase was shown to ameliorate angiotensin (ang)-II induced end-organ damage, thus highlighting the significance of aldosterone and the EGFR for general pathophysiology in renocardiovascular system.43 The underlying mechanism for the genomic interactions seems to be a stimulation of the EGFR promoter by Aldosterone-bound MR, which then leads to dose-dependently enhanced EGFR proteins that can be inhibited by Spironolactone.44 Therefore Spironolactone reverses cardiac hypertrophy and excessive extracellular matrix formation. Although it has been demonstrated that Spironolactone ameliorates anthracycline -induced cardiotoxicity in preclinical45 and clinical studies,46 there is no data regarding to effect of Spironolactone on both Trastuzumab and radiation-induced cardiotoxicity. In the present study we demonstrated that, Spironolactone had a protective effect on cardiotoxicity that is caused by the use of concomitant Trastuzumab and RT. The protective effect was observed with long-term use, and we could not demonstrate such effect on thoracic aorta.
The renin-angiotensin-aldosterone system (RAAS) is the most effective system in the remodeling of the myocardium. The RAAS facilitates cardiac oxidative damage and it has been proved in various studies that the remodeling process after this damage may be improved via blockage of this system.46, 47 The first major trial to assess the use of an aldosterone antagonist in patients with left ventricular dysfunction, the Randomized Aldactone Evaluation Study (RALES), assessed the effect of Spironolactone compared with placebo among optimally managed heart failure patients.48 In the RALES study it had been demonstrated that the administration of 25 mg/day Spironolactone in addition the routine treatment improved cardiac fibrosis and remodeling. Mortality was reduced by 30% in patients treated with Spironolactone. In the EPHESUS study, which is double-blind, placebo-controlled study, it was demonstrated that the use of an aldosterone antagonist on the third to fourth days after acute myocardial infarction in patients with left ventricular dysfunction who were already receiving standard therapy,49 led to a 15% reduction in overall mortality, 17% reduction in cardiovascular deaths and a %23 reduction in heart failure in patients treated with aldosterone antagonist. The mechanism of aldosterone antagonism on cardiac injury may be explained via (i) remodeling after myocardial damage due to antifibrotic effect; (ii) lowering oxidative stress which is also known as antifibrotic effect; and (iii) preventing apoptosis.46 By the light of these findings Akpek and colleagues investigated the protective effect of Spironolactone in patients with breast cancer who received antracycline.46 Their results suggested that Spironolactone, when given simultaneously with anthracycline group chemotherapeutics protects both myocardial systolic and diastolic functions. This finding was verified in a preclinical study by Liu and colleagues.45
Radiation-induced cardiac disease is an underdiagnosed but potentially lethal side effect of RT.13, 50 The manifestations of radiation-induced cardiac disease may include acute/chronic pericarditis, accelerated atherosclerosis, conduction abnormalities, valvular changes, and, notably, pericardial and myocardial fibrosis. The symptoms and signs of radiation-induced cardiac disease are, for the most part, indistinguishable from those encountered in patients with heart disease of other etiologies.50 As is explained above, RAAS plays a vital important role in cardiovascular diseases, such as atherosclerosis, vascular and cardiac remodeling (tissue repair and remodeling by regulation of cell growth and matrix synthesis), and cardiac fibrosis including proliferation of cardiac myocytes and fibroblasts and intense perivascular inflammation. The pathophysiology of radiation-induced cardiac disease is similar to those cardiovascular diseases induced by angiotensin–aldosterone.50, 51 Since angiotensin–aldosterone is involved in the process of almost all kinds of cardiovascular diseases, in the current study, we investigated whether Spironolactone ameliorate cardiovascular injury that is caused by RT and Trastuzumab or not. Although the cardiac mean fibrosis scores of RT control and RT + Spironolactone groups were 1.50 and 1 respectively; this difference did not reach statistically significant level. Therefore our results suggested that when RT or trastuzumab used alone Spironolactone did not reverse cardiovascular injury.
Cardiac cells do not divide after birth, but they often become “innocent bystander” targets of anticancer drugs designed to interfere with cell signaling pathways.52, 53 Trastuzumab is a recent addition to the anticancer armamentarium that improves survival rate in women with metastatic breast cancer; however, it causes heart failure, particularly when used in combination with anthracyclines.54 The mechanism of trastuzumab-induced cardiac toxicity has not been understood yet; however there is strong evidence indicating the importance of the EGF signaling system in the normal heart and suggests that Trastuzumab cardiotoxicity is directly related to HER2 blockade. The ErbB2 gene belongs to a family of EGFRs (EGFR, HER2, HER3 and HER4) that regulate many essential cell type-specific functions, particularly cell growth, proliferation and survival.55 ErbB2 is thought to participate in an important pathway for growth, repair, and survival of adult cardiomyocytes.55, 56 On the other hand Spironolactone, an Aldosterone receptor antagonist, has a cardioprotective effect. The cardioprotective mechanism of Spironolactone was associated with the EGFR25 and inhibition of Aldosterone synthase was shown to ameliorate angiotensin (ang)-II induced end-organ damage, thus highlighting the significance of aldosterone and the EGFR for general pathophysiology in renocardiovascular system.26 As the mechanisms of both spironolactone and trastuzumab on cardiac tissue use the same receptors, we thought that spironolactone would be able to ameliorate Trastuzumab- induced cardiac toxicity via inhibition of transactivation of the EGFR by aldosterone. However we could not demonstrate the positive effect of Spironolactone on Trastuzumab-induced cardiac toxicity in the present study. On the other hand when Trastuzumab used with concomitant RT, we observed that Spironolactone ameliorated cardiac injury by reducing the cardiac fibrosis and TGF-β expressions in the cardiac tissue.
The risk of toxicity of combined Trastuzumab and RT on cardiovascular structures has not yet been evaluated in detail. However there are same clinical evidences in the literature that Trastuzumab radiosensibilizes human healthy tissues and in this way it could increase the toxicity of the treatment.21 Currently the most important question remains whether the concomitant therapy with Trastuzumab and RT increases cardiotoxicity of the treatment. In the literature, there are limited data about the safety of concomitant therapy with RT and Trastuzumab.19 The observation period in the studies was short, the longest reported median observation period after the completion of concomitant treatment with RT and Trastuzumab was 3.7 years.8 Therefore currently there is no evidence that such therapy is safe after a long observation period.19 In our previous study, we have demonstrated that when combined with high dose RT, Trastuzumab may lead severe vascular damage.22 In the present study we also evaluated the impact of Trastuzumab on radiation-induced cardiovascular toxicity. Our results suggested that when used with concomitant Trastuzumab, radiation-induced cardiac toxicity may increase since we observed a significant difference between RT control group and RT + Trastuzumab group with respect to TGF-β expression levels. Additionally Spironolactone ameliorated the cardiac toxicity that was caused by concomitant Trastuzumab and RT; although it had no such effect when these agents used alone.
Our study has some limitations that should be mentioned as well. First of all, we could not demonstrate any significant differences with respect to the thoracic aorta samples. We used light microscopy instead of electron microscopy which was a limitation of our study. We think that electron microscopy may further enlighten the possible interaction of RT and Trastuzumab in this regard. Secondly, this study is an experimental study; therefore our results should be clarified with clinical studies.
In conclusion, our results suggested that Spironolactone may improve the cardiotoxicity that is induced by RT and Trastuzumab; however it does not have such effect on thoracic aorta. This finding should be clarified with further clinical studies.
Authors’ contribution
GY, RE, CY: Conception and design of the study; GY, EC, CE, RE: Acquisition of data, analysis and interpretation of data; GY, CY: Drafting the article; GY, CE, EC, RE: Acquisition of data. All authors read and approved the final manuscript.
Conflict of interest
None declared.
Financial disclosure
This work was supported by Selcuk University. There is no role of study sponsors in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript and in the decision to submit the manuscript for publication.
Contributor Information
Guler Yavas, Email: guler.aydinyavas@gmail.com.
Esin Celik, Email: dresincelik@hotmail.com.
Cagdas Yavas, Email: drcagdasyavas@gmail.com.
Cagdas Elsurer, Email: cagdaselsurer@yahoo.com.
Rengin Elsurer Afsar, Email: renginels@yahoo.com.
References
- 1.Benz C.C., O’Hagen R.C., Richter B. HER2/neu and the Ets transcription activator PEA3 are coordinately upregulated in human breast cancer. Oncogene. 1997;15:1513–1525. doi: 10.1038/sj.onc.1201331. [DOI] [PubMed] [Google Scholar]
- 2.Wonders K.Y., Reigie B.S. Trastuzumab and doxorubicin related cardiotoxicity and the cardioprotective role of exercise. Integr Cancer Ther. 2009;8:17–21. doi: 10.1177/1534735408330717. [DOI] [PubMed] [Google Scholar]
- 3.Albanell J., Bellmunt J., Moline R. Node negative breast cancers with p53 (-)/HER2-neu status may identify women with very good prognosis. Anticancer Res. 1996;16:1027–1032. [PubMed] [Google Scholar]
- 4.Romond E.H., Perez E.A., Bryan J. Trastuzumab plus adjuvant chemotherapy for operable HER-2 positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Perez E.A., Suman V.J., Davidson N. NCCTG N9831. May 2005 update Presented at the 45th annual meeting of the American Society of Clinical Oncology; Orlando FL; 2005 May 16. [Google Scholar]
- 6.Smith I., Procter M., Gelber R.D. 2-Year follow-up of trastuzumab after adjuvant chemotherapy in HER-2 positive breast cancer: a randomized controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H., Kellokumpu-Lehtienen P.L., Bono P. Adjuvant docetaxel or vineralbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 8.Halyard M.Y., Pisansky T.M., Dueck A.C. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG phase III trial N9831. J Clin Oncol. 2009;27(16):2638–2644. doi: 10.1200/JCO.2008.17.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogel C., Cobleigh M.A., Triphaty D.A. Adjuvant docetaxel or vineralbine with or without trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 10.Slamon D.J., Leyland-Jones B., Shak S. Use of chemotherapy plus monoclonal antibody against HER 2 for metastatic breast cancer that overexpresses HER 2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 11.Leyland Jones B., Gelmon K., Ayoub J.P. Pharmacokinetics, safety and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21:3965–3971. doi: 10.1200/JCO.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 12.Seiman A., Hudis C., Pierri M.K. Cardiac dysfunction in the Trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 13.Adams M.J., Hardenbergh P.H., Constine L.S., Lipshultz S.E. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 14.Gold H. Production of arteriosclerosis in the rat: effect of x-ray and a high-fat diet. Arch Pathol. 1961;71:268–273. [PubMed] [Google Scholar]
- 15.Lindsay S., Kohn H.I., Dakin R.L., Jew J. Aortic arteriosclerosis in the dog after localized aortic x-irradiation. Circ Res. 1962;10:51–60. doi: 10.1161/01.res.10.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Zidar N., Ferluga D., Hvala A., Popović M., Soba E. Contribution to the pathogenesis of radiation-induced injury to large arteries. J Laryngol Otol. 1997;111:988–990. doi: 10.1017/s0022215100139167. [DOI] [PubMed] [Google Scholar]
- 17.Rubin D.B. 1st edition. CRC Press; Chapman and Hall: 1998. The radiation biology of the vascular endothelium. [Google Scholar]
- 18.Stewart J.R., Fajardo L.F., Gillette S.M., Constine L.S. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1991;31:1205–1211. doi: 10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 19.Marinko T., Dolenc J., Bilban-Jakopin C. Cardiotoxicity of concomitant radiotherapy and trastuzumab for early breast cancer. Radiol Oncol. 2014;48(2):105–112. doi: 10.2478/raon-2013-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietras R.J., Poen J.C., Gallardo D., Wongvipat P.N., Lee H.J., Slamon D.J. Monoclonal antibody to HER-2/neu receptor modulates repair of radiation–induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59:1347–1355. [PubMed] [Google Scholar]
- 21.Law A.B., Evans T., Hayward R.L. Possible radiation sensitisation by trastuzumab leading to radiation-induced myelitis. Breast Care (Basel) 2009;4:40–42. doi: 10.1159/000193069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yavas G., Yildiz F., Guler S. Concomitant trastuzumab with thoracic radiotherapy: a morphological and functional study. Ann Oncol. 2011;22:1120–1126. doi: 10.1093/annonc/mdq590. [DOI] [PubMed] [Google Scholar]
- 23.Dorrance A.M., Osborn H.L., Grekin R., Webb R.C. Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R944–R950. doi: 10.1152/ajpregu.2001.281.3.R944. [DOI] [PubMed] [Google Scholar]
- 24.Florian J.A., Dorrance A., Webb R.C., Watts S.W. Mineralocorticoids upregulate arterial contraction to epidermal growth factor. Am J Physiol Regul Integr Comp Physiol. 2001;281:R878–R886. doi: 10.1152/ajpregu.2001.281.3.R878. [DOI] [PubMed] [Google Scholar]
- 25.Nakano S., Kobayashi N., Yoshida K., Ohno T., Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extyracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005;28:925–936. doi: 10.1291/hypres.28.925. [DOI] [PubMed] [Google Scholar]
- 26.Fiebeler A., Nussenberger J., Shagdarsuren E. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 29.Howlader N., Altekruse S.F., Li C.I. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju055. pii: dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albanell J., Bellmunt J., Molina R. Node-negative breast cancers with p53(−)/HER2-neu(−) status may identify women with very good prognosis. Anticancer Res. 1996;16:1027–1032. [PubMed] [Google Scholar]
- 31.Ellis M.J., Coop A., Singh B. Letrazole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1 and/or ErbB-2 positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808–3816. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 32.Ménard S., Valagussa P., Pilotti S. Response to cyclophosphamide, methotrexate, and fluorouracil in lymph node-positive breast cancer according to HER2 overexpression and other tumor biologic variables. J Clin Oncol. 2001;19:329–335. doi: 10.1200/JCO.2001.19.2.329. [DOI] [PubMed] [Google Scholar]
- 33.Esteva F.J., Valero V., Booser D. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Slamon D.J., Leyland-Jones B., Shak S. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 35.Romond E.H., Perez E.A., Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 36.Smith I., Procter M., Gelber R.D. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 37.Perez E.A., Romond E.H., Suman V.J. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamon D.J., Eiermann W., Robert N.J. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER 2 neu positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009;69:500S. [Google Scholar]
- 39.Piccart-Gebhart M.J., Procter M., Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 40.Klein P.M., Dybdal N. Trastuzumab and cardiac dysfunction: update on preclinical studies. Semin Oncol. 2003;30:49–53. doi: 10.1053/j.seminoncol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee P.J., Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiol Rev. 2005;13:80–86. doi: 10.1097/01.crd.0000131188.41589.c5. [DOI] [PubMed] [Google Scholar]
- 42.Nakano S., Kobayashi N., Yoshida K., Ohno T., Matsuoka H. Cardioprotective mechanisms of spironolactone associated with the angiotensin-converting enzyme/epidermal growth factor receptor/extyracellular signal-regulated kinases, NAD(P)H oxidase/lectin-like oxidized low-density lipoprotein receptor-1, and rho-kinase pathways in aldosterone/salt-induced hypertensive rats. Hypertens Res. 2005;28:925–936. doi: 10.1291/hypres.28.925. [DOI] [PubMed] [Google Scholar]
- 43.Fiebeler A., Nussenberger J., Shagdarsuren E. Aldosterone synthase inhibitor ameliorates angiotensin II-induced organ damage. Circulation. 2005;111:3087–3094. doi: 10.1161/CIRCULATIONAHA.104.521625. [DOI] [PubMed] [Google Scholar]
- 44.Krug A.W., Grossmann C., Schuster C. Aldosterone stimulates epidermal growth factor receptor (EGFR) expresssion. J Biol Chem. 2003;278:43060–43066. doi: 10.1074/jbc.M308134200. [DOI] [PubMed] [Google Scholar]
- 45.Liu G., Liu Y., Wang R. Spironolactone attenuates doxorubicin-induced cardiotoxicity in rats. Cardiovasc Ther. 2016;34(4):216–224. doi: 10.1111/1755-5922.12189. [DOI] [PubMed] [Google Scholar]
- 46.Akpek M., Ozdogru I., Sahin O. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail. 2015;17(1):81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 47.Solomon S.D., Pfeffer M.A. Aldosterone antagonism and myocardial infarction: from animals to man and back. J Am Coll Cardiol. 2003;42(9):1674–1676. doi: 10.1016/j.jacc.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Pitt B., Zannad F., Remme W.J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 49.Pitt B., Remme W., Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 50.Wu R1, Zeng Y. Does angiotensin II-aldosterone have a role in radiation-induced heart disease? Med Hypotheses. 2009;72(3):263–266. doi: 10.1016/j.mehy.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 51.Al-Waili NS1, Butler G.J. A combination of radiotherapy, nitric oxide and a hyperoxygenation sensitizing protocol for brain malignant tumor treatment. Med Hypotheses. 2007;68(3):528–537. doi: 10.1016/j.mehy.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 52.Ng R., Better N., Green M.D. Anticancer agents and cardiotoxicity. Semin Oncol. 2006;33(1):2–14. doi: 10.1053/j.seminoncol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Telli M.L., Hunt S.A., Carlson R.W., Guardino A.E. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25(23):3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 54.Feldman A.M., Lorell B.H., Reis S.E. Trastuzumab in the treatment of metastatic breast cancer: anticancer therapy versus cardiotoxicity. Circulation. 2000;102(3):272–274. doi: 10.1161/01.cir.102.3.272. [DOI] [PubMed] [Google Scholar]
- 55.Chen K.R. Myocyte survival pathways and cardiomyopathy: implications for Trastuzumab cardiotoxicity. Semin Oncol. 2000;28:20–27. [PubMed] [Google Scholar]
- 56.Force T., Krause D.S., Van Etten R.A. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]