Abstract
Aim
The purpose of this study was to determine the optimal mean liver biologically effective dose (BED) to prevent radiation-induced liver disease (RILD) in stereotactic body radiation therapy (SBRT).
Background
The actual mean doses appropriate for liver irradiation in modern radiotherapy techniques have not been adequately investigated, although SBRT is sometimes alternatively performed using fractionated regimens.
Materials and methods
SBRT treatment plans for liver tumors in 50 patients were analyzed. All distributions of the physical doses were transformed to BED2 using the linear-quadratic model. The relationship between physical doses and the BED2 for the liver were then analyzed, as was the relationship between the mean BED2 for the liver and the planning target volume (PTV).
Results
A significantly positive correlation was observed between the mean physical dose for the background liver and the mean BED2 for the whole liver (P < 0.0001, r = 0.9558). Using the LQ model, a mean BED2 of 73 and 16 Gy for the whole liver corresponded to the hepatic tolerable mean physical dose of 21 and 6 Gy for Child–Pugh A- and B-classified patients, respectively. Additionally, the PTV values were positively correlated with the BEDs for the whole liver (P < 0.0001, r = 0.8600), and the background liver (P < 0.0001, r = 0.7854).
Conclusion
A mean BED2 of 73 and 16 Gy for the whole liver appeared appropriate to prevent RILD in patients with Child–Pugh classes A and B, respectively. The mean BED2 for the liver correlated well with the PTV.
Keywords: Biologically effective dose, Child–Pugh classification, Intensity modulated radiation therapy, Stereotactic body radiation therapy, Radiation-induced liver disease, Hepatocellular carcinoma
1. Background
Surgical resection is the first choice treatment for hepatocellular carcinoma (HCC).1, 2 Transarterial chemoembolization, percutaneous ethanol injection, radiofrequency ablation, and radiotherapy have been used in cases of unresectable HCC. Additionally, conformal radiotherapy is a palliative option for HCC. However, recent advances in modern radiotherapy, including intensity modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), have made them suitable for curative treatments.3, 4, 5, 6, 7, 8, 9, 10
Radiation-induced liver disease (RILD) has traditionally been recognized as an almost fatal complication.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Several dosimetric models that make use of dose–volume histograms have been generated to quantitatively predict RILD in patients who receive three-dimensional conformal radiotherapy (3D-CRT). The mean liver dose has been thoroughly debated, and approximately 30 Gy was deemed to be the dose–volume limit of the liver.20 However, the actual mean doses appropriate for liver irradiation in modern radiotherapy techniques have not been adequately investigated.
Xu et al. reported a prediction model for RILD after 3D-CRT where the hepatic tolerable doses (TD5; the mean dose that produce a 5% incidence of RILD) for a normal liver were 21 Gy and 6 Gy for Child–Pugh A- and B-classified patients, respectively.19 In their study, the incidence and mortality rate of RILD were 16% and 76%, respectively. Currently, modern radiotherapeutic techniques can significantly reduce the doses to organs at risk (OARs) and provide effective doses to the target with high precision. RILD after SBRT has not been well described or understood compared to that after 3D-CRT.21, 22, 23, 24, 25, 26 Therefore, it is important to establish the probability of RILD due to radiotherapy. In addition, IMRT can be used in various regimens based on the biologically effective dose (BED) using a linear-quadratic (LQ) model.27, 28 Previously described mean physical doses may be insufficient for predicting RILD in modern radiotherapy. Based on this information, we chose to conduct a planning study to assess the mean liver BED in an SBRT plan to prevent RILD.
The purpose of this study was to assess the BED in SBRT using radiotherapy planning data for liver tumors, and to establish an SBRT protocol that does not cause RILD in treatment of such tumors.
2. Materials and methods
Our study was conducted according to the principles of the Declaration of Helsinki. The institutional review board of our clinic approved this retrospective study (Approval No. 9). We analyzed SBRT treatment plans for liver tumors in 50 patients at our institution between January 2010 and February 2014. We excluded patients who received re-irradiation in the same area. Patients’ characteristics are shown in Table 1. Radiotherapy of the liver tumors was performed as described previously.28 Computed tomography (CT) images and magnetic resonance imaging (MRI) for treatment planning were obtained using the 4-slice BrightSpeed Excel™ (GE Healthcare, Waukesha, WI, USA) and the SIGNA EXCITE HDx 1.5T™ (GE Healthcare), respectively. Planning contrast-enhanced four-dimensional CT scans and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI images were used to determine gross tumor volume. To account for respiratory tumor motion, an internal target volume (ITV) was generated by contouring the imaging data of the four-dimensional CT. The planning target volume (PTV) was typically created by adding a 4- to 8-mm margin to the ITV in all directions. PTV margins of 4, 5, 6, and 8 mm were applied in 11, 2, 9, and 28 patients, respectively. Moreover, additional 2- and 3-mm margins were added in the longitudinal direction in 4 and 2 patients based on their respiratory status, respectively. The prescription radiation doses were documented at the reference point using conformal beams in 6 patients or were designed to deliver 100% of the prescription dose to 95% of the PTV using IMRT in 44 patients. Among 6 patients who received conformal beam radiotherapy, the treatment plans incorporated 7, 8, 9, and 10 beams in 1, 1, 3, and 1 patients, respectively. Among 44 patients who received IMRT, the treatment plan was devised using 5, 6, 7, 8, and 9 beams in 5, 25, 10, 2, and 2 patients, respectively. Additionally, Pencil-beam and Monte Carlo dose calculation algorithms were used in 30 and 20 patients, respectively, including one who received conformal beam radiotherapy for planning calculations. Treatment planning was performed by using iPlan RT Image™ version 4.1.0 and iPlan RT Dose™ version 4.1.2 (BrainLAB AG, Germany) units.
Table 1.
Patients’ characteristics and summary of the radiotherapy.
| Characteristics | Number of patients | |
|---|---|---|
| Number of patients | 50 | |
| Age (years) | 73.5 (48–89) | |
| Tumor background | ||
| Hepatocellular carcinoma | 36 | |
| Metastatic liver tumor | 13 | |
| Cholangiocellular carcinoma | 1 | |
| Background liver | ||
| Normal liver (metastatic liver tumor) | 13 | |
| Child–Pugh classification | A | 29 |
| B | 8 | |
| Summary of radiotherapy | |
|---|---|
| Total prescription dose (Gy) | 50.0 (40.0–65) |
| Number of fractions | 9 (4–25) |
| Fraction size (Gy) | 5.7 (2.6–11) |
| BED10 (Gy) | 80.0 (56.0–115.5) |
| PTV (cm3) | 80.2 (17.1–1242.7) |
| Volume of the whole liver (cm3) | 1048.5 (683–1701) |
| Volume of the background liver (cm3) | 929.4 (574.2–1574.7) |
| Mean physical dose for the whole liver (Gy) | 14.8 (5.34–35.93) |
| Mean physical dose for the background liver (Gy) | 11.1 (3.91–24.96) |
| Mean BED2 for the whole liver (Gy) | 36.8 (9.26–99.91) |
| Mean BED2 for the background liver (Gy) | 22.5 (7.49–58.88) |
HCC = hepatocellular carcinoma; PTV = planning target volume; BED = biologically effective dose.
The background liver was defined as the whole liver minus the PTV.
All dose distributions of the physical doses were corrected to the BEDs using the LQ formulation with an assumed α/β ratio of 2 Gy for the liver.20
First, the differential dose–volume histogram (DVH) of the liver was acquired from the radiotherapy planning system. Next, the output of the physical dose that was assumed to be equivalent to the delivery of 55.2 Gy in 12 fractions (4.6 Gy per fraction) to the tumor was calculated, following the recommendations of a previous report, 19 because the dose distributions are independent of the dose fraction size. Each physical dose was then converted into the BED by using the α/β value of 2 Gy, and the irradiated liver volume was obtained at each BED2. Moreover, the mean DVH values for the liver were calculated in terms of BED and were defined as the mean BED of the liver. Finally, we assessed the relationship between the mean physical doses and BED2 for liver. The dose distribution of each BED2 in the liver was exported using the DICOM-RT image fusion software (ShioRIS™ 2.0, Osaka, Japan).29
Additionally, we analyzed the relationship between the mean BED2 values for the liver and the planning target volume (PTV), as well as those between the expected hepatic dysfunctional volume and the PTV in order to estimate the residual volume after radiotherapy. We defined the potential liver dysfunction volume as a liver volume that received a BED2 of 50 Gy or greater when cirrhotic, or received 75 Gy or greater when normal.28 The PTV intersection point with the liver was assessed by using the iPlan RT Image™ version 4.1.0 intersection function tool treatment planning system (BrainLAB AG, Feldkirchen, Germany).
The data are expressed as medians with the ranges in parentheses, unless otherwise indicated. Linear regression was used to estimate the BED2 values from the physical doses. A two-tailed Spearman test was performed to analyze the correlation between two variables. All statistical analyses were performed using GraphPad Prism version 6.0b (GraphPad Software Inc., San Diego, CA, USA). P values <0.05 were considered statistically significant.
3. Results
The process of conversion from physical doses to biological doses in a representative case is shown in detail in Fig. 1. In this planning study, the mean physical doses and transferred BEDs were evaluated in each patient. Mean physical doses and BED2 values for the whole liver and those for background liver, which was defined as the whole liver volume minus the PTV, are shown in Table 2.
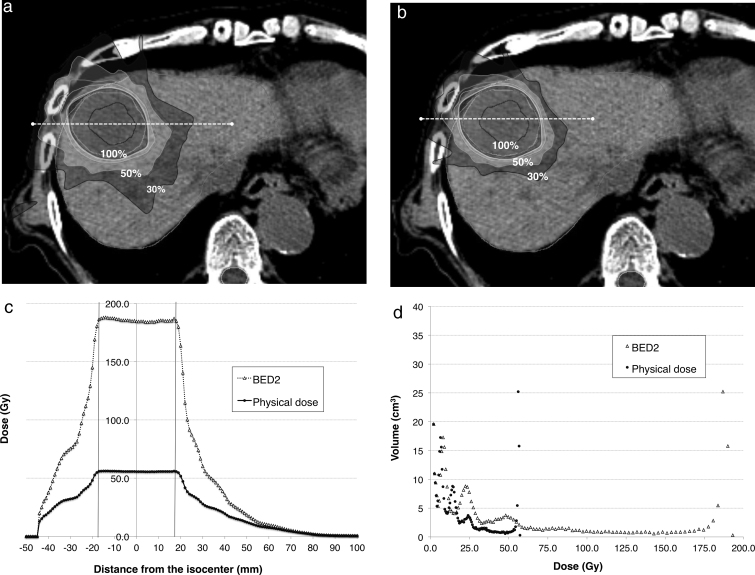
Fig. 1.
An 84-year-old man who presented with hepatocellular carcinoma in the S4 region had Child–Pugh B cirrhosis and received a total of 42.5 Gy in 5 fractions (8.5 Gy per fraction). The whole liver volume and planning target volume were 1062 and 61.2 cm3, respectively. The dose contribution lines are shown in physical (a) and biological (b) doses, and are normalized to prescription doses (100%). The original radiotherapy plan was revised to administer 12 fractions using the dose distribution devised for each plan in this study. The dose at each isodose line (white dashed line) was plotted (c); physical and biological doses were both plotted into the graph. The X and Y axes indicate the distance from the isocenter and the doses at each distance, respectively. Vertical solid lines indicate the edges of the planning target volumes. The liver volume that received each absorbed dose is shown in the scatter plot (d), in which the physical (dot) and biological doses (triangle) were plotted. The X and Y axes in the scatter plot indicate the absorbed dose and volume that received each dose, respectively. The mean physical doses for the whole liver and background liver were 8.51 and 5.83 Gy, respectively. Transferred biological doses for the whole liver and background liver were 20.91 and 11.3 Gy, respectively. Finally, the mean physical and biological doses for the liver in each patient were evaluated.
Table 2.
Summary of the liver mean doses.
| Mean physical dose for the whole liver (Gy) | 14.8 (5.34–35.93) |
| Mean physical dose for the background liver (Gy) | 11.1 (3.91–24.96) |
| Mean BED2 for the whole liver (Gy) | 36.8 (9.26–99.91) |
| Mean BED2 for the background liver (Gy) | 22.5 (7.49–58.88) |
BED = biologically effective dose.
The background liver was defined as the whole liver minus the PTV. Each physical dose was transformed to BED2 using the linear linear-quadratic model.
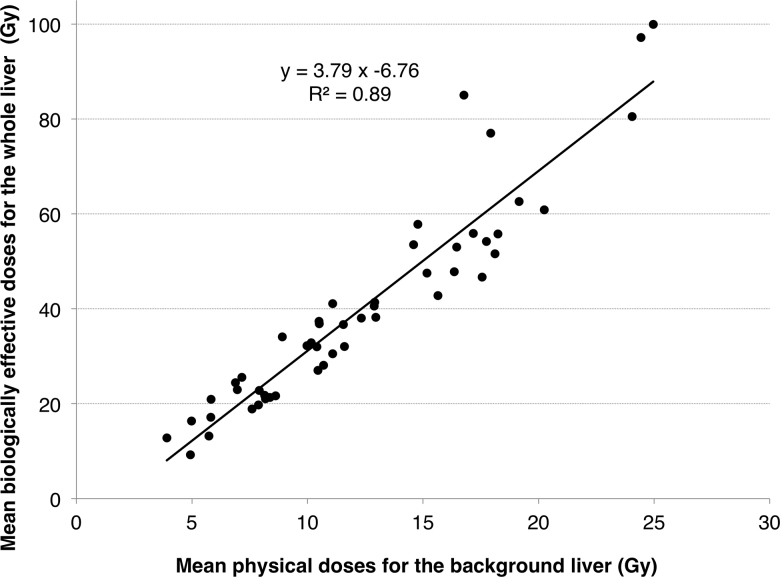
Fig. 2 shows a significant positive relationship between the mean physical doses for the background liver and the mean BED2 for the whole liver (P < 0.0001, r = 0.9558). Using the LQ model, a mean BED2 of 73 and 16 Gy for the whole liver corresponded to a mean physical dose of 21 and 6 Gy, respectively, for the background liver.
Fig. 2.
Correlation between physical doses and biologically effective doses (BEDs) for a liver. Mean liver dose in each treatment plan was plotted on a scatter diagram. The X and Y axes indicate the mean physical dose and the mean BED2 for the liver, respectively.
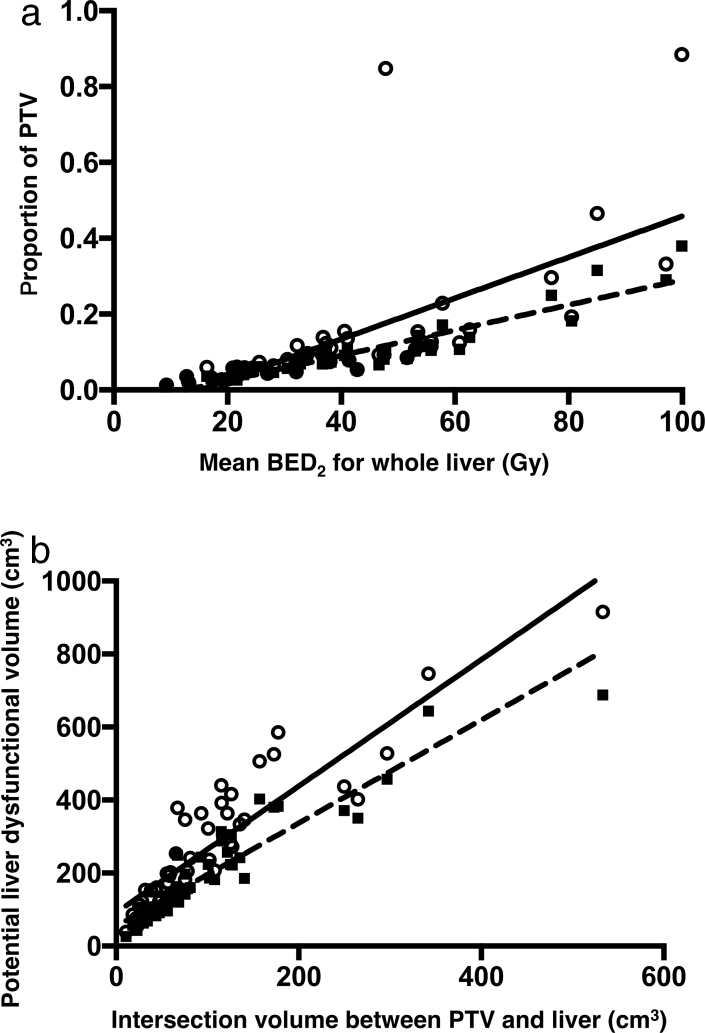
The mean BED2 for the whole liver was positively correlated with the PTV (Fig. 3a). Additionally, a significantly positive correlation was detected between the mean BED2 for the whole liver and the PTV proportions in the liver (P < 0.0001, r = 0.8857) as well as the intersection of the PTV in the liver (P < 0.0001, r = 0.9424). Moreover, the PTV values were positively correlated with the physical doses for the whole liver (P < 0.0001, r = 0.8528) and the background liver (P < 0.0001, r = 0.7915), as well as the BEDs for the whole liver (P < 0.0001, r = 0.8600), and the background liver (P < 0.0001, r = 0.7854). The potential liver dysfunctional volume was positively correlated with the volume of the PTV intersection (Fig. 3b). A significant positive correlation was observed between liver dysfunctional volume and a BED2 of 50 Gy (P < 0.0001, r = 0.934) and 75 Gy (P < 0.0001, r = 0.9544) in cirrhotic and normal livers, respectively.
Fig. 3.
(a) The correlation between the biologically effective doses for the whole liver and the PTV. X and Y axes indicate the mean BED2 for liver (Gy) and the proportion of PTV (circles; the solid line denotes the standard curve) and that of the overlap between the PTV and the liver (squares; the dashed line denotes the standard curve) in the whole liver, respectively. (b) The correlation between the potential liver dysfunctional volume (cm3) (Y axis) and the intersection volume between the PTV and the liver (cm3) (X axis). The circles (with the solid line denoting the standard curve) indicate the liver volumes that received a BED2 of 50 Gy or greater. The squares (with the dashed line denoting the standard curve) indicate the liver volumes that received a BED2 of 75 Gy or greater PTV = planning target volume, BED = biologically effective dose.
4. Discussion
SBRT is a promising technology in radiation oncology that is minimally invasive, and is well-tolerated for the management of liver tumors.3, 4, 5, 6, 7, 8, 9, 10 Successful SBRT requires the delivery of ablative radiation to a tumor while maximally sparing normal tissue. More studies are required to define the optimal application of SBRT in cancer therapy and normal tissue tolerance. SBRT requires a high degree of accuracy for target localization and treatment delivery to achieve tumor control while minimizing normal tissue toxicity. Radiotherapy of liver tumors is associated with two types of risks: fatal RILD and partial liver damage.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 28, 30, 31, 32, 33, 34 Because RILD is an often fatal complication of radiotherapy, and since modern radiotherapeutic techniques significantly reduce doses to OARs, it may be ethically unfeasible to perform prospective trials that test for RILD-inducing doses.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 We therefore used the data reported by Xu et al. as a reference when analyzing dose-volume histograms for radiotherapy planning.
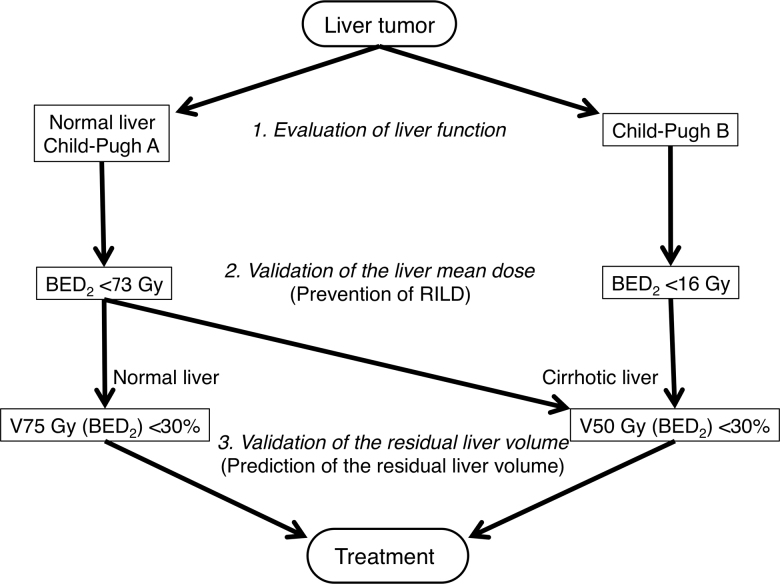
Herein, we propose a safe treatment protocol for SBRT of liver tumors (Fig. 4). First, liver function is evaluated according to the Child–Pugh classification in a cirrhotic liver (typically in HCC patients) or a normal liver (typically in patients with liver metastasis), since numerous reports have stated that patients with Child–Pugh B liver function have higher radiosensitivity than those with Child–Pugh A liver function.17, 18, 19, 20 Next, the liver doses are evaluated to prevent RILD, which can be lethal. A mean BED2 of less than 73 and 16 Gy for the whole liver should be maintained to prevent RILD in patients with Child–Pugh A and B liver function, respectively. Finally, the volume of hepatic dysfunction is assessed to estimate the residual liver volume. Less than 30% of the liver volume should receive BED2 values of 50 and 75 Gy in cirrhotic and normal livers, respectively, since it was previously shown that the tolerance dose of hepatocyte dysfunction was not influenced by the Child–Pugh score in those with cirrhotic livers.28
Fig. 4.
The current recommended treatment protocol based on our data. To prevent radiation-induced liver disease and minimize liver damage, two different checkpoints were included in the protocol BED = biologically effective dose.
Our protocol has two different checkpoints to ensure safe treatment. One is the evaluation of the mean dose and the other is the assessment of lost functional volume of the liver. To prevent RILD-related mortality, we evaluate the mean doses for the whole liver first, and then analyze the potential loss of hepatic function. Although several protocols for definitive radiotherapy for liver tumors have been reported, only a few comparison studies of such treatment regimens exist. Furthermore, some protocols require radiotherapy in alternative fractionated regimens to spare OARs such as the intestines. Therefore, a standard scale to evaluate the biological dose for the liver is required separately from the physical dose. Our evaluation protocol for the liver can be highly flexible and adaptive in alternative fractionated regimens of radiotherapy for liver tumors based on the BED, using the LQ model.
The prediction of residual liver volume after treatment has been reported after surgical resection.2, 35 The 15-min retention rate of indocyanine green may provide significant information with which to estimate the residual liver volume. Additionally, portal vein embolization is often performed to induce compensatory hypertrophy in the remnant liver area in order to prevent hepatic failure. As a result, surgical resection of a liver tumor can be expanded from enucleation to wide resection; i.e., up to 80% of the whole liver.35
Radiotherapy has several advantages compared to other radical treatment options. It can overcome anatomical borders such as the vascular, great vessel, and hepatic portal region; IMRT can also irradiate a target that has an irregular shape while reducing doses to OARs. SBRT has been reported to be safe as long as 700 mL of background liver remains uninjured.20, 24 However, no standard method exists for evaluation of residual liver volume after focal radiotherapy of liver tumors, despite the fact that liver tumors may require repeat curative treatments. Moreover, the physical constitution of a patient should be taken into account when considering the functional liver volume. We administer SBRT for liver tumors when we estimate that less than 30% of the whole liver volume will lose functional hepatocytes.28
We acknowledge several limitations in our study. Mean doses were calculated for the whole liver, including the tumor, to make evaluations objective and uncomplicated. Liver tolerance to radiotherapy is typically evaluated by using the liver volume, considered the whole liver minus the gross tumor volume.15, 16, 17, 18, 20 However, mean liver doses are defined according to the total liver volumes minus the PTV, per Xu et al.19 To compare different treatment settings such as PTV margins and prescription doses, we used a mean dose for the whole liver, including the target. As a result, a clear positive relationship was detected between the physical dose for background liver and the whole liver BED2 in our study.
We determined the mean liver BEDs using the whole liver for several reasons. First, the internal target volume is commonly used to evaluate the radiotherapeutic doses for the liver tumor in order to compensate for motion during breathing. Second, the PTV calculation margin depends on the required clinical and setup margins. Moreover, the PTV had a significantly strong correlation with the mean dose for both background and whole livers in this study; hence, PTV may be considered a predictive factor for the mean liver doses and residual liver volume after treatment in our study. Lastly, larger PTV margins can lead to underestimating both the liver dose that is actually delivered and the functional hepatocyte population around the tumor. We therefore considered that the evaluation of mean doses for the whole liver would be sufficiently reliable and safe.
Our data can be instrumental in clinical practice. However, further clinical trials are required to validate the applicability of our protocol to a larger pool of patients with greater homogeneity in order to establish a tolerable liver volume loss after partial radiotherapy using modern radiotherapy. A protocol based on our data is currently under safety evaluation; the results will be reported in due course.
5. Conclusion
We suggest that the mean BED2 of 73 and 16 Gy for whole liver were led in this planning study to prevent RILD in patients with Child–Pugh A and B, respectively. PTV had a positive relationship with the mean physical doses and BED2 for the liver.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.National Comprehensive Cancer Network . 2016. Hepatobiliary cancers (version 1. 2016) http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [accessed 15.03.16] [Google Scholar]
- 2.Nakayama H., Takayama T. Role of surgical resection for hepatocellular carcinoma based on Japanese clinical guidelines for hepatocellular carcinoma. World J Hepatol. 2015;7:261–269. doi: 10.4254/wjh.v7.i2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu E., Stenmark M.H., Schipper M.J. Stereotactic body radiation therapy for primary and metastatic liver tumors. Transl Oncol. 2013;6:442–446. doi: 10.1593/tlo.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tree A.C., Khoo V.S., Eeles R.A. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 5.Nouhaud E., Créhange G., Cueff A. Stereotactic body radiation therapy for liver tumors with or without rotational intensity modulated radiation therapy. BMC Res Notes. 2013;6:492. doi: 10.1186/1756-0500-6-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scorsetti M., Clerici E., Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol. 2014;5:190–197. doi: 10.3978/j.issn.2078-6891.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanuki N., Takeda A., Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3100–3111. doi: 10.3748/wjg.v20.i12.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huertas A., Baumann A.S., Saunier-Kubs F. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115:211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Wang P.M., Chung N.N., Hsu W.C., Chang F.L., Jang C.J., Scorsetti M. Stereotactic body radiation therapy in hepatocellular carcinoma: optimal treatment strategies based on liver segmentation and functional hepatic reserve. Rep Pract Oncol Radiother. 2015;20:417–424. doi: 10.1016/j.rpor.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comito T., Clerici E., Tozzi A., D’Agostino G. Liver metastases and SBRT: a new paradigm? Rep Pract Oncol Radiother. 2015;20:464–471. doi: 10.1016/j.rpor.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence T.S., Ten Haken R.K., Kessler M.L. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence T.S., Robertson J.M., Anscher M.S., Jirtle R.L., Ensminger W.D., Fajardo L.F. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 14.McGinn C.J., Ten Haken R.K., Ensminger W.D., Walker S., Wang S., Lawrence T.S. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246–2252. doi: 10.1200/JCO.1998.16.6.2246. [DOI] [PubMed] [Google Scholar]
- 15.Dawson L.A., Normolle D., Balter J.M., McGinn C.J., Lawrence T.S., Ten Haken R.K. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J.C., Wu J.K., Huang C.M. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41–45. doi: 10.1016/s0167-8140(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 17.Cheng J.C., Wu J.K., Lee P.C. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Liang S.X., Zhu X.D., Xu Z.Y. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z.Y., Liang S.X., Zhu J. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:189–195. doi: 10.1016/j.ijrobp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Pan C.C., Kavanagh B.D., Dawson L.A. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guha C., Kavanagh B.D. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park C., Papiez L., Zhang S., Story M., Timmerman R.D. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 23.Jung J., Yoon S.M., Kim S.Y. Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol. 2013;8:249. doi: 10.1186/1748-717X-8-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schefter T.E., Kavanagh B.D., Timmerman R.D., Cardenes H.R., Baron A., Gaspar L.E. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Son S.H., Choi B.O., Ryu M.R. Stereotactic body radiotherapy for patients with unresectable primary hepatocellular carcinoma: dose-volumetric parameters predicting the hepatic complication. Int J Radiat Oncol Biol Phys. 2010;78:1073–1080. doi: 10.1016/j.ijrobp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Cárdenes H.R., Price T.R., Perkins S.M. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 27.Hall E.J., Giaccia A.J. 7th ed. Lippincott Williams & Wilkins; Philadelphia: 2011. Radiobiology for the radiologist. [Google Scholar]
- 28.Doi H., Shiomi H., Masai N. Threshold doses and prediction of visually apparent liver dysfunction after stereotactic body radiation therapy in cirrhotic and normal livers using magnetic resonance imaging. J Radiat Res. 2016;57:294–300. doi: 10.1093/jrr/rrw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosu K., Sumida I., Shiomi H. A robust measurement point for dose verification in delivery quality assurance for a robotic radiosurgery system. J Radiat Res. 2016 doi: 10.1093/jrr/rrw103. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen C.C., Welsh J., Kavanagh B.D. Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1414–1424. doi: 10.1016/j.ijrobp.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 31.Rühl R., Lüdemann L., Czarnecka A. Radiobiological restrictions and tolerance doses of repeated single-fraction HDR-irradiation of intersecting small liver volumes for recurrent hepatic metastases. Radiat Oncol. 2010;5:44. doi: 10.1186/1748-717X-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda A., Oku Y., Sanuki N. Dose volume histogram analysis of focal liver reaction in follow-up multiphasic CT following stereotactic body radiotherapy for small hepatocellular carcinoma. Radiother Oncol. 2012;104:374–378. doi: 10.1016/j.radonc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Sanuki N., Takeda A., Mizuno T. Tumor response on CT following hypofractionated stereotactic ablative body radiotherapy for small hypervascular hepatocellular carcinoma with cirrhosis. Am J Roentgenol. 2013;201:W812–W820. doi: 10.2214/AJR.12.10169. [DOI] [PubMed] [Google Scholar]
- 34.Sanuki N., Takeda A., Mizuno T. Threshold doses for focal liver reaction after stereotactic ablative body radiation therapy for small hepatocellular carcinoma depend on liver function: evaluation on magnetic resonance imaging with Gd-EOB-DTPA. Int J Radiat Oncol Biol Phys. 2014;88:306–311. doi: 10.1016/j.ijrobp.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 35.Wagener G. Assessment of hepatic function, operative candidacy, and medical management after liver resection in the patient with underlying liver disease. Semin Liver Dis. 2013;33:204–212. doi: 10.1055/s-0033-1351777. [DOI] [PubMed] [Google Scholar]