Abstract
Background
Methamphetamine (METH) is one of the most popular psychostimulants which produce long lasting learning and memory impairment. Previous studies have indicated that estrogen and progesterone replacement therapy attenuate cognitive impairment against a wide array of neurodegenerative diseases. Present study was designed to figure out the effects of estrogen, progesterone alone or in combination, on early long-term potentiation (E-LTP) at the cornu ammonis (CA1) area of the hippocampus in METH-exposed ovariectomized (OVX) rat.
Methods
Twenty-one days after ovariectomy, the OVX rats received vehicle, estrogen [1 mg/kg, intraperitoneal (IP)] or progesterone (8 mg/kg, IP) and co-administration of estrogen plus progesterone during 14 consecutive days. On the 28th day, animals were exposed to neurotoxic METH regimens [four injections 6 mg/kg, subcutaneous (SC), 2 h intervals] 30 min after the hormones replacement. Finally, we investigated the effect of those ovarian hormones on synaptic plasticity using in vivo extracellular recording in the CA1 area of the hippocampus 2 days after last treatment.
Findings
The findings showed that the induction and maintenance phase of E-LTP was impaired in the METH exposed animals compared to the saline group. Data from this study demonstrated that treatment with estrogen and progesterone showed a significant facilitation for induction and enhancement of the maintenance of LTP in animals that received METH. In addition, co-administration of estrogen plus progesterone did not significantly affect the hippocampal synaptic plasticity in METH-exposed OVX rats in comparison with METH-exposed animals that received vehicle injections.
Conclusion
The present findings provide new insight about treatment with ovarian hormones on synaptic plasticity deficits induced by METH.
Keywords: Methamphetamine, Estrogen, Progesterone, Long-term potentiation, Rat
Introduction
Amphetamines are one of the most powerful and addictive psychostimulant drugs in many countries and modern societies.1-3 One of the well-known forms of amphetamines, i.e. methamphetamine (METH), leads to not only irreparable financial loss to government but also emotional and psychological toll on METH abuser by producing cognitive impairment.4 Among the detrimental neurotoxic effects of exposure to METH on cognitive ability is learning and memory impairment that remains long after drug withdrawal.5,6 Although the neural mechanisms of METH neurotoxicity have yet to be identified, recent studies have suggested that functional long-lasting alteration in hippocampal neurons can be associated with this cognitive impairments.7,8 In this aspect, previous works revealed the noxious effects of METH on hippocampus-dependent learning, memory and long-term potentiation (LTP), which is an electrophysiological model of synaptic plasticity accepted as a basic and early step in the formation of memory.7,9-11
Gonadal hormones like estrogen and progesterone exert wide-ranging actions that are not directly associated with sexual responses and reproductive behaviors.12 Converging evidence from animal studies indicated that ovarian hormone severely influence brain regions which have crucial role in learning and memory, such as the hippocampus.13,14 Due to their neurotrophic and neuroprotective actions, estrogen and progesterone replacement attenuate hippocampal learning and memory deficits in the various neurodegenerative diseases.15-18 Accumulative data from histological and electrophysiological investigations indicated that steroid hormones produce structural synaptic remodeling including increasing the number of dendritic spines and synapses and promoting the magnitude of LTP in hippocampal cornu ammonis (CA1) pyramidal cells.19-21 In this context, recently, Dai et al.15 showed that treatment with estrogen (1 mg/kg) promotes LTP in hippocampal CA1 following a global ischemia. Moreover, clinical and experimental evidence show that treatment with estrogen plus progesterone ameliorate performance of learning and memory tasks both in animal models and in humans.13,22 In cycling female rats, it was observed that LTP varies across estrus cycle, and is greater during proestrus than estrus in female rats.21
A series of reports revealed the beneficial effects of ovarian hormones on METH-induced neurotoxicity.23,24 Taking the importance of neurotoxicity in cognitive function into account, Ghazvini et al.25 demonstrated that treatment with estrogen or progesterone can partially improve METH-induced spatial learning and memory impairments in ovariectomized (OVX) female rats. Although co-administration of estrogen and progesterone did not influence METH-induced cognitive deficits. However, the probable effect of gonadal hormones on METH-induced synaptic plasticity impairment remains largely unknown. Considering the fact that an alteration in synaptic efficacy and function in area CA1 of hippocampus is associated with learning and memory mechanisms, the current study was designed to figure out the possible effects of estrogen and progesterone therapy on prevention of the synaptic plasticity deficits induced by neurotoxic dose of METH in OVX rats.
Methods
Female Wistar rats (200-250 g) were bred and kept in the Kerman Neuroscience Research Center animal facility, Iran, and placed in a temperature-controlled room with an ambient temperature set to 22 ± 1 °C. Rats were maintained with food and water available on a 12-h light-cycle (light beginning at 7:00 a.m.). All experimental protocols were conducted with the approval of the Ethics Committee of Kerman Neuroscience Research Center (Ethics Code: KNRC/EC/92-65).
The drugs included METH hydrochloride (Sigma, St Louis, MO), 17b-estradiol (Sigma, St Louis, MO), progesterone (Sigma, St Louis, MO). Ketamine and xylazine were obtained from the Alfasan Company (Holland). Estrogen and progesterone (Sigma) were each dissolved in sterile sesame oil.
Surgical operations were conducted under general anesthesia with an intraperitoneally (IP) injection of ketamine and xylazine (60 mg/kg, IP ketamine and 10 mg/kg, IP xylazine). The ovaries were removed by making two small bilateral incisions (2 cm) under aseptic conditions.25,26 All rats were OVX 3 weeks before the experiments in order to avoid hormonal influence. Drug treatment began 3 weeks after full recovery from surgery.
Animals were randomly divided into 6 groups. The treatment groups (n = 6-7) were as follows: 1. Saline group: OVX rats that received normal saline subcutaneous (SC), 2. METH-exposed group: OVX rats that received METH (6 mg/kg, SC)25, 3. Oil + METH-treated group: OVX rats that received injections of estrogen or progesterone vehicle (sesame oil) and METH (6 mg/kg, SC), 4. estrogen + METH-treated group: OVX rats that received injections of estrogen (1 mg/kg, IP)25,26 and METH (6 mg/kg, SC.), 5. Progesterone + METH-treated group: OVX rats that received injections of progesterone (8 mg/kg, IP)25,26 and METH (6 mg/kg, SC), 6. Estrogen+ Progesterone + METH-treated group: OVX rats that received injections of combined estrogen (1 mg/kg, IP) and progesterone (8 mg/kg, IP) and METH (6 mg/kg, SC).
As described by Ghazvini et al.,25 21 days after the ovariectomy, experimental groups were treated with estrogen, progesterone, estrogen + progesterone or vehicle for 14 consecutive days. On the 28th day, rats were exposed to a single-day METH regimen (four injections of 6 mg/kg, SC at 2 h intervals) 30 min after the hormone therapy. Hormone injection was continued for the next 6 days. As illustrated in figure 1, two days after the last injections at day 37, synaptic plasticity by in vivo field potential recording in CA1 area of hippocampus was examined.
Figure 1.
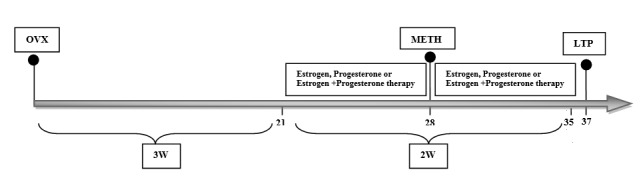
Overview and timeline of experimental procedures used in this study LTP: Long-term potentiation; OVX: Ovariectomized; METH: Methamphetamine
In vivo electrophysiological recording of field excitatory postsynaptic potentials (fEPSPs) from CA1 region was carried out as described previously.27,28 The female Wistar rats were nesthetized using urethane (1.2 g/kg) (Sigma-Aldrich) and mounted in a stereotaxic apparatus. Two burr holes were drilled into the skull to allow electrodes insertions. During the experiment, body temperature was monitored by a rectal thermistor probe and maintained at 36.5 ± 0.5 ◦C by a heating pad. A concentric bipolar stimulating electrode was placed into the Shaffer collateral pathway [anterior-posterior (AP) = 3.0 mm; medial-lateral (ML) = 3.5 mm; dorsal-ventral (DV) = 2.8-3.0 mm] and a stainless steel recording electrode positioned into the stratum radiatum of area CA1 of right hippocampus (AP = 4.1; ML = 3.0 mm; DV = 2.5 mm) according to the atlas of Paxinos and Watson.29 After connecting the stimulating electrode to a stimulator and recording electrode to an amplifier, fEPSP was measured by stimulating the Schaffer collateral pathway and recording in area CA1.
The location of the electrodes was optimized in order to maximize the amplitude of the evoked field potentials and then input-output (I/O) curve were constructed by gradually ascending stimulus intensity (input) and recording the fEPSPs response (output). The elicited field potentials were amplified and filtered (1 Hz to 3 kHz band pass) by applying differential amplifier. A baseline recording was obtained by giving a test stimulus every 10 s during 20 min adjusted to evoke about 50% of the maximal response. Moreover, paired-pulse facilitation (PPF) was measured by applying identical pairs of pulses at four inter-stimulus interval (20, 50, 70 and 100 ms) to the Schaffer collateral pathway in order to analyze presynaptic function. Then, PPF ratio were calculated as the ratio of the slope of the second fEPSP to that of the first (fEPSP 2/fEPSP 1).
LTP was induced by applying high frequency stimulation (HFS) consisting a trains of 10 pulses at 400 Hz with an inter-stimulus interval of 7 s during 70 s with the same stimulation intensity used for baseline recordings. Finally, for evaluating the maintenance of LTP, a test stimulus at the same intensity was presented every 10 s and continued for 2 h. The average of 10 consecutive traces at each time point was considered as values of the fEPSP slope. Computer-based stimulation and recording was obtained using NeuroTrace software version 9 and Electromodule 12 (Science Beam Institute, Tehran, Iran). Also for analyzing the fEPSP slopes, the software Potentialise from the same institute was used.10,30
A repeated-measure analysis of variance (ANOVA) was conducted to determine overall differences in LTP time points (group and time as the factors). Results from single time point among groups were also analyzed with unpaired Student’s t-test or one-way ANOVA followed by Tukey’s test for multiple comparisons, when required. All values were presented as means ± standard error of mean (SEM) and P-values less than 0.05 were regarded as significant.
Results
CA1 basal synaptic transmission and PPF relationship
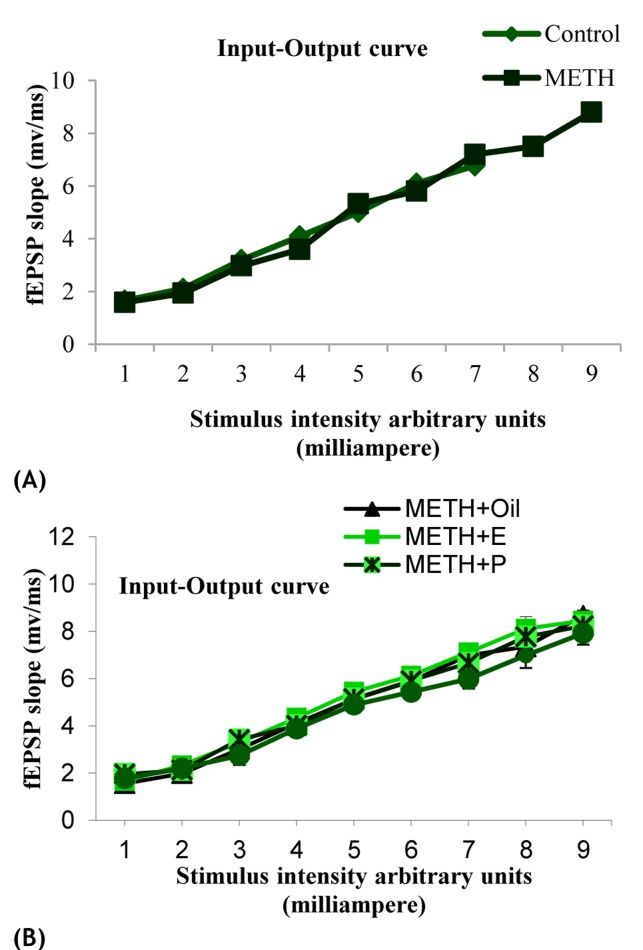
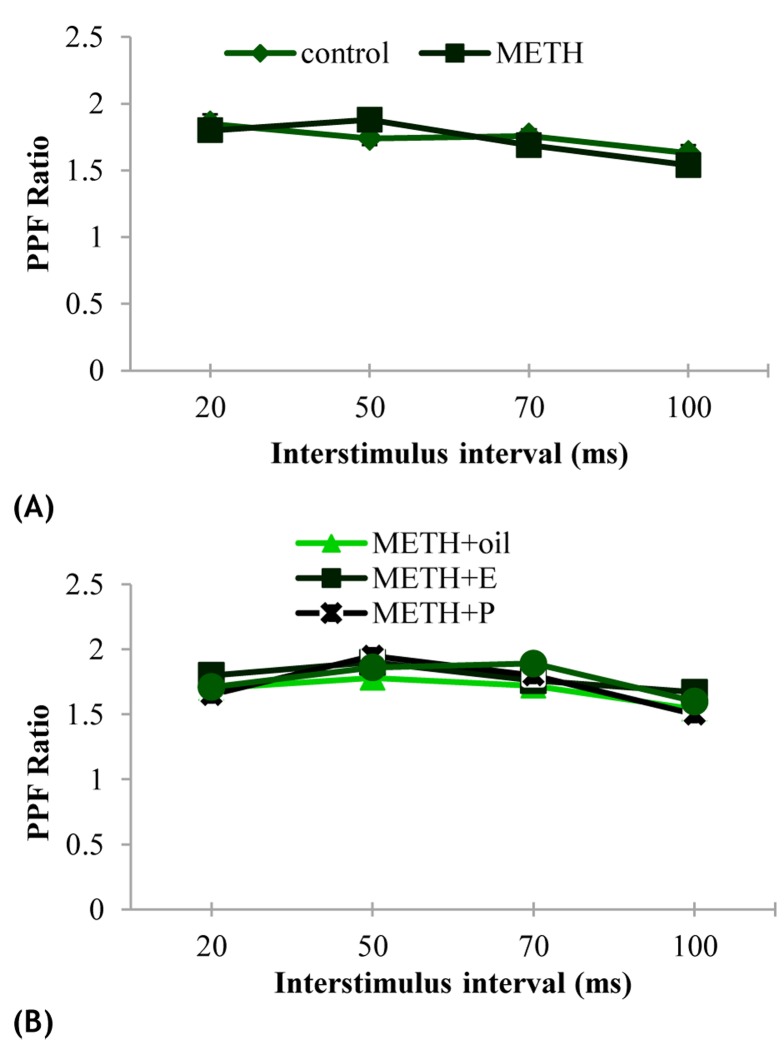
The effects of exposure to METH or ovarian hormones on basal synaptic transmission were determined using I/O curve which was constructed by plotting in the slope of the (fEPSP) in response to increasing stimulus intensities. Overall, no significant difference was observed in the I/O relationship among all female rats (P > 0.05) (Figure 2, A and B). Furthermore, we were interested in evaluating whether presynaptic mechanisms are involved in the effect of METH or ovarian hormones on synaptic plasticity by measuring paired-pulse facilitation (PPF) ratio values. Results indicated that PPF ratio values were not significantly affected in experimental groups (P > 0.05) (Figure 3, A and B).
Figure 2.
Input–output curves were constructed from responses in a range of stimulus intensities in the CA1 area of the hippocampus. Notably, the units of stimulus intensity are arbitrary where 1 is the intensity that exerts the minimum responses, and 9 is the intensity that exerts the maximum responses. I/O (Input-Output) was not significantly different between all groups (P > 0.05)
Figure 3.
The effects of METH administration and hormone replacement on paired pulse facilitation (PPF) ratio in the cornu ammonis (CA1) area of the hippocampus over a range of interstimulus intervals (20, 50, 70 and 100 ms). There was no significant difference among the groups (P > 0.05)
Early long-term potentiation (E-LTP)
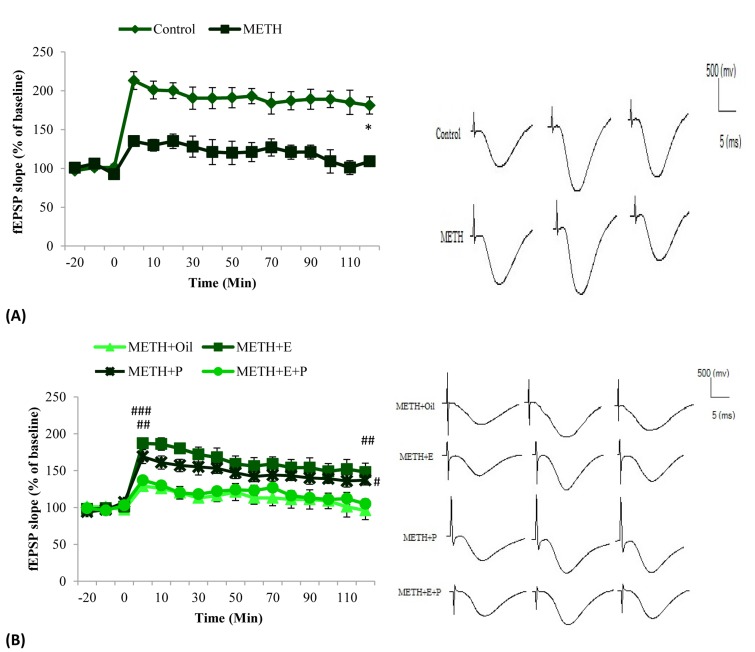
The increase in the slope of the fEPSP after HFS and its maintenance over 2 h were considered as E-LTP. Representative baseline and after HFS traces for experimental groups are shown in figure 4 (A and B).
Figure 4.
The effects of METH (A) hormone replacement + METH (B) administration on LTP induction and maintenance over a period of 120-min (early phase) in OVX female rats. METH administration produced a significant reduction of long-term potentiation (LTP) induction and maintenance compared to the saline group (P < 0.001). Treatment with estrogen alone and progesterone alone partially improved LTP induction and maintenance in animals that received METH (P < 0.050). Moreover, the hippocampal synaptic plasticity in animals that received combination of estrogen and progesterone in METH-exposed rats did not significantly differ from that of METH-exposed animals that received vehicle injections. Each point is the mean ± SEM. All recorded responses were averaged from normalized to the mean of baseline slopes. Calibrations, 500 mv/5 ms, were used during the recording session *P < 0.001, in comparison with the saline group, #P < 0.05, ##P < 0.01, ###P < 0.001 compared to oil + METH group
As illustrated in figure 4, all groups revealed LTP induction. The repeated measures ANOVA demonstrated an overall significant reduction in LTP induction and maintenance in METH group in comparison with saline group (P < 0.001). This overall reduction was ameliorated by treatment with estrogen and progesterone in METH exposed animals compared with oil + METH group (P < 0.001). After applying HFS, the mean fEPSP slope immediately increased which was considered as E-LTP induction. An unpaired Student’s t-test analysis showed that METH-exposed animals exhibited a significant reduction in fEPSP slope (135.00 ± 5.11% of baseline) in comparison with saline group (214.0 ± 11.5% of baseline) (F = 3.08, P < 0.001) (Figure 4, A).
Moreover, the current results showed that following HFS, treatment with either estrogen (187.00 ± 5.37% of baseline) (F = 12.1, P < 0.001) or progesterone (169.00 ± 9.51% of baseline) (F = 12.1, P < 0.010) could enhance the mean fEPSP slope in METH-exposed rats (Figure 4, B). On the other hand, there were no differences in fEPSP slope percent change from baseline between oil + METH (130.0 ± 6.2% of baseline) and estrogen + progesterone + METH (138.00 ± 5.41% of baseline). In addition, further analysis showed that LTP was impaired in METH-exposed animals compared with saline group, (P < 0.001). Our findings indicated that in the METH-exposed rats, after an initial increase in fEPSP slope, fEPSP slope approximately reduced to the baseline during 2 h compared to saline group (Saline = 181.0 ± 11.1, METH = 109.00 ± 6.64% of baseline respectively) (F = 1.18, P < 0.001). Furthermore, 2 h after applying HFS, estrogen + METH (138.0 ± 12.1% of baseline) (F = 6.66, P < 0.010) and progesterone + METH (138.00 ± 5.66% of baseline) (F = 6.66, P < 0.050) groups showed a sustained increase in the fEPSP slope compared with oil + METH group (101.0 ± 11.8% of baseline). However, there were no significant differences in fEPSP slope between estrogen + progesterone + METH (106.00 ± 6.63% of baseline) and oil + METH (101.0 ± 11.8% of baseline) groups.
Discussion
Current study was designed to investigate the possible effect of estrogen and progesterone treatment on METH-induced LTP impairment. The findings presented here indicated that METH (6 mg/kg, SC) has a disruptive effect on hippocampal synaptic plasticity in OVX rats. As our results showed, treatment with estrogen (1 mg/kg, IP) in METH-exposed rats was able to attenuate METH-induced LTP deficit. Furthermore, similar significant improvement was observed in treatment with progesterone (8 mg/kg, IP) compared to METH group. Conversely, findings of therapy with estrogen plus progesterone and therapy with vehicle in METH exposed rats did not significantly differ.
A growing literature indicates that exposure to neurotoxic METH regimen causes learning and memory impairments which remains long after drug withdrawal.5,6,25 Cumulative data from animal studies emphasized that there is a strong correlation between synaptic efficacy and function in area CA1 of hippocampus and learning and memory mechanisms.31,32 Hori et al.7 indicated that repeated METH administration resulted in deficient LTP in the Schaffer collateral-CA1 pathway in the hippocampus in male rats compared with saline group. Similar results were obtained in another study by Swant and et al.9 using in vitro hippocampal slice preparation. They showed that exposure to METH (10 mg/kg) caused a reduction in LTP in rat hippocampus.
In agreement with previous findings, present data revealed that LTP induction within the CA1 region of the hippocampus was significantly reduced in METH exposed OVX female rats and also after applying HFS, the fEPSP slopes were approximately decreased to baseline levels.7,9 These results demonstrated that METH-induced neurotoxicity affects behavior and memory functions.
Although the exact mechanisms involved in deleterious effects of METH on LTP and synaptic plasticity is not fully determined yet, these neurocognitive deficits can be attributed to alterations in structural and functional plasticity of hippocampal neurons. One of the probable mechanisms is the decrease in dopamine and serotonin transporter activity following METH-administration.33,34 In this aspect, McCann et al.34 showed that METH-induced memory impairment was related to a reduction in dopamine transporter activity. In addition, recent studies demonstrated that administration of a neurotoxic METH regimen led to morphological changes ranging from neuronal degeneration to changes in the density of spines and damage of the blood-brain barrier.8,35-37 In this manner, a study from Thompson et al.38 revealed that METH consumption led to memory dysfunction, which was related to a decrease of hippocampal volume and white-matter hypertrophy. In cellular and molecular approach, METH-induced neurotoxicity is accompanied with oxidative stress that eventually leads to biochemical changes including decline in endogenous antioxidant defense in hippocampus such as nuclear factor (erythroid-derived 2)-like 2 (Nrf2) which is correlated with cognitive function.39-42 Since the hippocampus is highly susceptible to METH, there is a probable association between METH-induced toxicity and METH-induced memory deficit.
Several lines of evidence have demonstrated that ovarian hormone including estrogen and progesterone had neuroprotective effects on deleterious behavioral, synaptic and molecular changes induced by wide-ranging neurodegenerative diseases.5,16 In OVX rats, when estrogen was administered in daily injections for 15 days, the recognition and spatial memory functions was profoundly enhanced following a global ischemia.16 Furthermore, previous works showed that progesterone treatment (8 mg/kg) promoted spatial learning performance in the Morris water maze task and protected neuronal damage in dorsal hippocampus following some neurological disorder such as traumatic brain injury (TBI) and ischemia.17,43 As previous studies have shown, it has been established that there is a strong association between synaptic efficacy and function in area CA1 of hippocampus and learning and memory mechanisms.44-46 In this aspect, Dai et al.15 showed that an administration of estrogen (1 mg/kg) was able to prevent the reduction in basal synaptic transmission and impairment of induction and maintenance of LTP following global ischemia. In agreement with other studies, the present findings showed that treatment with estrogen and progesterone prevented the deleterious effect of METH on E-LTP in CA1 region of the hippocampus. Moreover, in our experiments, sesame oil and saline had no significant impact on LTP magnitude or maintenance in OVX rats. It is worth pointing out that similar to other works, we find that I/O curve and PPF ratios were not altered among groups. This observation can be explained as there was no change in the possibility of release of neurotransmitters from hippocampal presynaptic terminals under the present experimental conditions.7,9,21 Taken together, these results support our previous study that showed treatment with estrogen (1 mg/kg) and progesterone (8 mg/kg) attenuated METH-induced spatial learning and memory impairments in OVX rats.25
Although exact mechanisms of the potentiating effects of ovarian hormones responsible for the long lasting increase in synaptic strength have remained unknown, recent works discovered some of the mechanisms through which ovarian hormones exert their effects. Gonadal steroids, in particular estradiol, can profoundly enhance LTP through its impact on hippocampal synaptic plasticity by increasing number of dendrites and spine density along with synaptic remodeling including neurite outgrowth and synaptogenesis.19,20,47,48
Recent studies have found evidences that acute administration of METH mimics traumatic brain injury that leads to some processes such as induction of oxidative stress and euroinflammation, which could cause neuronal damages and cell death.49,50 Noxious effects of METH might be attenuated if the treatment included potential neuroprotective pharmacological approaches like ovarian hormone replacement similar to those used in TBI. In this point of view, Khaksari et al.26 showed that treatment with pharmacological doses of estrogen (1 mg/kg) and progesterone (8 mg/kg) could reduce proinflammatory cytokines and brain edema following traumatic brain injury. Further studies are required to evaluate the effects of gonadal hormones on processes such as neuroinflammation in METH-exposed female rats.
On the other hand, the effects of combination therapy of estrogen and progesterone on cognitive ability is poorly understood and almost contradictory. A series of evidence reported that progesterone does not influence with the protective effects of estrogen.25,51,52 In this context, progesterone (10 mg/kg) did not significantly alter protective effects of estrogen on spatial learning and memory in OXV female rats.51 Furthermore, in OVX female rats, co-treatment with estrogen and progesterone exhibited either basal synaptic transmission or LTP to similar levels observed in gonadally intact animals.53 On the other hand, in cycling female rat, quantification of morphological alteration showed that the number of dendritic spines and the density of synapses in hippocampal CA1 increased during proestrus when estrogen and progesterone are at their higher levels, then reduced during estrus when estrogen and progesterone are at lower levels.54,55 In addition, a study by Warren et al.21 revealed that LTP varied across estrus cycle and LTP was greater during proestrus compared to estrus in female rats. In contrast to previous studies, recent observations from other works indicated that progesterone antagonizes the beneficial effects of estrogen. In one study, Murphy and et al.56 revealed that progesterone counteracted the effect of estrogen on hippocampal spine density. Moreover, using middle-age female rats, treatment with progesterone reverses the estrogen-induced spatial memory improvement in Morris water maze.57 The present findings showed that combination of estrogen plus progesterone did not significantly impair synaptic plasticity in METH-exposed animals. This is consistent with our recent observations that co-treatment with estrogen and progesterone did not affect METH-induced spatial learning and memory impairment in Morris water maze.25 Due to this fact that either estrogen or progesterone alone had protective effects on synaptic plasticity, the complexity of their interactions which may influence cognitive function was probably responsible for the observed discrepancies. On one hand, it seems that some of the noxious effects of progesterone on protective effects of estrogen comes from a direct or indirect interaction with GABA-A receptors, which is thought to counteract the estrogen effects.53 On the other hand, Bimonte-Nelson et al.57 showed that progesterone reverses the estrogen elevation in neurotrophic proteins levels such as NGF, BDNF and NT3 in brain regions critically involved in spatial learning and memory. The reason for this discrepancy is unclear, additional studies are needed to clarify the neural mechanism of this apparent divergence. However, effectiveness of combined estrogen and progesterone on modulating the properties of neuroplasticity and LTP may be dependent on age, dose, experimental procedure used and somehow could be attributed to the duration and timing of treatment.
Conclusion
The results of the present study indicated that exposure to METH caused impairment in hippocampal LTP. Moreover, the present findings demonstrated that treatment with either estrogen or progesterone can partially attenuate METH-induced synaptic plasticity deficits in CA1 area of the hippocampus in METH-exposed OVX rats. On the other hand, combination therapy of estrogen and progesterone did not influence METH-evoked effects on LTP. However, the beneficial effects of ovarian hormones treatment on synaptic plasticity may be affected by numerous factors including doses of used hormones, duration and timing of treatment, age and etc. Further studies are required to fully define the cellular and molecular mechanisms mediating protective effects of these hormones against METH-induced cognitive impairment.
Acknowledgments
This research article is part of first author PhD thesis. This work was supported financially by the Kerman Neuroscience Research Center (Grant No: KNRC/EC/92-65).
Footnotes
Conflicts of Interest
The Authors have no conflict of interest.
REFERENCES
- 1.Amiri S, Alijanpour S, Tirgar F, Haj-Mirzaian A, Amini-Khoei H, Rahimi-Balaei M, et al. NMDA receptors are involved in the antidepressant-like effects of capsaicin following amphetamine withdrawal in male mice. Neuroscience. 2016;329:122–33. doi: 10.1016/j.neuroscience.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Tirgar F, Rezayof A, Zarrindast MR. Central amygdala nicotinic and 5-HT1A receptors mediate the reversal effect of nicotine and MDMA on morphine-induced amnesia. Neuroscience. 2014;277:392–402. doi: 10.1016/j.neuroscience.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Kalant OJ. The amphetamines; toxicity and addiction. Toronto, ON: University of Toronto Press; 1973. [Google Scholar]
- 4.US Centers for Disease Control and Prevention. Acute public health consequences of methamphetamine laboratories--16 states, January 2000-June 2004. MMWR Morb Mortal Wkly Rep. 2005;54(14):356–9. [PubMed] [Google Scholar]
- 5.Camarasa J, Rodrigo T, Pubill D, Escubedo E. Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol Res. 2010;62(5):450–6. doi: 10.1016/j.phrs.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Nagai T, Takuma K, Dohniwa M, Ibi D, Mizoguchi H, Kamei H, et al. Repeated methamphetamine treatment impairs spatial working memory in rats: reversal by clozapine but not haloperidol. Psychopharmacology (Berl) 2007;194(1):21–32. doi: 10.1007/s00213-007-0820-1. [DOI] [PubMed] [Google Scholar]
- 7.Hori N, Kadota MT, Watanabe M, Ito Y, Akaike N, Carpenter DO. Neurotoxic effects of methamphetamine on rat hippocampus pyramidal neurons. Cell Mol Neurobiol. 2010;30(6):849–56. doi: 10.1007/s10571-010-9512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19(12):3165–70. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swant J, Chirwa S, Stanwood G, Khoshbouei H. Methamphetamine reduces LTP and increases baseline synaptic transmission in the CA1 region of mouse hippocampus. PLoS One. 2010;5(6):e11382. doi: 10.1371/journal.pone.0011382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheibani V, Afarinesh M, Hajializadeh Z, Abbasnejad M, Haghpanah T, Arabnezhad R, et al. Evaluation of Origanum Vulgare L. ssp. viridis leaves extract effect on discrimination learning and ltp induction in the ca1 region of the rat hippocampus. Iran J Basic Med Sci. 2011;14(1):177–84. [Google Scholar]
- 11.Haghpanah T, Esmailpour Bezanjani K, Afarinesh Khaki MR, Sheibani V, Abbasnejad M, Masoomi Ardakani Y. Effect of intra-hippocampal injection of Origanum vulgare L. ssp. viridis leaf extract on Spatial learning and memory consolidation. Feyz. 2011;14(4):380–7. [Google Scholar]
- 12.Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118(2):306–13. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21(1):107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 14.Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–42. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- 15.Dai X, Chen L, Sokabe M. Neurosteroid estradiol rescues ischemia-induced deficit in the long-term potentiation of rat hippocampal CA1 neurons. Neuropharmacology. 2007;52(4):1124–38. doi: 10.1016/j.neuropharm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Plamondon H, Morin A, Charron C. Chronic 17beta-estradiol pretreatment and ischemia-induced hippocampal degeneration and memory impairments: a 6-month survival study. Horm Behav. 2006;50(3):361–9. doi: 10.1016/j.yhbeh.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382(3):286–90. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 18.Nezhadi A, Sheibani V, Esmaeilpour K, Shabani M, Esmaeili-Mahani S. Neurosteroid allopregnanolone attenuates cognitive dysfunctions in 6-OHDA-induced rat model of Parkinson's disease. Behav Brain Res. 2016;305:258–64. doi: 10.1016/j.bbr.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: Implications for cognition and aging. Neurology. 1997;48(5 Suppl 7):S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 20.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18(7):2550–9. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703(1-2):26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 22.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women's Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 23.Gajjar TM, Anderson LI, Dluzen DE. Acute effects of estrogen upon methamphetamine induced neurotoxicity of the nigrostriatal dopaminergic system. J Neural Transm (Vienna) 2003;110(11):1215–24. doi: 10.1007/s00702-003-0045-3. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Liao PC. Estrogen and progesterone distinctively modulate methamphetamine-induced dopamine and serotonin depletions in C57BL/6J mice. J Neural Transm (Vienna) 2000;107(10):1139–47. doi: 10.1007/s007020070027. [DOI] [PubMed] [Google Scholar]
- 25.Ghazvini H, Khaksari M, Esmaeilpour K, Shabani M, Asadi-Shekaari M, Khodamoradi M, et al. Effects of treatment with estrogen and progesterone on the methamphetamine-induced cognitive impairment in ovariectomized rats. Neurosci Lett. 2016;619:60–7. doi: 10.1016/j.neulet.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 26.Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can J Physiol Pharmacol. 2011;89(1):31–40. doi: 10.1139/y10-103. [DOI] [PubMed] [Google Scholar]
- 27.Hajali V, Sheibani V, Ghazvini H, Ghadiri T, Valizadeh T, Saadati H, et al. Effect of castration on the susceptibility of male rats to the sleep deprivation-induced impairment of behavioral and synaptic plasticity. Neurobiol Learn Mem. 2015;123:140–8. doi: 10.1016/j.nlm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Saadati H, Sheibani V, Esmaeili-Mahani S, Hajali V, Mazhari S. Prior regular exercise prevents synaptic plasticity impairment in sleep deprived female rats. Brain Res Bull. 2014;108:100–5. doi: 10.1016/j.brainresbull.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Philadelphia, PA: Elsevier; 2007. [Google Scholar]
- 30.Khodamoradi M, Asadi-Shekaari M, Esmaeili-Mahani S, Esmaeilpour K, Sheibani V. Effects of genistein on cognitive dysfunction and hippocampal synaptic plasticity impairment in an ovariectomized rat kainic acid model of seizure. Eur J Pharmacol. 2016;786:1–9. doi: 10.1016/j.ejphar.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Geinisman Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb Cortex. 2000;10(10):952–62. doi: 10.1093/cercor/10.10.952. [DOI] [PubMed] [Google Scholar]
- 32.Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27(11):2738–49. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62(2):1119–26. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 35.Schmued LC, Bowyer JF. Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Res. 1997;759(1):135–40. doi: 10.1016/s0006-8993(97)00173-x. [DOI] [PubMed] [Google Scholar]
- 36.Dawirs RR, Teuchert-Noodt G, Busse M. Single doses of methamphetamine cause changes in the density of dendritic spines in the prefrontal cortex of gerbils (Meriones unguiculatus). Neuropharmacology. 1991;30(3):275–82. doi: 10.1016/0028-3908(91)90155-5. [DOI] [PubMed] [Google Scholar]
- 37.Sharma HS, Kiyatkin EA. Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: An experimental study using light and electron microscopy. J Chem Neuroanat. 2009;37(1):18–32. doi: 10.1016/j.jchemneu.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Granado N, Lastres-Becker I, Ares-Santos S, Oliva I, Martin E, Cuadrado A, et al. Nrf2 deficiency potentiates methamphetamine-induced dopaminergic axonal damage and gliosis in the striatum. Glia. 2011;59(12):1850–63. doi: 10.1002/glia.21229. [DOI] [PubMed] [Google Scholar]
- 40.Li XH, Li CY, Lu JM, Tian RB, Wei J. Allicin ameliorates cognitive deficits ageing-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Neurosci Lett. 2012;514(1):46–50. doi: 10.1016/j.neulet.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 41.Khalifeh S, Oryan S, Digaleh H, Shaerzadeh F, Khodagholi F, Maghsoudi N, et al. Involvement of Nrf2 in development of anxiety-like behavior by linking Bcl2 to oxidative phosphorylation: Estimation in rat hippocampus, amygdala, and prefrontal cortex. J Mol Neurosci. 2015;55(2):492–9. doi: 10.1007/s12031-014-0370-z. [DOI] [PubMed] [Google Scholar]
- 42.Khalifeh S, Oryan S, Khodagholi F, Digaleh H, Shaerzadeh F, Maghsoudi N, et al. Complexity of compensatory effects in nrf1 knockdown: Linking undeveloped anxiety-like behavior to prevented mitochondrial dysfunction and oxidative stress. Cell Mol Neurobiol. 2016;36(4):553–63. doi: 10.1007/s10571-015-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: A progesterone dose-response study. Pharmacol Biochem Behav. 2003;76(2):231–42. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–38. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 45.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29(2):117–30. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, et al. Tracking the estrogen receptor in neurons: Implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98(13):7093–100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, Wang J, Liu C, Jiang C, Zhao C, Zhu Z. Progesterone influences postischemic synaptogenesis in the CA1 region of the hippocampus in rats. Synapse. 2011;65(9):880–91. doi: 10.1002/syn.20915. [DOI] [PubMed] [Google Scholar]
- 49.Warren MW, Kobeissy FH, Liu MC, Hayes RL, Gold MS, Wang KK. Concurrent calpain and caspase-3 mediated proteolysis of alpha II-spectrin and tau in rat brain after methamphetamine exposure: A similar profile to traumatic brain injury. Life Sci. 2005;78(3):301–9. doi: 10.1016/j.lfs.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 50.Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, et al. Methamphetamine- and trauma-induced brain injuries: Comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66(2):118–27. doi: 10.1016/j.biopsych.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28(4):602–10. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Mannella P, Sanchez AM, Giretti MS, Genazzani AR, Simoncini T. Oestrogen and progestins differently prevent glutamate toxicity in cortical neurons depending on prior hormonal exposure via the induction of neural nitric oxide synthase. Steroids. 2009;74(8):650–6. doi: 10.1016/j.steroids.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Baudry M, Bi X, Aguirre C. Progesterone-estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–94. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286–91. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12(7):2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72(3):133–43. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- 57.Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]