Abstract
Most of the cancers are still incurable human diseases. According to recent findings, especially targeting cancer stem cells (CSCs) is the most promising therapeutic strategy. CSCs take charge of a cancer hierarchy, harboring stem cell-like properties involving self-renewal and aberrant differentiation potential. Most of all, the presence of CSCs is closely associated with tumorigenesis and therapeutic resistance. Despite the numerous efforts to target CSCs, current anti-cancer therapies are still impeded by CSC-derived cancer malignancies; increased metastases, tumor recurrence, and even acquired resistance against the anti-CSC therapies developed in experimental models. One of the most forceful underlying reasons is a “cancer heterogeneity” due to “CSC plasticity” A comprehensive understanding of CSC-derived heterogeneity will provide novel insights into the establishment of efficient targeting strategies to eliminate CSCs. Here, we introduce findings on mechanisms of CSC reprogramming and CSC plasticity, which give rise to phenotypically varied CSCs. Also, we suggest concepts to improve CSC-targeted therapy in order to overcome therapeutic resistance caused by CSC plasticity and heterogeneity.
Keywords: Cancer, Cancer stem cell, Cancer therapy, Plasticity, Reprogramming
INTRODUCTION
Revealing the origin of cancer has been a topic of much interest in that it might shed light on a complete treatment of cancer. For the past 20 years, plenty of studies have suggested that only a small subpopulation of the cancer cells with tumor-initiating capability is the core origin of the tumorigenesis and the subset of cancer cells was named cancer stem cells (CSCs). As it can be inferred from its nomenclature, CSCs share several features of normal stem cells. They can self-renew to form identical daughter cells by cell division and differentiate into various types of progenies (1).
Early researches on CSCs have been focused on verifying the existence of CSCs in certain types of cancer and finding molecular markers for isolation of CSCs. Several years after the conceptual suggestion of the existence of stem-like cancer cells, experimental evidence was first provided in a leukemia model, confirming that CD34+CD38− leukemic cells show bone marrow hematopoietic stem cell characteristics (2, 3). Solid tumor CSCs were first identified in breast cancer (CD44+CD24−/lowLin−), followed by their establishment in other common cancer types, including brain, ovary, prostate, colon, pancreas, liver, skin, and lung cancers, and common or unique CSC markers have been suggested for the tumors (4, 5).
Currently, it is widely accepted that CSCs are closely related to pathological features which result in worse clinical prognosis. Resistance to the conventional anti-cancer therapies is a characteristic of CSCs which is most important from a clinical point of view. CSCs harbor endogenous resistance mechanisms against radiation and chemotherapy which gives CSCs a survival advantage over differentiated counterparts (6, 7). Also, CSCs can lead to the diverse composition of cells in a tumor tissue which results in the generation of phenotypically varied subclones, thereby increasing the chances of leaving a resistant fraction after anti-cancer therapy (8).
The surrounding microenvironment critically affects cancers by regulating CSC physiologies. The tumor microenvironment not only supplies growth-promoting signals, but it also takes part in therapeutic resistance by protecting tumor cells from the therapy-induced damages (9). Earlier studies have demonstrated the role of microenvironments such as perivascular, hypoxic and invasive niches, in the generation and maintenance of CSCs (10). However, subsequent studies have shown evidence that CSCs also contribute to the reconstitution of the microenvironment through transdifferentiation into lineages which resemble normal stroma such as blood vessel endothelial cells, pericytes or fibroblasts (11–13).
Increased infiltration to the surrounding area and metastasis to the secondary organs are the most remarkable features of malignant tumors (14). The presence of CSCs within a tumor is often connected to the enhanced invasiveness and metastatic capability. Many studies have demonstrated the promotive roles of CSCs in tumor invasiveness and metastasis through in vitro and in vivo gain-or-loss-of-function approaches (15–18). Besides, recent studies are focusing on the plasticity of CSCs; a dynamic transition of the cellular phenotype between epithelial-like and mesenchymal-like depending on the stages of invasion or metastasis (19). Corresponding to the characteristics of CSCs mentioned above, bioinformatics-based studies have shown that a worse prognosis of the patient correlates with higher expression of the molecular signatures related to CSCs (20).
Two representative concepts about the origin of the CSC were suggested; one postulating transformed adult stem cell as a CSC source and the other demonstrating that differentiated cancer cells can be reprogrammed to become CSC (10). Recent findings reported that reprogramming occurs in the variety of the tumors and it affects CSC heterogeneity by two ways; reprogramming of genetically diverse non-CSCs and dynamic state-switching of CSCs (1, 21, 22). Thus, this review article focuses on the CSC reprogramming, giving explanations on the molecular mechanism of reprogramming discovered through varying previous studies. Also, this review demonstrates the limitations of current strategies targeting CSCs and the proposed remedies to overcome those limits.
REPROGRAMMING MECHANISMS
Core stemness signals and transcription factors (TFs) for reprogramming
It is known that normal stem cells and CSCs share core stemness signaling such as Notch, Hedgehog, WNT/β-Catenin, JAK/STAT, and NFκB (23). They have vital roles in maintaining stem cell properties or regulating their differentiation during numerous developmental processes and tumor progression. Recently, some papers suggested that an activation of these signals functions in regulating stem cell plasticity in both normal and cancer tissues. In the normal cerebral cortex, glial cell types like astrocytes give rise to reactive astrocytes, which have multipotencies like neural stem cells in vivo and in vitro via Sonic Hedgehog (SHH) signaling induction after invasive injury, and re-differentiate into neurons (24). It implies that certain types of differentiated cells act as tissue progenitors via dedifferentiation to repair tissue injuries. Similarly, SHH secreted by endothelial cells promotes CSC-like properties of glioma cells (25). Therefore, exposure to appropriate stemness signals can induce dedifferentiation mechanisms in normal tissues, and cancer uses them to build a cellular hierarchy.
Recent studies have identified that the most representative reprogramming process in physiological conditions is a transformation of the epithelial cell into mesenchymal type, namely epithelial-to-mesenchymal transition (EMT). Because mesenchymal type cells facilitate to migrate through the extracellular matrix (ECM), it is critically important to embryogenesis and further developmental process (26). Importantly, this phenomenon appears in both normal and cancer cells. Both mammary epithelial cells and mammary carcinomas underwent EMT, acquiring many stem cell phenotypes (27). Moreover, mechanisms of EMT and CSCs share many identical TFs, such as Twist, ZEB1/2, and HIFs, and signaling pathways of TGF-β, WNT/β-Catenin, Notch, and Hedgehog (28). During recent two decades, growing number of studies have shown that the importance of NFκB-mediated inflammatory signal has been issued in CSC biology, especially in MET (29). For example, breast cancer induces EMT program by NFκB-Twist axis activated by TNFα stimulation (30).
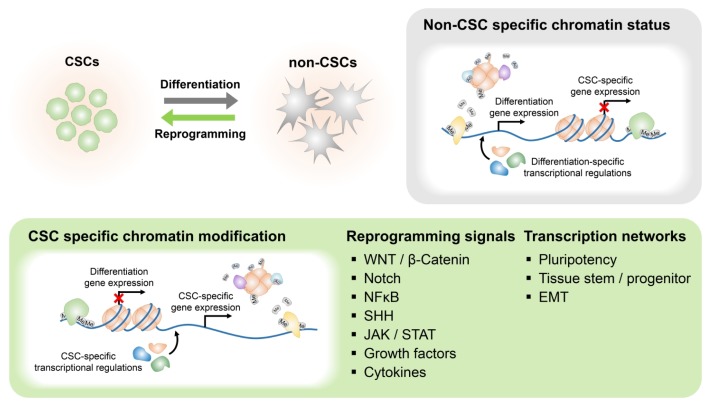
Although CSCs activate such core stemness signaling pathways, the most important point is that final alteration of gene expression pattern is directly controlled by TFs. For examples, HGF-cMET-mediated reprogramming network requires the function of Nanog, which is one of the embryonic TFs (31). Likewise, many studies have explained links between CSC reprogramming mehanisms and the major stem cell TF networks. Its importance has been suggested in induced pluripotent stem cells (iPSCs) generation from somatic cells by ectopic expression of 4 TFs, OCT3/4, SOX2, KLF4, and cMYC (32). They regulate various genes required for pluripotency. Activation of iPSC reprogramming factors has identified in many types of cancers including glioblastoma and carcinomas of breast, liver, prostate, and lung, especially in CSCs (33–36). More specifically, in brain tumors, core neurodevelopmental TFs containing POU3F2, SOX2, SALL2, and OLIG2 play crucial roles in stem-like glioma cells and their ectopic expression induces stem cell properties (37), indicating that it is necessary to understand the functions of TFs related to tissue stem/progenitors. Moreover, many studies dealing with such stemness-associated TFs have demonstrated their roles in the acquisition of CSC properties by their gain-of-function experiments. So, to target CSCs, it is necessary to understand comprehensively about extracellular reprogramming signal inducers like ligands, their downstream signal cascades, and finally corresponding TFs in CSCs (Fig. 1). Hereafter, we introduce in-depth several mechanisms which account for cancer cell reprogramming into CSC.
Fig. 1.
Core signaling pathways and epigenetic modifications regulating CSC reprogramming and differentiation.
Microenvironmental factors
Although genetic mutations are closely associated with cancer, the plasticity of cancer is more affected by their microenvironment rather than mutation during the reprogramming process. For normal tissue homeostasis, stem cells are regulated by various signaling derived from specialized microenvironments, called stem cell niches (38). Similarly, numerous studies have suggested CSCs require their CSC niches to maintain stem cell properties. These niches consist of endothelial cells, immune cells, fibroblasts, ECM, and their secreted factors like growth factors or cytokines (39). The most studied niches are perivascular and hypoxic niches, but other microenvironments composed of various stromal cells have been identified (39). Interestingly, recent studies have suggested that CSC niches or individual microenvironments are important to not only CSC maintenance but reprogramming into CSCs.
Perivascular niche
The best-studied niche is a perivascular niche, meaning microenvironments around blood vessels. Along with numerous studies, Kiel et al. firstly concluded hematopoietic stem cells resided in the perivascular region in spleen and bone marrow and defined it as a stem cell niche (40). Likewise, this niche is crucial for maintenance of CSC populations in cancer tissue by direct cell-cell interactions or secreted soluble factors (41). Glioma is the best known human cancer about the perivascular niche for CSCs. In 2007, it was firstly suggested vascular microenvironments help maintenance of self-renewing CSC pool in brain tumor (42). Recently, endothelial cells are known to enhance stemness properties of CSCs in glioma by Notch signal activation and the nitric oxide (NO)-signaling pathway (43). Similarly, the increase in inhibitor of differentiation 4 (ID4) by platelet-derived growth factor (PDGF)-driven NO signaling promotes Jagged1-Notch activity, resulting in self-renewal properties and tumorigenesis of glioblastoma (44). SHH-positive endothelial cells increase various stemness factors like SOX2, OLIG2, and BMI1 in glioma cells, generating CD133+ CSC-like glioma cell (45).
Besides brain tumors, reprogramming mechanisms of other types of cancer in the vascular niche have also been identified. Vascular endothelial growth factor (VEGF) in the niche promotes cancer stemness properties in skin squamous cell carcinoma (46). In head and neck squamous cell carcinoma, epidermal growth factor (EGF) secreted from endothelial cells induces EMT of cancer cells and leads them to acquire stem cell characteristics (47).
Hypoxia
Since oxygen is an essential factor for cellular metabolism and various physiologies, the body consistently requires this gas. Importantly, as it accepts a final electron in oxidative phosphorylation, physiological condition of low oxygen, named hypoxia, causes harmful damages to cells. It has been identified that various cells have many response and adaptation mechanisms to hypoxia, which is mainly mediated by oxygen sensor protein, Hypoxia-inducible factors (HIFs). Hypoxia also has a beneficial effect on embryonic development or in maintaining stem cell functions (48). Unfortunately, cancer utilizes these stem cell-related programs to maintain or generate CSCs in hypoxia. In neuroblastomas, HIF1α and HIF2α stabilized in hypoxia change gene expression patterns and induce dedifferentiation into neural crest sympathetic progenitor-like cells expressing Notch-1 and c-Kit (49). Similarly, increased ID2 by HIF1 plays a role in dedifferentiation of neuroblastoma cells (50). Some studies demonstrated direct regulation of well-known stemness TFs in hypoxia. Hypoxia and HIFs induce ALKBH5-mediated m6A-demethylation of Nanog mRNA and its stabilization in breast cancer (51).
Since hypoxia causes a depletion of nutrients as well as oxygen, it is an unfavorable condition for cellular growth or lots of biosynthetic processes even in cancer cells. Not only an adaptation but an evasion from the hypoxia condition may be a possible way to survive. EMT is the most relevant phenomenon with cellular invasiveness and cancer reprogramming in hypoxia. HIF1α transcriptionally regulates well-known EMT-related TFs such as ZEB1, and Twist (52, 53). Also, some studies have indicated that core stemness signaling pathways involving Notch, WNT/β-Catenin, Hedgehog, and NFκB are potentially associated with hypoxia and EMT (54). In breast cancer, Jagged2-Notch signaling induced by hypoxia stimulates EMT programs, causing metastasis and acquisition of stem cell properties (55). These results suggest that hypoxia-mediated EMT programs play a pivotal function in activating metastatic cells that have CSC properties.
Other stromal cells
A recent trend in cancer biology is to identify mechanisms governing microenvironment-mediated tumor malignancy. There are numerous types of stromal cells including immune cells, mesenchymal stem cells and even fibroblasts in tumor tissues, promoting CSC plasticity. A tumor modulates immune cells, in particular, by secretion of various cytokines to make help tumor progression rather than attack them. It is suggestive that inflammatory-associated factors may activate reprogramming network leading to the generation of CSCs. Recruited monocytes and macrophages into tumor tissue induce invasion and metastasis and create immuno-suppressive environment via secretion of TGF-β, known as a potent stimulator of EMT (56). Activated NFκB and STAT3 signaling pathways via inflammatory cytokines like IL-6 and TNFα also induce EMT (57, 58). These immune-associated microenvironments are inevitably occurred in tumor tissues and participate in cancer plasticity regardless intended or unintended.
Fibroblasts in tumor tissue called cancer-associated fibroblasts (CAFs) promote tumor progression, and some studies demonstrated their role in dedifferentiation. CAF promotes malignancy of breast cancer through EMT induced by TGF-β secretion (59). Myofibroblast-secreted factor including HGF enhances WNT signal activity and stemness properties of LGR5-positive colorectal CSCs (60). A stellate cell which is myofibroblast-like cell in pancreas promotes CSC phenotype via Nodal/Activin (61).
These reports have shown varying cytokines or growth factors from various stromal cells activate stem cell properties of cancer cells and induce metastasis. Importantly, because most of the cytokine-mediated signaling pathways can be associated with inflammation responses, damages induced by various therapies may cause such inflammatory microenvironment, rather leading cancer malignancies. One study showed CAF secrets IL-17A, which enhances stem cell properties of colorectal cancer after chemotherapy, resulting in a chemo-resistance and a recurrence (62).
Epigenetic alteration
Beyond these signaling cascades, a final determination of cell type is dependent on the epigenetic status of lineage determinant factors. During iPSCs generation, iPSC TFs consist of embryonic stem cell (ESC) chromatin network along with various epigenetic modulators, driving specialized epigenetic mechanisms which play crucial roles in resetting their identities during reprogramming process (63, 64). Likewise, such stemness TFs and epigenetic modifications are considered to function as critical elements for reprogramming cancer cells into CSCs. In fact, many recent studies have reported relevance of various epigenetic modifiers in cancers. For example, cancer cells repress differentiation-related genes or tumor suppressor genes through epigenetic silencing of Polycomb-group proteins, which function in cellular differentiation and development via histone modification-driven transcriptional repression (65, 66). Although methylation status of each cancer type varies, hypermethylated gene set of a particular type of cancer is sharing with ESC signature (67). It has been identified that key factors of polycomb repressive complex 2 (PRC2), such as enhancer of zeste homolog 2 (EZH2) and suppressor of zeste 12 homolog (SUZ12), were overexpressed in ovarian, breast, prostate, and colon cancers and they were crucial for maintenance of their CSC population (68–71). Ectopic expression of SUZ12 in differentiated breast cancer cell resulted in the CSC formation (69). BMI1, a key subunit of PCR1 complex, is upregulated by controlling methylation pattern on its promoter by embryonic transcription factor SALL4 in leukemic stem cells (72). In glioblastoma, BMI1 and EZH2 are highly expressed in tumor-initiating CD133-positive cells, and their knockdown disrupts stem cell properties (73). Besides PRC complex subunit, numerous chromatin regulators have been reported in human cancer. DNA methyltransferases (DNMTs) containing DNMT1 essential for maintenance of existing methylation patterns and DNMT3 for de novo methylations at CpG islands are also potential factors for CSC reprogramming. For incidence, DNMT1 and DNMT3A have a crucial function in regulating malignancies of breast CSCs and various leukemia stem cells, respectively (74, 75). Another histone methyltransferase, mixed-lineage leukemia 1 (MLL1), is required for hypoxia-induced self-renewal properties (76), whereas one of histone demethylases, JARID1B, is engaged in the dynamics of CSC population in melanomas (77).
In conclusion, an aberrant epigenetics induce or suppress transcription of stemness or differentiation factors, resulting in an activation of various stemness signaling pathways in differentiated cancer cells. Furthermore, to explain variable cancer plasticity, chromatin status also may be closely associated with their surrounding microenvironments, rather than a one-time genetic mutation. For example, differentiated basal breast cancers acquire CSC characteristics by ZEB1 increased by TGFβ signaling (78). Altogether, dynamics of the chromatin status are controlled by regular cellular programs which, in turn, are controlled by stimuli recognizers, signal mediators, and TFs under physiological conditions and with proper environmental factors.
PERSPECTIVES ON CSC TARGET THERAPY IN THE PRESENT AND THE FUTURE
Current CSC targeting strategies and their limitations
As CSCs take critical roles in cancer progression and therapeutic resistance as the apex of cancer hierarchy, anti-cancer therapy targeting CSCs has been suggested to be a promising therapeutic modality to effectively eliminate the origin of cancer development and reduce the risk of recurrence (79). There are several studies showing CSC targeting strategies including targeting CSC-marker, CSC-specific cellular signaling pathways, and CSC microenvironment. Since several prominent CSC surface markers have been discovered in various cancer types, researchers speculated that it would be promising to target those markers for the CSC-specific drug delivery and direct inhibition of CSC maintenance. Many studies tried CD133-mediated CSC targeting, for instance, drug conjugation to CD133 antibody, immune-mediated clearing with CD133-recognizing bi-specific antibodies bound to immune cells and nanoparticle-conjugated CD133 aptamer, showed modest anti-CSC effect (80). Researchers also tried to abrogate CSC-specific signaling nodes by chemical- or antibody-dependent inhibition. Recent reports demonstrated positive clinical and pre-clinical outcomes of CSC-specific signaling component inhibitors such as OMP-18R5 targeting WNT receptor Frizzled, BMS-906024 targeting γ-secretase to block Notch signaling, and vismodegib and BMS-833923 which block SHH signal receptor Smoothened (81–83).
Despite the multilateral approaches, recent studies have pointed out the limitations of CSC targeting strategies. CSC marker-negative or differentiation marker-positive cancer cells could initiate tumor formation (84, 85). Single cell transcriptome analysis revealed that the cells positive for the different CSC markers or the cells harboring activation of the distinct CSC-specific signaling nodes, could co-exist within a population of tumor cells, and many CSC or cancer subtype markers can be expressed by a cell at the same time. This demonstrates that CSCs are heterogeneous and that a single CSC marker does not properly segregate CSCs and non-CSCs (86). Also, activation of CSC-specific signaling pathways could be different within a tumor, implying that abrogation of a single pathway may not critically affect whole CSCs (87). It is plausible that diversity of CSCs may be generated by distinct stemness or reprogramming signaling activations, resulting in divergent expression patterns or CSC markers. Therefore, development of CSC-specific targeting strategies using marker-dependently sorted CSCs and targeting of a single CSC marker or signaling node is not proper strategy due to CSC heterogeneity.
Necessity for comprehensive understanding of CSC dynamics: Diversity of phenotypes and distinct reprogramming process
In the past, we commonly defined CSC as a cell at a “fixed” status consistently maintaining so-called “CSC phenotypes”. However, some evidence suggests that we should put more weight to the plasticity of CSCs, a dynamic conversion of phenotypic status by trans-differentiation and reprogramming, rather than if CSCs remain in the steady-state (1). In the breast CSC model, both ALDH+, and CD44+/CD24− populations are stem-like, but their phenotypes differ; one being more quiescent resembling luminal type of normal breast stem cells and the other being more mesenchymal-like similar to basal type of breast stem cells, even though those populations are capable of interconversion between each other (8, 88).
Recently, several cancers are subdivided into “subtypes” by distinct gene expression patterns and characteristics, even though they were formed from identical tissues. Thus, a subtypical conversion of CSCs may be a potent cause of CSC dynamics. This phenomenon has been demonstrated in various cancers and showed a clear example of their plasticity. The phenotypic transition of the proneural type of brain CSCs into mesenchymal CSC type is well-characterized, and ALDH1A3 and NFκB signaling activation are identified to be key modulators for this transition (89–91). Another study showed CSC plasticity in the prostate cancer, which is strongly related to metastatic capability (92). Recent findings suggested that poor prognostic outcome of the castration-resistant prostate cancer accounts for the dynamic switching of prostate CSCs between epithelial-like and mesenchymal-like states by androgen signaling, histone modification, and miRNAs which eventually promotes metastatic spread (92–94). A capability for these dynamic transitions leads CSCs to adapt to environmental changes in the process of invasion and metastasis thereby affecting tumor progression and imparting therapeutic resistance. These studies have suggested that the rapid and repetitive reprogramming process generates a hierarchical organization and a mixed composition of phenotypically distinct subclones. Importantly, each subtype may require activations of distinct and specific signaling pathways, because they show their specific gene expression patterns. Although we still narrowly understand about subtypical interconversions of CSCs, it is likely that distinct signaling activators or specific microenvironmental conditions may be required for the transition into specific subtypes.
Necessity for comprehensive understanding of CSC dynamics: Status of CSC sources
Given that CSCs could originate from differentiated non-CSCs by reprogramming signals, it is reasonable that these signals dedifferentiate non-CSCs harboring different genetic content giving rise to genetically heterogeneous CSCs or that they may not give rise to CSCs even in an existence of potent reprogramming activators. During iPSC generation, reprogramming is affected by various factors, including epigenetic factors and TFs, acting as reprogramming barriers or enhancers (95). A previous study reported that each of the clones with different genetic alterations requires activation of distinct signaling nodes, which can promote stem cell-like properties and tumor propagation. This result suggests that, even though CSCs within a tumor may share some of CSC features, diversity in the genetic background would give rise to a variety of CSC phenotypes (21). One of the standard features of cancers is genomic instability, including mutations and aberrant epigenetics, and it is known that each of the cells consisting tumor bulk harbors various genetic alterations thus presenting genetic heterogeneity (14). Cancer cells with diverse background status may reach to different CSC hierarchical stages or become different CSC types even in identical conditions. Despite various mechanisms governing stemness or reprogramming, it seems that they converge towards several stemness TFs to regulate stem cell gene signatures. For example, epigenetic modifiers interacting stemness TFs may function as crucial elements to do this, because genes being epigenetically tied-up status, called “heterochromatin,” should be open to facilitate their transcription in non-CSCs. Therefore, it is plausible that identifying transcription factor and epigenetic modifier networks involving in CSCs and reprogramming process should be a potential approach to developing CSC targeting strategy. Furthermore, development of a CSC-specific therapy that targets molecular mechanisms controlling CSC heterogeneity should be an important future goal.
CONCLUSION
As mentioned above, developing therapeutic strategies to target CSCs is necessary considering its impact on cancer progression and prognosis of patients. However, targeted elimination of pre-existing CSCs is not enough as plenty of recent findings demonstrates that the CSCs can be newly generated from the differentiated non-CSCs by reprogramming mechanism through which even CSCs with different characteristics could emerge. That is, CSCs not only serve as the origin of tumor formation but also drive heterogeneity of cell composition inside the tumor and CSCs themselves as well. Since CSC diversity renders tumor resistant to the anti-cancer therapies eventually resulting in recurrence, it is necessary to gain new insight from a comprehensive understanding of CSC plasticity based on molecular genetics and biology.
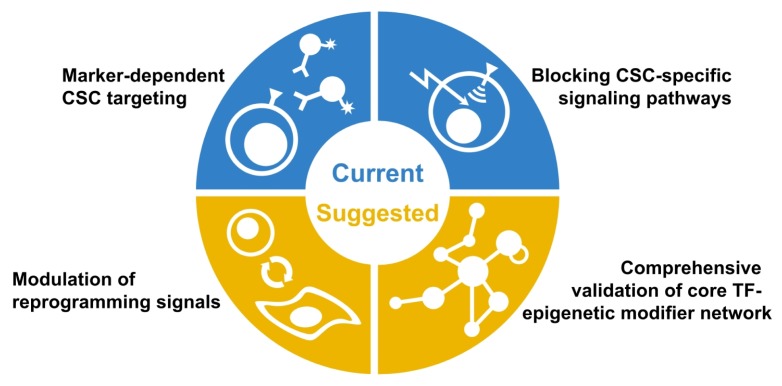
Thus, our perspectives on establishing novel CSC-targeting strategy suggest that we should consider the following respects (Fig. 2). 1) Since populations of CSCs already reside in the tumor, eliminating them by marker-dependent targeting or inhibition of CSC-specific signaling nodes should be initial and essential regimens as is currently accepted. 2) Also, controlling a variety of reprogramming mechanisms should be combined to prevent de novo generation of the different types of CSCs. Unfortunately, it is impossible to modulate all the reprogramming signals at the same time, 3) therefore certain microenvironment-specific or subtype-specific core TF-epigenetic modifier networks should be identified and considered as a potential target. Although CSCs are regulated by diverse signaling depending on their types, we may speculate that CSCs would share common transcriptional programs mediated by core TF-epigenetic modifier networks, as described in similar gene expression signature among CSCs of same subtype.
Fig. 2.
A schematic diagram showing CSC targeting strategies.
In summary, interconnected networks consisting of various TFs, microenvironmental factors, and epigenetic alterations modulate CSC reprogramming and differentiation. Further, dynamic regulation of CSC reprogramming results in CSC plasticity and heterogeneity. Therefore, as this review suggests, the future direction for targeting CSCs should include both CSC and de novo CSC generation. Thus it must be based on recent findings of CSC plasticity and the comprehensive validations on the networks of related signaling pathways.
ACKNOWLEDGEMENTS
We are grateful to all members of the Cell Growth Regulation Laboratory for their helpful discussion. This work was supported by grants from the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A5A1009024), from Next-Generation Biogreen21 Program (PJ01107701), and from Korea University.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medema JP. Cancer stem cells: The challenges ahead. Nat Cell Biol. 2013;15:338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 7.Jeon HM, Sohn YW, Oh SY, et al. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res. 2011;71:3410–3421. doi: 10.1158/0008-5472.CAN-10-3340. [DOI] [PubMed] [Google Scholar]
- 8.Brooks MD, Burness ML, Wicha MS. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell. 2015;17:260–271. doi: 10.1016/j.stem.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Campisi J, Higano C, et al. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153:139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen OW, Nielsen HL, Gudjonsson T, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao X, Katiyar S, Willmarth NE, et al. c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion. J Biol Chem. 2010;285:8218–8226. doi: 10.1074/jbc.M110.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin YH, Bian BS, Yang XJ, et al. OU5F1 enhances the invasiveness of cancer stem-like cells in lung adenocarcinoma by upregulation of MMP-2 expression. PLoS One. 2013;8:e83373. doi: 10.1371/journal.pone.0083373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Fan J, Chen H, et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. 2014;4:5911. doi: 10.1038/srep05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Eppert K, Takenaka K, Lechman ER, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 21.Piccirillo SG, Colman S, Potter NE, et al. Genetic and functional diversity of propagating cells in glioblastoma. Stem Cell Reports. 2015;4:7–15. doi: 10.1016/j.stemcr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Huang Y-h, Chen J-l. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirko S, Behrendt G, Johansson PA, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell. 2013;12:426–439. doi: 10.1016/j.stem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Yan G-N, Yang L, Lv Y-F, et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay ED. An overview of epithelial-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 27.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci. 2010;67:2605–2618. doi: 10.1007/s00018-010-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karin M, Greten FR. NF-kappa B: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 30.Li CW, Xia W, Huo L, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappaB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Li A, Glas M, et al. c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A. 2011;108:9951–9956. doi: 10.1073/pnas.1016912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Suva ML, Rheinbay E, Gillespie SM, et al. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Jeter CR, Liu B, Liu X, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011;30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suvà ML, Rheinbay E, Gillespie SM, et al. Reconstructing and reprogramming the tumor propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 39.Plaks V, Kong N, Werb Z. The Cancer Stem Cell Niche: How Essential is the Niche in Regulating Stemness of Tumor Cells? Cell stem cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 41.Oh M, Nör JE. The Perivascular Niche and Self-Renewal of Stem Cells. Front Physiol. 2015;6:367. doi: 10.3389/fphys.2015.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabrese C, Poppleton H, Kocak M, et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Charles N, Ozawa T, Squatrito M, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeon HM, Kim SH, Jin X, et al. Crosstalk between glioma-initiating cells and endothelial cells drives tumor progression. Cancer Res. 2014;74:4482–4492. doi: 10.1158/0008-5472.CAN-13-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan GN, Yang L, Lv YF, et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck B, Driessens G, Goossens S, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nor JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014;74:2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jogi A, Ora I, Nilsson H, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lofstedt T, Jogi A, Sigvardsson M, et al. Induction of ID2 expression by hypoxia-inducible factor-1: a role in dedifferentiation of hypoxic neuroblastoma cells. J Biol Chem. 2004;279:39223–39231. doi: 10.1074/jbc.M402904200. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Nat Acad Sci U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 53.Joseph JV, Conroy S, Pavlov K, et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1alpha-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Bao B, Azmi AS, Ali S, et al. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochimica et biophysica acta. 2012;1826:272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing F, Okuda H, Watabe M, et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30:4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Condeelis J, Pollard JW. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan NJ, Sasser AK, Axel AE, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-beta signalling. Br J Cancer. 2014;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vermeulen L, De Sousa E, Melo F, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 61.Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349–354. doi: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell. 2011;145:835–850. doi: 10.1016/j.cell.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49–69. doi: 10.1038/cr.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Widschwendter M, Fiegl H, Egle D, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 66.Ohm JE, McGarvey KM, Yu X, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet. 2007;39:237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Easwaran H, Johnstone SE, Van Neste L, et al. A DNA hypermethylation module for the stem/progenitor cell signature of cancer. Genome Res. 2012;22:837–849. doi: 10.1101/gr.131169.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizzo S, Hersey JM, Mellor P, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 2011;10:325–335. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to Polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crea F, Hurt EM, Mathews LA, et al. Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumorigenicity and tumor progression in prostate cancer. Mol Cancer. 2011;10:40. doi: 10.1186/1476-4598-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benoit YD, Witherspoon MS, Laursen KB, et al. Pharmacological inhibition of polycomb repressive complex-2 activity induces apoptosis in human colon cancer stem cells. Exp Cell Res. 2013;319:1463–1470. doi: 10.1016/j.yexcr.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang J, Chai L, Liu F, et al. Bmi-1 is a target gene for SALL4 in hematopoietic and leukemic cells. Proc Nat Acad Sci U S A. 2007;104:10494–10499. doi: 10.1073/pnas.0704001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pathania R, Ramachandran S, Elangovan S, et al. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun. 2015;6:6910. doi: 10.1038/ncomms7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15:152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heddleston JM, Wu Q, Rivera M, et al. Hypoxia-induced mixed-lineage leukemia 1 regulates glioma stem cell tumorigenic potential. Cell Death Differ. 2012;19:428–439. doi: 10.1038/cdd.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaffer CL, Marjanovic ND, Lee T, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmohl JU, Vallera DA. CD133, Selectively Targeting the Root of Cancer. Toxins (Basel) 2016;8:165. doi: 10.3390/toxins8060165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gurney A, Axelrod F, Bond CJ, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717–11722. doi: 10.1073/pnas.1120068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gavai AV, Quesnelle C, Norris D, et al. Discovery of Clinical Candidate BMS-906024: A Potent Pan-Notch Inhibitor for the Treatment of Leukemia and Solid Tumors. ACS Med Chem Lett. 2015;6:523–527. doi: 10.1021/acsmedchemlett.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin Cancer Res. 2015;21:505–513. doi: 10.1158/1078-0432.CCR-14-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang SD, Yuan Y, Tang H, et al. Tumor cells positive and negative for the common cancer stem cell markers are capable of initiating tumor growth and generating both progenies. PLoS One. 2013;8:e54579. doi: 10.1371/journal.pone.0054579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim J, Villadsen R, Sorlie T, et al. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc Natl Acad Sci U S A. 2012;109:6124–6129. doi: 10.1073/pnas.1203203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyer M, Reimand J, Lan X, et al. Single cell-derived clonal analysis of human glioblastoma links functional and genomic heterogeneity. Proc Natl Acad Sci U S A. 2015;112:851–856. doi: 10.1073/pnas.1320611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mao P, Joshi K, Li J, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013;110:8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhat KP, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das R, Gregory PA, Hollier BG, Tilley WD, Selth LA. Epithelial plasticity in prostate cancer: principles and clinical perspectives. Trends Mol Med. 2014;20:643–651. doi: 10.1016/j.molmed.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Banyard J, Chung I, Wilson AM, et al. Regulation of epithelial plasticity by miR-424 and miR-200 in a new prostate cancer metastasis model. Sci Rep. 2013;3:3151. doi: 10.1038/srep03151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruscetti M, Dadashian EL, Guo W, et al. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene. 2016;35:3781–3795. doi: 10.1038/onc.2015.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ebrahimi B. Reprogramming barriers and enhancers: strategies to enhance the efficiency and kinetics of induced pluripotency. Cell Regen (Lond) 2015;4:10. doi: 10.1186/s13619-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]