Abstract
This study aimed to identify N-acylhomoserine lactone (AHL) produced by Hafnia alvei H4, which was isolated from spoiled instant sea cucumber, and to investigate the effect of AHLs on biofilm formation. Two biosensor strains, Chromobacterium violaceum CV026 and Agrobacterium tumefaciens KYC55, were used to detect the quorum sensing (QS) activity of H. alvei H4 and to confirm the existence of AHL-mediated QS system. Thin layer chromatography (TLC) and high resolution triple quadrupole liquid chromatography/mass spectrometry (LC/MS) analysis of the AHLs extracted from the culture supernatant of H. alvei H4 revealed the existence of at least three AHLs: N-hexanoyl-l-homoserine lactone (C6-HSL), N-(3-oxo-octanoyl)-l-homoserine lactone (3-oxo-C8-HSL), and N-butyryl-l-homoserine lactone (C4-HSL). This is the first report of the production of C4-HSL by H. alvei. In order to determine the relationship between the production of AHL by H. alvei H4 and bacterial growth, the β-galactosidase assay was employed to monitor AHL activity during a 48-h growth phase. AHLs production reached a maximum level of 134.6 Miller unites at late log phase (after 18 h) and then decreased to a stable level of about 100 Miller unites. AHL production and bacterial growth displayed a similar trend, suggesting that growth of H. alvei H4 might be regulated by QS. The effect of AHLs on biofilm formation of H. alvei H4 was investigated by adding exogenous AHLs (C4-HSL, C6-HSL and 3-oxo-C8-HSL) to H. alvei H4 culture. Biofilm formation was significantly promoted (p < 0.05) by 5 and 10 µM C6-HSL, inhibited (p < 0.05) by C4-HSL (5 and 10 µM) and 5 µM 3-oxo-C8-HSL, suggesting that QS may have a regulatory role in the biofilm formation of H. alvei H4.
Keywords: Hafnia alvei, quorum sensing, AHLs, instant sea cucumber, biofilm formation
1. Introduction
Food spoilage is a major socio-economic problem that occurs mainly as a result of the biochemical activity of a microbial community that renders a product undesirable or unacceptable for consumption [1]. Moreover, food spoilage is mostly caused by bacteria, and quorum sensing (QS) is thought to play a role in bacterial-linked food spoilage and food safety [2,3,4]. Increased interest in the connection between QS and food spoilage has led to more in-depth study of the signal molecules in relation to microbes that cause food spoilage. Several classes of signaling molecules of microbial origin, especially N-acyl homoserine lactones (AHLs), have now been identified and detected in different kinds of food, such as milk, meat, and vegetables [5,6].
AHLs are a kind of fatty acid derivatives that are generally recognized as autoinducer-1 (AI-1). These compounds are secreted by Gram-negative bacteria and used as the signals for intraspecies communication [7]. The structure of AHLs consist of a homoserine lactone ring, which is N-acylated, and a fatty acyl group linked to the position of C-1, with the acyl chains typically ranging from four to 18 carbons [8]. Bacteria are known to produce several kinds of AHLs, and one type of AHL can also be synthesized by various genera [1]. Several environmental conditions, including temperature, pH, and NaCl, may influence the concentrations and types of AHLs being produced [9]. The amount of AHLs detected in rotten foods seems to correlate with the expression of certain proteases and the growth of spoilage bacteria [10].
In recent years, instant sea cucumber, which can be eaten without further preparation, is becoming more and more popular among sea cucumber consumers. These sea cucumbers can better maintain their nutritional qualities and have more beneficial amino acid composition compared to traditional dried sea cucumber [11]. Instant sea cucumbers are generally stored at 0–5 °C for three months, making them susceptible to spoilage caused mainly by bacteria. Microbial transmission occurring during food processing is a primary reason why food can become contaminated by microbes, and biofilm formation can benefit the process of this transmission [12,13]. Biofilms are aggregates of microorganisms within a matrix composed of extracellular biopolymers that adhere to a solid surface such as food, or food-processing equipment, resulting in reduced effectiveness of disinfectants. Decontamination of biofilms on food-processing equipment is particularly difficult as biofilms frequently slough off, releasing cells into contacted food products [14] and induce food spoilage. Therefore, biofilms play an important part in the spoilage of instant sea cucumber. Since QS molecules, it is important to know the effect they have on biofilm formation. Previous studies have verified the effect of AHLs on biofilm formed by Pseudoalteromonas ulvae TC14 and Shewanella baltica has been verified [15,16]. According to our best knowledge, there are few reports on QS and biofilm formation caused by H. alvei isolated from instant seafood.
Hafnia alvei is an opportunistic pathogen belonging to the Enterbacteriaceae family [17]. It is a Gram-negative bacterium and a common food contaminant capable of producing AHLs. In addition, H. alvei is considered as the most commonly isolated from contaminant vacuum-packed chilled meat samples [18]. Production of N-(3-oxohexanoyl) homoserine lactone (3-oxo-C6-HSL) by H. alvei has been reported by Viana et al. [19]. Furthermore, food spoilage and biofilm formation have been linked to the QS activity of H. alvei. In this study, three strains of bacteria (H2, H4, and H7) were isolated from spoiled instant sea cucumber and their ability to produce AHLs was assessed. H4 exhibited stronger AHL activity than the other two strains. It was identified as an H. alvei strain. Considering the link of QS to food spoilage and biofilm formation, the AHLs produced by H. alvei H4 were identified and their effect on biofilm formation was also investigated.
2. Experimental Section
2.1. Sample Collection and Bacterial Strains Isolation
The bacterial strain used in this study was isolated from spoiled instant sea cucumber. Each sample was cut into 25-g pieces and minced with sterile knife, then mixed with 225 mL of normal saline under sterile condition. The sample mixture was homogenized for 60 s and 100 μL samples were spread onto LB (10 g Tryptone, 5 g Yeast extract power, 10 g NaCl, dissolved in 1 L deionized water) agar plates as previously described [20]. The plates were incubated at 28 °C for 24 to 48 h. Several morphologically distinct colonies that appeared on the plates were randomly chosen and subcultured at 28 °C for 24 to 48 h to obtain a pure culture, which was then maintained on LB agar plate at 4 °C.
2.2. Screening for Bacterial Isolates for AHLs Production
Two AHLs bacterial biosensors, Chromobacterium violaceum CV026 and Agrobacterium tumefaciens KYC55 which respond to short-chain and long-chain AHLs, respectively, were used in the preliminary screening of AHL produced by the bacteria isolated from spoiled instant sea cucumber. Agar plate diffusion assay was used in the AHL screening process that multiple AHLs-producting bacteria were screened in one culture dish by Anbazhagan et al. [21], to avoid the false positives due to possible contamination between these isolated strains in one dish, some modifications were carried out. Briefly, the bacterial strain to be tested for the production of AHLs as well as C. violaceum CV026 were streaked parallel to each other on a LB agar plate. Detection of AHLs production was also carried using A. tumefaciens KYC55, and the assay was basically performed in the same way, except that the agar was supplemented with 50 μg/mL X-gal (Sangon Biotech, Shanghai, China). In both cases, the plates were incubated at 28 °C for overnight. Production of exogenous AHLs was indicated by the formation of the purple pigment violacein or blue coloration by β-galactosidase activity.
2.3. Identification and Phylogenetic Analysis of AHL-Producing Bacteria
Genomic DNA of H2, H4, and H7 strains was extracted with TIANamp bacteria DNA kit (Tiangen Biotech, Beijing, China) and then used as template to amplify the 16S rDNA gene. The primers used were 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR sample contained 1 μL of each primer (10 μM), 1 μL DNA template solution, 10 μL Premix Ex Taq DNA polymerase buffer (Takara, Tokyo, Japan), and 7 μL ultra-pure water. PCR condition consisted of 95 °C 5 min; 30 cycles of 95 °C 40 s, 55 °C 1 min, and 72 °C 2 min, and a final elongation step at 72 °C 10 min. PCR products were purified and sequenced by BGI (Shenzhen, China). 16S rDNA sequences were used to identify bacterial genera by comparing the sequences with those in the GenBank database (http://blast.ncbi.nlm.nih.gov). To construct a phylogenetic tree based on its 16S rDNA sequence, CLUSTAL W [22] was used to align the nucleotide sequences of the AHL-producing bacterial isolates (http://www.genome.jp/tools/clustalw/). Molecular Evolutionary Genetic Analysis version 3.1 (MEGA3.1) was used to construct the phylogenetic tree, and the aligned complete 16S rDNA sequences were subjected to phylogenetic analysis using MEGA 3.1. To ensure the robustness and reliability of trees constructed, Neighbor-Joining algorithm method and bootstrap analysis for 500 resamplings were conducted to generate the tree [23].
2.4. Extraction of AHLs
In order to identify the AHLs produced by the isolated bacterial strain with strong QS activity from spoiled instant sea cucumber, AHLs were first extracted from the corresponding bacterial culture. Aliquot (100 mL) of an overnight culture was centrifuged at 8000× g for 15 min at 4 °C, and the supernatant was added to an equal volume of ethyl acetate containing 0.1% acetic acid (v/v) followed by thorough mixing. The mixture was then incubated at 25 °C for 2 h with shaking at 180 rpm. After that, the ethyl acetate layer was removed and freeze-dried under vacuum, while the residue was dissolved in 1 mL ultra-pure water, and then dried in a vacuum drier. The sample was dissolved in 100 μL 25/75 (v/v) acetonitrile/water and subjected to TLC and LC-MS analyses as described below.
2.5. Identification of AHLs by TLC and HPLC/MS
Reverse phrase C18 TLC plate (Merck, Darmstadt, Germany) was cut into a 4 × 10 cm strip, AHL extracted and standard AHLs (C4-HSL (100 μg/mL), C6-HSL (50 μg/mL) and 3-oxo-C8-HSL (100 μg/mL) obtained from Sigma–Aldrich (St. Louis, MI, USA) were spotted onto the strip about 1 cm from the bottom of the strip, and with a spacing of 1 cm between spots. Methanol/water (50%, v/v) was used as an eluting solvent to separate the AHLs. After development, the strip was air dried at 25 °C, and the dried strip was then put into a culture dish and overlaid with LB agar containing C. violaceum CV026. After setting, the plate was incubated at 28 °C for overnight. The presence of AHL would result in the appearance of purple spots in the agar, indicative of the production of a purple pigment by C. violaceum CV026 induced by AHL [24].
For mass spectrometry analysis, individual AHLs were first purified from the extract by solid phase extraction (SPE) as optimized by Li et al. [25] using Varian Bond Elut C18 (1 g, 6 mL) (Agilent Technolgies, Palo Alto, CA, USA). Identification of AHLs extracted from the bacterial culture were performed as described by Okutsu et al. [26], but with some modification, using a triple quadrupole/linear ion trap instrument (LIT) (QTRAP4000; AB Sciex, Foster, CA, USA) with an electrospray ionization (ESI) source equipped with a RRHD SB-C18 column (3.0 mm × 100 mm, 1.8 μm particle size) kept at 30 °C. The sample (6 μL) was loaded onto the column and eluted with acetonitrile and water, both of which contained 0.1% (v/v) acetic acid. The flow rate was set at 0.3 mL/min for 30 min, and the acetonitrile gradient was set from 30% (v/v) at 0 min to 70% (v/v) at 30 min. The eluent was monitored by absorbance at 210 nm. LIT was used to record the MS/MS spectra with product ion scan mode. Ion source was kept at 450 °C, curtain gas was at 10, collisional activated dissociation (CAD) gas at medium, ion source gas 1 at 30 psi, and ion source gas 2 at 30 psi. Ionspray voltage was set at 5500 V in positive ion mode. Declustering Potential was set at 100 V, Entrance Potential 10 V, Collision Potential 15 V, and Collision Potential cell exit potentials was maintained at 10 V. Positive ion mode was performed to scan precursor ion with Q1 set to scan a mass range from m/z 100 to 250 Da. In addition, Q3 was set at m/z 102 Da to monitor the lactone ring of the product ion. Standard AHLs were used as references: C6-HSL, 3-oxo-C8-HSL and C4-HSL (Sigma–Aldrich, St. Louis, MI, USA) and analyzed by HPLC/MS with the same settings described above.
2.6. AHLs Quantification by β-Galactosidase Assay
The quantity of AHLs produced by an AHL-positive strain was measured by determining the level of β-galactosidase activity with o-nitrophenyl-D-galactopyranoside (ONPG, Sigma–Aldrich, St. Louis, MI, USA) as a substrate as previously described [27]. Briefly, 100 µL sterile-filtered culture supernatant of an overnight culture of H. alvei H4 was added to 1 mL of diluted KYC55 culture (106–107 CFU/mL) and the mixture was inoculated at 30 °C until the OD600 of the culture reached 0.2–1.0. The cells were harvested from 200 μL culture and resuspended in 1 mL of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, pH 7.0) followed by the addition of one drop of 0.1% SDS and three drops of chloroform to lyse the bacterial cells. The tubes were then placed in a 30 °C water bath and allowed to equilibrate for about 5 min. Then, 0.2 mL ONPG (final concentration of 4 mg/mL in 0.1 M phosphate buffer pH 7.0) was added to each tube followed by vortexing. The reaction was stopped by adding 0.6 mL of 1 M Na2CO3 after the appearance of a yellow color. The mixture was centrifuged at 12,000× g for 15 min and the absorbance of the supernatant was measured at 420 nm using a UV spectrophotometer. The activity of β-galactosidase was calculated as Equation (1) below. AHL production was monitored over a 48-h period, with measurement taken at every 6 h. For the negative control, 100 μL LB medium was used instead of sterile bacterial culture supernatant.
| (1) |
2.7. Biofilm Formation Assay
The quantification of biofilm formation was performed using a regular 96-well microtiter plates as described previously [28], but with minor modifications. Briefly, an overnight culture of H. alvei H4 was inoculated (1:100 dilution) in LB medium and 200 μL of the culture was added to a 96-well microtiter plate (Corning, Corning, NY, USA) followed by the addition of exogenous synthetic AHL (C4-HSL, C6-HSL or 3-oxo-C8-HSL; Sigma–Aldrich, St. Louis, MI, USA) to a final concentration of 5, 10, 20, or 40 μM. For control, deionized water was used instead of AHL. The plate was incubated at 30 °C for 48 h. After incubation, the culture suspension was discarded and the plate was washed three times with PBS (pH 7.4, 0.01 M) using 200 μL per well. The cells were then fixed with 200 μL methanol for 15 min, dried at 60 °C and stained with 200 μL of 0.1% crystal violet for 15 min. The plate was again washed three times with deionized water to remove the excess dye and then dried at 60 °C. Biofilm formed on the plate was solubilized by addition of 200 µL of 33% acetic acid (per well) followed by 20 min incubation at room temperature. The absorbance of the plate was then measured at 590 nm with a spectrophotometer (Molecular Devices, San Francisco, CA, USA).
2.8. Statistical Analysis
Each sample was conducted by three replicate trials, and all experiments were repeated three times. Results were presented as mean ± standard deviation (SD) and analyzed by T-test using SPSS 16.0 software and the graphics were made by origin pro8.6 version.
3. Result
3.1. Isolation and Identification of AHL-Producing Bacteria
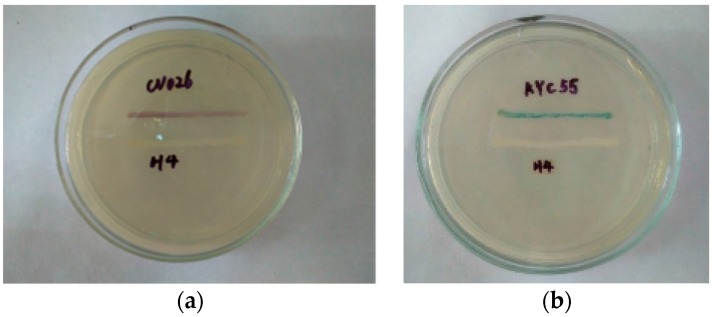
Three dominant food spoilage bacteria (H2, H4, and H7) were isolated from spoiled instant sea cucumber and their ability to produce AHLs was determined. AHL production was only detected for strain H4 when the assay was based on color changes produced by the reporter strains: purple in the case of C. violaceum 026 and blue for A. tumefaciens KYC55 (Figure 1). H2 and H7 strains were only found to produce AHLs when assayed with A. tumefaciens KYC55 as the reporter strain (data not shown).
Figure 1.
Screening of N-acylhomoserine lactone (AHL)–producing bacteria by (a) C. violaceum CV026 and (b) A. tumefaciens KYC55 strain. H4: bacterium isolated from spoiled sea cucumber; CV026 and KYC55: biosensor strains used to detect AHL-producing strains.
3.2. Identification of AHL-Producing Bacteria
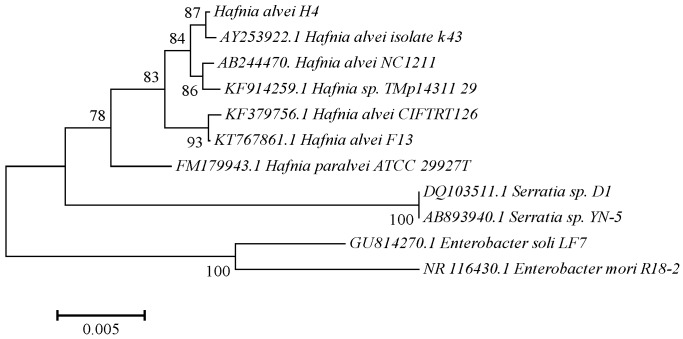
Comparison of the complete 16S rDNA gene sequences from the three bacteria with the sequences available in the NCBI database by nucleotide blast analysis indicated that H2, H4, and H7 belong to Pesudomonas, Hafnia and Acinetobacter, respectively. Since H4 appeared to have a stronger quorum sensing activity than the other two strains, it was subjected phylogenetic analysis. The phylogeny tree obtained by MEGA 3.1 software using the 16S rDNA sequences of 10 bacterial strains from NCBI database was presented as Figure 2. The result from the well-supported phylogeny with high resolution inner branches indicated that H4 was a Hafnia alvei strain, and therefore it was referred to as H. alvei H4. The AHLs extracted from H. alvei H4 culture were subjected to further analysis to identify the individual AHLs.
Figure 2.
Phylogenetic analysis of AHL-producing bacteria isolated from spoiled ready-to-eat sea cucumber. Phylogenetic analysis was performed with MEGA4.1 software.
3.3. AHL Profile Analysis by TLC and HPLC-MS/MS
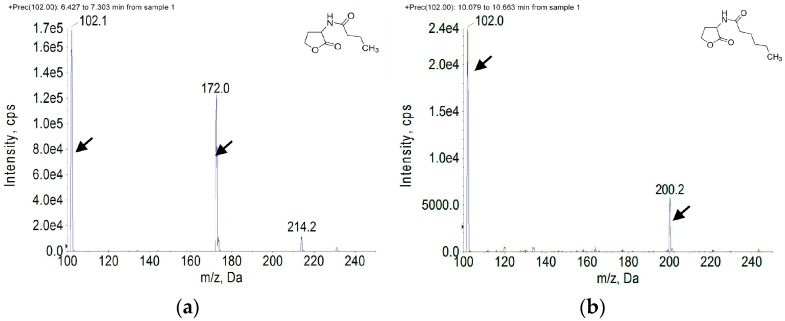
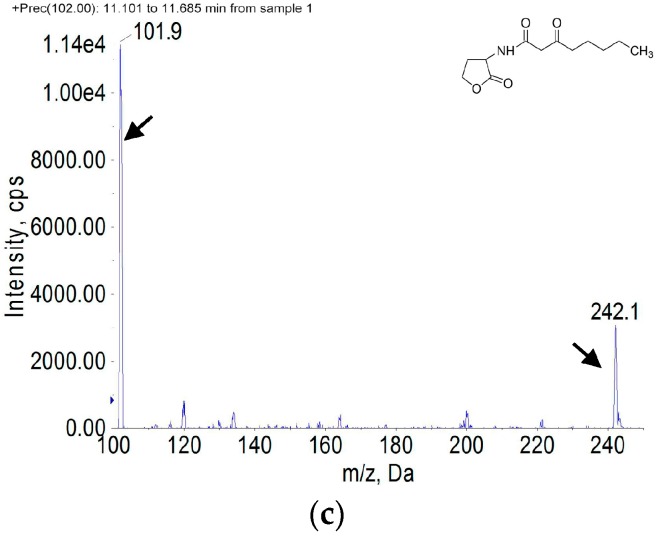
TLC analysis of the AHLs extracts revealed two purple spots with the retention factors (Rf) corresponding to those of C4-HSL (Rf 0.62) and C6-HSL AHLs (Rf 0.38) (Figure 3). Further identification was conducted by HPLC/MS, comparison of the spectra (Figure 4 ) generated on the LC/MS platform compared to the standard AHLs revealed that H. alvei H4 produced three types of AHLs: C6-HSL (m/z 200), C4-HSL (m/z 172), and 3-oxo-C8-HSL (m/z 242).
Figure 3.

TLC bioassay of AHLs produced by H. alvei H4 with C. violaceum CV026 Lane 1: Standard AHLs of C4-HSL; Lane 2: Standard AHLs of C6-HSL; Lane 3: AHLs extracted from H. alvei H4 culture supernatant.
Figure 4.
Mass spectrometry analysis of AHLs extracted from H. alvei H4 culture supernatant; (a) Spectrum of C4-HSL (m/z 172.0); (b) Spectrum of C6-HSL (m/z 200.2) (marked by arrow); (c) Spectrum of 3-oxo-C8-HSL (m/z 242.1) (marked by arrow).
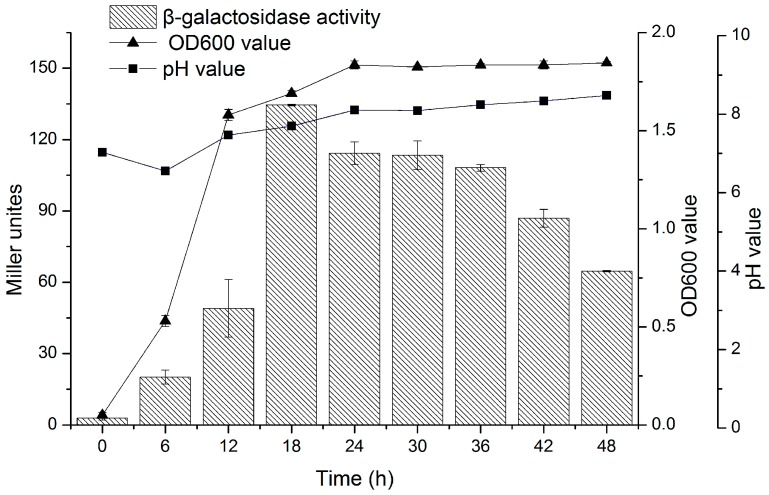
3.4. AHLs Production by H. alvei H4 in Growth Phase
H. alvei H4 produced AHLs throughout the 48 h of incubation in LB at 30 °C, with the level of AHLs reaching a maximum of 135 Miller unites after 18 h (Figure 5), and gradually decreased at late log and stationary phases to 110 Miller unites, followed by rapid decrease until 36 h, and down to 67 Miller unites at the end of 48 h. At the same time, the pH of the bacterial culture also increased gradually during incubation and reached 8.47 after 48 h.
Figure 5.
Production of AHLs by H. alvei H4 at different stages of growth in LB medium. AHLs in the culture supernatant of H. alvei H were quantitated by the β-galactosidase assay with A. tumefaciens KYC55 as the reporter strain.
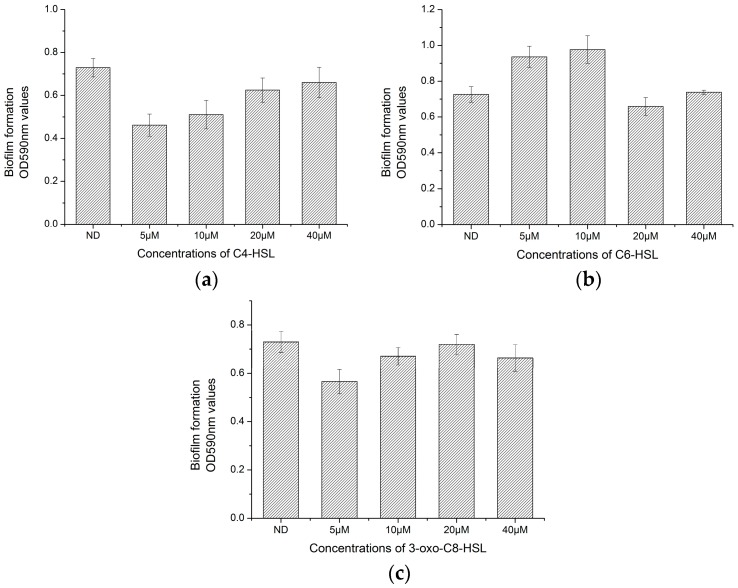
3.5. Effect of AHLs on Biofilm Formation of H. alvei H4
Growth of H. alvei H4 was not affected by the addition of AHLs as evidenced by a lack of change in OD600 values. Addition of C4-HSL to the culture significantly (p < 0.05) inhibited the biofilm formed by H. alvei H4, but only at low concentrations of C4-HSL (5 and 10 µM), since at high concentrations (20 and 40 µM) no significant difference was observed compared to the control. In contrast to the effect of C4-HSL, low concentrations of C6-HSL significantly (p < 0.05) promoted biofilm formation by H. alvei H4, although high concentration of C6-HSL slightly inhibited biofilm formation. 3-oxo-C8-HSL exhibited slight inhibition against the biofilm formed by H. alvei H4, with the greatest inhibition achieved by 5 µM 3-oxo-C8-HSL (p < 0.05).
4. Discussion
In this study, three bacteria were isolated from spoiled instant sea cucumber, and subsequently identified as members of the Pesudomonas, Hafnia and Acinetobacter genera by 16S rDNA analysis. All three strains are considered as food-spoilage bacteria [29]. Pesudomonas is widely present in chill-stored proteinaceous raw foods, vacuum-packed food and aquatic food products, but has also been isolated from spoiled pasteurized milk [6], whereas Hafnia is often found in vacuum-packed, refrigerated spherical fish paste and fresh meat [3,30]. Acinetobacter is found in brand beef and on food processing surfaces [31,32]. Quorun sensing system has been shown to play a role in bacteria-mediated food spoilage, especially those mediated by gram-negative bacteria, and the production of AHLs is thought to be an important contributing factor. Therefore, the bacterial strains isolated from spoiled instant sea cucumber were screened by C. violaceum CV026 and A. tumefaciens KYC55 for the ability to produce AHLs so that to determine their quorum sensing activity. H4 strain was found to secret AHLs as detected by both the two sensor strains, which presented a strong quorum sensing activity and subsequently identified as a Hafina alvei strain was chosen for further study.
Three kinds of AHL molecules were detected in the culture supernatant of H. alvei H4, and these were identified as C4-HSL, C6-HSL, and 3-oxo-C8-HSL by the TLC and HPLC-MS methods (Figure 3 and Figure 4), and the charge-to-mass ratio (m/z) values of these detected compounds are consistent with the m/z values of C4-HSL, C6-HSL, and 3-oxo-C8-HSL reported by Ortori et al. [33]. A report has been published that the production of C6-HSL by H. alvei 071 isolated from cooled raw milk by Viana et al. [19], and 3-oxo-C8-HSL can be secreted by H. alvei FB1 as reported by Tan et al. [3], while the production of C4-HSL by H. alvei was demonstrated for the first time in this study. Spoilage of food caused by bacteria is mainly associated with the production of exoenzymes that cause the degradation of food ingredients. Production of proteolytic and lipolytic enzymes in Serratia proteamaculans B5a depends on the lipB gene, a process that is regulated by 3-oxo-C6-HSL as described by Christensen et al. [34]. Production of extracellular protease and biofilm formation were observed to be regulated by ahyI, which codes for AHLs synthase in Aeromonas hydrophila. A positive effect on these phenotypes was observed with a supplement of C4-HSL to Aeromonas hydrophila ahyI mutant, indicating the existence of a regulatory role for C4-HSL [35]. Both H. alvei and Serratia are members of the Enterobacteriacea family, and since H. alvei and Aeromonas hydrophila are opportunistic pathogens that are also closely related, a similar regulatory function of AHLs might exist in H. alvei as mentioned by Tan et al. [3]. However, this speculation needs to be verified by further study.
In most Gram-negative bacteria, Quorum sensing is mediated by AHL molecules [4]. The trend in AHLs activity was consistent with the growth of H. alvei H4 within the first 18 h of incubation (Figure 6), since at late log phase, quorum sensing may play a more central role in bacterial physiology by slowing down its growth rate. This suggested that QS pathways may converge with starvation-sensing pathways, causing the cells to switch from the log phase to the stationary phase [36,37]. Thus the growth of H. alvei H4 might also be controlled by AHLs-mediated QS as explained by Nackerdien et al. [38] and Han et al. [36]. The reduction in AHLs activity that occurred when the pH of the culture became more alkaline was consistent with the fact that AHLs are unstable in alkaline condition [39].
Figure 6.
Effect of AHLs on the biofilm formation of H. alvei H4. (ND: No addition of AHLs). (a) Addition of 5–40 µM C4-HSL; (b) Addition of 5–40 µM C6-HSL; (c) Addition of 5–40 µM 3-oxo-C8-HSL.
To preliminarily verify the function of QS signaling molecules in H. alvei H4, the effect of each of the three AHLs produced by H. alvei H4 on its own biofilm formation appeared to vary, depending on the type of molecule. Except for C6-HSL, which promoted biofilm formation at low concentration, the other two all inhibited biofilm formation at low concentrations (Figure 6). Similar results were obtained by Nievas et al. [40], who showed that biofilm by Peanut-Nodulating Bradyrhizobia P8A strain can be inhibited by lower concentrations of (5 and 10 µM) 3-oxo-C10-HSL and 3-oxo-C14-HSL, consistent with the observed effects of C4-HSL and 3-oxo-C8-HSL on H. alvei H4 (Figure 6). In addition, the biofilm produced by Peanut-Nodulating Bradyrhizobia 62B strain can be increased by low concentrations of 3-oxo-C12-HSL (5 and 10 µM), also consistent with the result of C6-HSL on H. alvei H4 (Figure 6). A promoting effect was also observed on Acinetobacter baumannii ATCC19606 when C6-HSL was added [41]. Biofilm formation of Serratia A2 strain was inhibited by 10 µM C4-HSL and Aeromonas B1 strain was promoted by 10 µM C6-HSL reported by Zhang et al. [20], which were also in agreement to our finding. However, a contrary effect was obtained by Zhao et al. [16] when biofilm formation of Shewanella baltica was inhibited by 10 µM 3-oxo-C8-HSL, and biofilms of Flavobacterium sp and Klebsiella sp1 was promoted by low concentrations of C4-HSL (10–100 nM) [42]. Therefore, considering the results achieved in this study and the description above, conclusions can be drawn that the effect of AHLs on biofilm formation varies to different bacteria, which were dose-dependent and type-dependent.
LuxR/I homologs in H. alvei are considered as halR/I. AHLs are synthesized by AHL synthase and regulated by halI. A Hafnia alvei 071 halI mutant that is unable to produce AHL is also deficient in biofilm formation [31]. This suggests an important role of AHLs in biofilm formation, which is linked to AHLs-mediated QS system. Although no direct evidence has been obtained to support a mechanistic relationship between QS and biofilm formation in H. alvei H4, the effect of AHLs on biofilm formed by H. alvei H4 was confirmed in our data.
5. Conclusions
Three spoilage bacteria were isolated from spoiled instant sea cucumber and identified as Pesudomonas H2, Hafnia H4, and Acinetobacter H7. Only Hafnia H4 exhibited strong AHL activity, and further identification showed that it was H. alvei H4 that produced three kinds of AHLs (C4-HSL, C6-HSL, and 3-oxo-C8-HSL). Biofilm formation H. alvei H4 could be significantly promoted or inhibited by the addition of the three kinds of AHLs with different concentrations. Further study will focus on constructing AHLs synthase mutant strain to research whether the production of extracellular protease and biofilm formation of H. alvei H4 were regulated by AHLs-mediated QS system.
Acknowledgments
This work was supported financially by “The National Key Technology Support Program (Grant No. 2015BAD17B00)” and “The Science and Technology Foundation of Liaoning Province (201602049)”.
Author Contributions
Hong-Man Hou and Gong-Liang Zhang conceived of and designed the experiments; Hong-Man Hou and Yao-Lei Zhu performed the experiments; Hong-Man Hou and Yao-Lei Zhu analyzed the data; Jia-Ying Wang, Wen-Yan Qu, and Feng Jiang contributed reagents/materials/analysis tools; Hong-Man Hou and Hong-Shun Hao wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Skandamis P.N., Nychas G.J. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012;78:5473–5482. doi: 10.1128/AEM.00468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dourou D., Ammor M.S., Skandamis P.N., Nychas G.J. Growth of Salmonella enteritidis and Salmonella typhimurium in the presence of quorum sensing signalling compounds produced by spoilage and pathogenic bacteria. Food Microbiol. 2011;28:1011–1018. doi: 10.1016/j.fm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Tan J.Y., Yin W.F., Chan K.G. Quorum sensing activity of Hafnia alvei isolated from packed food. Sensors. 2014;14:6788–6796. doi: 10.3390/s140406788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu S., Wu H., Zeng M., Liu Z., Wang Y. The involvement of bacterial quorum sensing in the spoilage of refrigerated Litopenaeus vannamei. Int. J. Food Microbiol. 2015;192:26–33. doi: 10.1016/j.ijfoodmicro.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 5.Liu M., Gray J.M., Griffiths M.W. Occurrence of proteolytic activity and N-acyl-homoserine lactone signals in the spoilage of aerobically chill-stored proteinaceous raw foods. J. Food Prot. 2006;69:2729–2737. doi: 10.4315/0362-028X-69.11.2729. [DOI] [PubMed] [Google Scholar]
- 6.Pinto U.M., De Souza V.E., Martins M.L., Vanetti M.C.D. Detection of acylated homoserine lactones in gram-negative proteolytic psychrotrophic bacteria isolated from cooled raw milk. Food Control. 2007;18:1322–1327. doi: 10.1016/j.foodcont.2006.09.005. [DOI] [Google Scholar]
- 7.Smith D., Wang J.H., Swatton J.E., Davenport P., Price B., Mikkelsen H., Stickland H., Nishikawa K., Gardiol N., Spring D.R., et al. Variations on a theme: diverse N-acyl homoserine lactone-mediated quorum sensing mechanisms in gram-negative bacteria. Sci. Prog. 2006;89:167–211. doi: 10.3184/003685006783238335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitehead N.A., Barnard A.M., Slater H., Simpson N.J., Salmond G.P. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 9.Popat R., Cornforth D.M., McNally L., Brown S.P. Collective sensing and collective responses in quorum-sensing bacteria. J. R. Soc. Interface. 2015;12 doi: 10.1098/rsif.2014.0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rul F., Monnet V. How microbes communicate in food: A review of signaling molecules and their impact on food quality. Curr. Opin. Food Sci. 2015;2:100–105. doi: 10.1016/j.cofs.2015.03.003. [DOI] [Google Scholar]
- 11.Liu Q., Sun J., Pang Y., Jia Z. Optimization of Processing Technology of Instant Sea Cucumber with Fuzzy Mathematic Comprehensive Evaluation by Response Surface Methodology and Exploration on Nutritional Value of Instant Sea Cucumber. Food Sci. Technol. Res. 2016;22:583–593. doi: 10.3136/fstr.22.583. [DOI] [Google Scholar]
- 12.Bai A.J., Rai V.R. Bacterial quorum sensing and food industry. Compr. Rev. Food Sci. Food Saf. 2011;10:183–193. doi: 10.1111/j.1541-4337.2011.00150.x. [DOI] [Google Scholar]
- 13.Kerekes E.B., Deak E., Tako M., Tserennadmid R., Petkovits T., Vagvolgyi C., Krisch J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Micrbiol. 2013;115:933–942. doi: 10.1111/jam.12289. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Stanford K., McAllister T.A., Johnson R.P., Chen J., Hou H., Niu Y.D. Biofilm Formation, Virulence Gene Profiles, and Antimicrobial Resistance of Nine Serogroups of Non-O157 Shiga Toxin—Producing Escherichia coli. Foodborne Pathog. Dis. 2016;13:316–324. doi: 10.1089/fpd.2015.2099. [DOI] [PubMed] [Google Scholar]
- 15.Aye A.M., Bonnin-Jusserand M., Brian-Jaisson F., Ortalo-Magné A., Culioli G., Nevry R.K., Molmeret M. Modulation of violacein production and phenotypes associated with biofilm by exogenous quorum sensing N-acylhomoserine lactones in the marine bacterium Pseudoalteromonas ulvae TC14. Microbiology. 2015;161:2039–2051. doi: 10.1099/mic.0.000147. [DOI] [PubMed] [Google Scholar]
- 16.Zhao A., Zhu J., Ye X., Ge Y., Li J. Inhibition of biofilm development and spoilage potential of Shewanella baltica by quorum sensing signal in cell-free supernatant from Pseudomonas fluorescens. Int. J. Food Microbiol. 2016;230:73–80. doi: 10.1016/j.ijfoodmicro.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Savini V., Santarelli A., Polilli E., Astolfi D., Pompilio A., Di Bonaventura G., Fazii P., D’Antonio D. Hafnia alvei from the farm to the delivery room. Vet. Microbiol. 2013;163:202–203. doi: 10.1016/j.vetmic.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Bruhn J.B., Christensen A.B., Flodgaard L.R., Nielsen K.F., Larsen T.O., Givskov M., Gram L. Presence of acylated homoserine lactones (AHLs) and AHL-producing bacteria in meat and potential role of AHL in spoilage of meat. Appl. Environ. Microbiol. 2004;70:4293–4302. doi: 10.1128/AEM.70.7.4293-4302.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viana E.S., Campos M.E., Poce A.R., Mantovani H.C., Vanetti M.C. Biofilm formation and acyl homoserine lactone production in Hafnia alvei isolated from raw milk. Biol. Res. 2009;42:427–436. [PubMed] [Google Scholar]
- 20.Zhang C., Zhu S., Jatt A.N., Zeng M. Characterization of N-acyl homoserine lactones (AHLs) producing bacteria isolated from vacuum-packaged refrigerated turbot (Scophthalmus maximus) and possible influence of exogenous AHLs on bacterial phenotype. J. Gen. Appl. Microbiol. 2016;62:60–67. doi: 10.2323/jgam.62.60. [DOI] [PubMed] [Google Scholar]
- 21.Anbazhagan D., Mansor M., Yan G.O.S., Yusof M.Y.M., Hassan H., Sekaran S.D. Detection of quorum sensing signal molecules and identification of an autoinducer synthase gene among biofilm forming clinical isolates of Acinetobacter spp. PLoS ONE. 2012;7:e36696. doi: 10.1371/journal.pone.0036696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh S.Y., Khan S.A., Tee K.K., Kasim N.H.A., Yin W.F., Chan K.G. Quorum sensing activity of Citrobacter amalonaticus L8A, a bacterium isolated from dental plaque. Sci. Rep. 2016;6:20702. doi: 10.1038/srep20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J.W., Koh C.L., Sam C.K., Yin W.F., Chan K.G. Short chain N-acyl homoserine lactone production by soil isolate Burkholderia sp. strain A9. Sensors. 2013;13:13217–13227. doi: 10.3390/s131013217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., George J.E., McCarty C.L., Wendelken S.C. Compliance analysis of phenylurea and related compounds in drinking water by liquid chromatography/electrospray ionization/mass spectrometry coupled with solid-phase extraction. J. Chromatogr. A. 2006;1134:170–176. doi: 10.1016/j.chroma.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 26.Okutsu N., Morohoshi T., Xie X., Kato N., Ikeda T. Characterization of N-Acylhomoserine Lactones Produced by Bacteria Isolated from Industrial Cooling Water Systems. Sensors. 2015;16:E44. doi: 10.3390/s16010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packiavathy I.A., Sasikumar P., Pandian S.K., Veera Ravi A. Prevention of quorum-sensing-mediated biofilm development and virulence factors production in Vibrio spp. by curcumin. Appl. Microbiol. Biotechnol. 2013;97:10177–10187. doi: 10.1007/s00253-013-4704-5. [DOI] [PubMed] [Google Scholar]
- 28.Alhede M., Bjarnsholt T., Givskov M., Givskov M. Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Adv. Appl. Microbiol. 2014;86:1–40. doi: 10.1016/B978-0-12-800262-9.00001-9. [DOI] [PubMed] [Google Scholar]
- 29.Ammor M.S., Michaelidis C., Nychas G.J.E. Insights into the role of quorum sensing in food spoilage. J. Food Prot. 2008;71:1510–1525. doi: 10.4315/0362-028X-71.7.1510. [DOI] [PubMed] [Google Scholar]
- 30.Jay J.M., Vilai J.P., Hughes M.E. Profile and activity of the bacterial biota of ground beef held from freshness to spoilage at 5–7 °C. Int. J. Food Microbiol. 2003;81:105–111. doi: 10.1016/S0168-1605(02)00189-7. [DOI] [PubMed] [Google Scholar]
- 31.Chung M.S., Lee J.H., Min D.B. Effects of Pseudomonas putrifaciens and Acinetobacter spp. on the flavor quality of raw ground Beef. J. Food Sci. 2002;67:77–83. doi: 10.1111/j.1365-2621.2002.tb11362.x. [DOI] [Google Scholar]
- 32.Møretrø T., Sharifzadeh S., Langsrud S., Heir E., Rickard A.H. Coaggregation between Rhodococcus and Acinetobacter strains isolated from the food industry. Can. J. Microbiol. 2015;61:503–512. doi: 10.1139/cjm-2015-0210. [DOI] [PubMed] [Google Scholar]
- 33.Ortori C.A., Dubern J.F., Chhabra S.R., Cámara M., Hardie K., Williams P., Barrett D.A. Simultaneous quantitative profiling of N-acyl-l-homoserine lactone and 2-alkyl-4 (1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal. Bioanal. Chem. 2011;399:839–850. doi: 10.1007/s00216-010-4341-0. [DOI] [PubMed] [Google Scholar]
- 34.Christensen A.B., Riedel K., Eberl L., Flodgaard L.R., Molin S., Gram L., Givskov M. Quorum-sensing-directed protein expression in Serratia proteamaculans B5a. Microbiology. 2003;149:471–483. doi: 10.1099/mic.0.25575-0. [DOI] [PubMed] [Google Scholar]
- 35.Khajanchi B.K., Sha J., Kozlova E.V., Erova T.E., Suarez G., Sierra J.C., Chopra A.K. N-acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology. 2009;155:3518–3531. doi: 10.1099/mic.0.031575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y., Li X., Qi Z., Zhang X.H., Bossier P. Detection of different quorum-sensing signal molecules in a virulent Edwardsiella tarda strain LTB-4. J. Appl. Microbiol. 2010;108:139–147. doi: 10.1111/j.1365-2672.2009.04405.x. [DOI] [PubMed] [Google Scholar]
- 37.Lazazzera B.A. Quorum sensing and starvation: Signals for entry into stationary phase. Curr. Opin. Microbiol. 2000;3:177–182. doi: 10.1016/S1369-5274(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 38.Nackerdien Z.E., Keynan A., Bassler B.L., Lederberg J., Thaler D.S. Quorum sensing influences Vibrio harveyi growth rates in a manner not fully accounted for by the marker effect of bioluminescence. PLoS ONE. 2008;3:e1671. doi: 10.1371/journal.pone.0001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li T., Cui F., Bai F., Zhao G., Li J. Involvement of Acylated Homoserine Lactones (AHLs) of Aeromonas sobria in Spoilage of Refrigerated Turbot (Scophthalmus maximus L.) Sensors. 2016;16:1083. doi: 10.3390/s16071083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nievas F., Bogino P., Sorroche F., Giordano W. Detection, characterization, and biological effect of quorum-sensing signaling molecules in peanut-nodulating bradyrhizobia. Sensors. 2012;12:2851–2873. doi: 10.3390/s120302851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo L.M., Wu L.J., Xiao Y.L., Zhao D., Chen Z.X., Kang M., Xie Y. Enhancing pili assembly and biofilm formation in Acinetobacter baumannii ATCC19606 using non-native acyl-homoserine lactones. BMC Microbiol. 2015;15:62. doi: 10.1186/s12866-015-0397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z., Wang Q., Guo F., Zhang S., Jiang Q., Yu X. Responses of bacterial strains isolated from drinking water environments to N-acyl-l-homoserine lactones and their analogs during biofilm formation. Front. Environ. Sci. Eng. 2014;8:205–214. doi: 10.1007/s11783-013-0492-5. [DOI] [Google Scholar]