Abstract
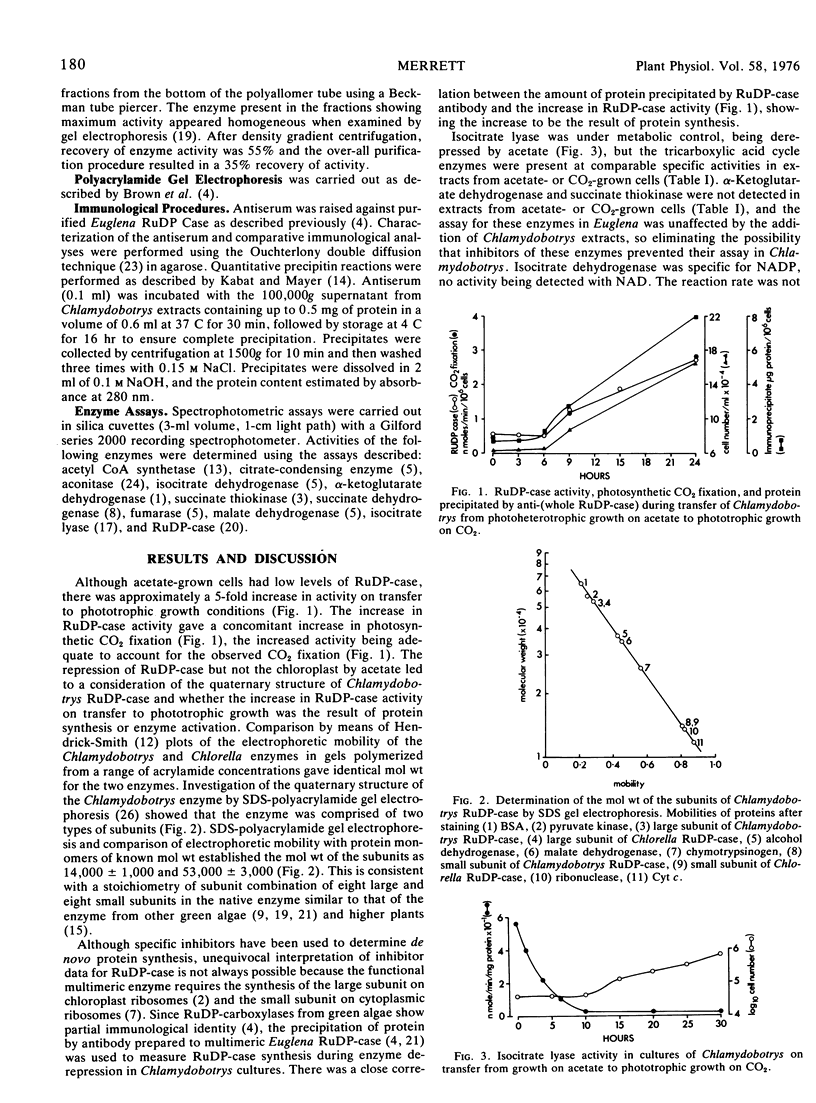
During the transition from photoheterotrophic growth on acetate to phototrophic growth on carbon dioxide, there is a decrease in isocitrate lyase and increase in ribulose-1,5-diphosphate carboxylase activity in Chlamydobotrys stellata cultures. The increase in ribulose-1,5-diphosphate carboxylase activity is the result of protein synthesis, there being a close correlation between increase in enzyme activity and protein precipitated by antibody to ribulose-1,5-diphosphate carboxylase. The purified ribulose-1,5-diphosphate carboxylase was similar to the constitutive enzyme from other green algae having a molecular weight of 530,000 and composed of two types of subunit of molecular weight 53,000 and 14,000.
Enzyme assays demonstrated an incomplete tricarboxylic acid cycle in cells growing photoheterotrophically on acetate or phototrophically on carbon dioxide. Although these cells lack α-ketoglutarate dehydrogenase and succinate thiokinase, a cyclic flow of acetate carbon is possible in the presence of the glyoxylate cycle enzymes but the yield of adenosine triphosphate from acetate oxidation may be insufficient to support heterotrophic growth, so rendering Chlamydobotrys an obligate phototroph.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amarasingham C. R., Davis B. D. Regulation of alpha-ketoglutarate dehydrogenase formation in Escherichia coli. J Biol Chem. 1965 Sep;240(9):3664–3668. [PubMed] [Google Scholar]
- Blair G. E., Ellis R. J. Protein synthesis in chloroplasts. I. Light-driven synthesis of the large subunit of fraction I protein by isolated pea chloroplasts. Biochim Biophys Acta. 1973 Aug 24;319(2):223–234. doi: 10.1016/0005-2787(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Collins N., Brown R. H., Merrett M. J. Oxidative phosphorylation during glycollate metabolism in mitochondria from phototrophic Euglena gracilis. Biochem J. 1975 Sep;150(3):373–377. doi: 10.1042/bj1500373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N., Merrett M. J. The localization of glycollate-pathway enzymes in Euglena. Biochem J. 1975 May;148(2):321–328. doi: 10.1042/bj1480321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle R. S., Dau B., Kleinkopf G. E., Huffaker R. C. Differential synthesis of ribulosediphosphate carboxylase subunits. Biochem Biophys Res Commun. 1970 Nov 9;41(3):621–627. doi: 10.1016/0006-291x(70)90058-6. [DOI] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- Givan A. L., Criddle R. S. Ribulosediphosphate carboxylase from Chlamydomonas reinhardi: purification, properties and its mode of synthesis in the cell. Arch Biochem Biophys. 1972 Mar;149(1):153–163. doi: 10.1016/0003-9861(72)90309-8. [DOI] [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., MADSEN N. B. The metabolism of C2 compounds in microorganisms. 3. Synthesis of malate from acetate via the glyoxylate cycle. Biochem J. 1958 Mar;68(3):549–557. doi: 10.1042/bj0680549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem J. 1966 Apr;99(1):1–11. doi: 10.1042/bj0990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIN R. A. Utilization of acetate by wild-type and mutant Chlamydomonas dysosmos. J Gen Microbiol. 1954 Dec;11(3):459–471. doi: 10.1099/00221287-11-3-459. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Brown R. H. Purification and Some Properties of Chlorella fusca Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Feb;55(2):360–364. doi: 10.1104/pp.55.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. Ribulose diphosphate carboxylase synthesis in euglena: increased enzyme activity after transferring regreening cells to darkness. Plant Physiol. 1975 May;55(5):890–892. doi: 10.1104/pp.55.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Lord J. M., Rowe A., Dilks S. Composition, quaternary structure, and catalytic properties of D-ribulose-1, 5-bisphosphate carboxylase from Euglena gracilis. Eur J Biochem. 1975 May;54(1):195–206. doi: 10.1111/j.1432-1033.1975.tb04129.x. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


