Abstract
The Mexican black-tailed rattlesnake Crotalus molossus nigrescens is distributed in the Mexican plateau. Its venom is known to cause hemolysis and presents fibrinogen coagulase, collagenase and fibrinolytic activities. These activities may be associated with hemostatic alterations, such as platelet aggregation, hemolysis and fibrinolysis, often described in ophidic accidents. However, the mechanisms of action of the C. m. nigrescens venom remain unclear. In this study we investigated the in vitro hemotoxic, neurotoxic, and vasculotoxic effects of the venom. We found that this venom produces two types of hemolytic responses, Oxyhemoglobin release and Methemoglobin formation. As a result of the cytotoxicity to endothelial cells produces morphological biphasic toxicity. The first step in this process is characterized by morphological changes, as well as the loss of cellular adhesion and reduction in thickness. The second phase is characterized by massive cellular aggregation and death. It also induced laminin, type IV collagen, perlecan and nidogen degradation. However, the venom did not modulate the muscular fetal and neuronal nicotinic acetylcholine receptors activity. Thus, we concluded that the C. m. nigrescens venom produced hemolysis and hemorrhages via degradation of the basement membrane components and endothelial cell cytotoxicity, but not by neurotoxicity at the receptor level in nicotinic acetylcholine receptors.
Keywords: Crotalus molossus nigrescens, hemolysis, hemorrhage, neurotoxicity, snake venom
Introduction
The Mexican black-tailed rattlesnake Crotalus molossus nigrescens is found in the Mexican plateau from Southern Chihuahua to North of Oaxaca (Lemos-Espinal and Smith, 2009), and is morphologically characterized by a black coloration in the tail. As in other venomous snakes, its venom is a complex mix of proteins, such as metalloproteinases (SVMP), serine proteinases, phospholipases A2 (PLA2), L-aminoacid oxidases (LAAO), disintegrins, C-type lectins, Cys-rich secretory proteins and other minor proteases toxins. This suggests that the toxicity of this venom is of predominantly proteolytic nature, such as fibrinogenolysis, fibrinolysis, hemorrhages and platelet aggregation (Mackessy, 2010). Crotalus molossus venom generates hemostatic alterations as platelet aggregation, hemolysis and fibrinolysis, as well as hemorrhages and necrosis (Hardy et al, 1982; Yarema and Curry, 2005).
The Mexican black-tailed rattlesnake venom is known to have protease, phospholipase, phosphodiesterase, deoxyribonuclease, fibrinogen coagulase, collagenase and fibrinolytic activities; only two toxins have been isolated, proteinase E, a 21.39kDa SVMP and 75kDa thrombin-like serineproteinase (Ramírez et al, 1990). In vitro studies demonstrate that C. m. nigrescens venom is more hemolytic than Crotalus atrox and Crotalus tigris venoms (Macias-Rodríguez et al, 2014) via plasmatic membrane degradation catalyzed by PLA2. The fibrinogen and collagen degradation previously mentioned suggest that C. m. nigrescens venom could be involved in hemorrhage formation and hemostatic alterations (Gutiérrez et al, 2005). Neurotoxicity in Crotalus genera has been described for two PLA2 toxins: i) Crotoxin, isolated from Crotalus durissus venom that can function as a presynaptic neurotoxin, inhibiting the chlorine uptake in neurons (Kattah et al, 2000), and a postsynaptic neurotoxin, modulating the nicotinic acetylcholine receptor (Bon et al, 1979); and ii) Mojave toxin, isolated from Crotalus scutulatus venom, can act as a Calcium channel inhibitor (Valdes et al, 1989) but there is no clear evidence of neurotoxicity in C. m. nigrescens venom. Thus due to a lack of data on C. m. nigrescens venom toxicity, this study was designed to investigate venom hemotoxicity, α-neurotoxicity and vasculotoxicity using in vitro techniques.
MATERIAL AND METHODS
Venom
Venom samples were obtained from 13 C. m. nigrescens specimens maintained at the Universidad Autónoma de Querétaro Herpetary. Venom extraction was performed manually as described previously (Meléndez-Martínez et al, 2014). The C. m. nigrescens venom was pooled, lyophilized and stored at -20oC until used. Protein concentration in venom was measured by Lowry protein assay (Lowry et al, 1951) using bovine serum albumin as protein standard.
Hemotoxicity
Blood collection
Blood was collected from at least three healthy, O+ blood type donors, who had not taken any medication in the previous 48hr. Blood samples were collected in BD Vacutainer® sodium heparin tubes for leukocyte cytotoxicity assay and buffered sodium citrate tubes for hemolytic activity assay. Blood collected in heparinized tubes were allowed to separate in the leukocyte rich plasma from Red Blood Cells (RBC) by gravity (Verma and Babu, 1995) and tubes were maintained at room temperature for ~40min at 45 degree inclination.
Hemolytic activity
Hemolytic activity was determined according to Das et al (2013) with some modifications. RBCs were isolated from blood collected in buffered sodium citrate tubes, washed 5 times by centrifugation at 1100xg for 15min, and resuspended in 0.9% (w/v) saline to a final concentration of 10% (v/v) RBC. Crotalus m. nigrescens venom concentrations tested (0-640μg/ml) were incubated for 24hr to give sufficient time for hemolysis, at 37°C with 37.5μl of 10% RBC to a final volume of 500μl with 0.9% (w/v) saline. Then, the tubes were centrifuged at 6400xg for 10min and supernatant was measured at 540nm for oxyhemoglobin (Oxy-Hb) release and 630nm for methemoglobin (Met-Hb) formation in a Helios Omega UV-Vis spectrophotometer (Thermo Scientific, USA). Distilled H2O was used as positive control of Oxy-Hb and H2O2 as positive control of Met-Hb.
Leukocyte cytotoxicity
Leukocyte rich plasma was washed three times at 500xg for 30min with PBS and resuspended in 5ml of DMEM (high glucose) media supplemented with 10% (v/v) fetal bovine serum and Penicillin-Streptomycin 100U/ml. Leukocyte cultures of 1x106 cells were prepared and exposed to different C. m. nigrescens venom concentrations (0-10μg/ml). The cells were incubated with 5% (v/v) CO2 at 37°C, at different time intervals (2-12hr). The cell counting was carried out in a Neubauer chamber by diluting the cells 1:1 with 0.1% (w/v) Evan’s blue dye.
α-Neurotoxicity
RP-HPLC
Lyophilized C. m. nigrescens venom was reconstituted in 0.1% (v/v) trifluoroacetic acid (TFA) and 4.5% (v/v) acetonitrile (ACN) solution. 250μg of reconstituted venom was fractioned with a GraceVydac C18 analytical reverse phase column (218TP54, 5μm, 4.6x250mm) equipped with a Vydac C18 guard column (218GK54, 5μm, 4.6x10mm). Solution A was 0.1% (v/v) aqueous TFA, and solution B was 0.085% (v/v) TFA in 90% aqueous ACN. Upon injection of the sample, a linear gradient from 5 to100% solution during 95min was applied at flow rate of 1ml/min. Venom fractions were manually collected and monitored at 280nm, the solvent was eliminated by vacuum concentration, dried fractions were dissolved in water and protein concentration was measured at 280nm by spectrophotometry.
Phospholipase A2 assay
The PLA2 enzymatic activity was performed in egg yolk plates. A 10% (v/v) egg yolk solution was prepared using phospholipase buffer (0.1M Tris-HCl, pH 7.5, 5mM CaCl2, and 0.5%, v/v, Triton X-100), stirring by 10min and centrifuged at 180xg for 5min to eliminate the non-dissolved egg yolk. The solution was poured into plastic Petri dishes with final concentration of 1% (v/v) of egg yolk solution with 0.5% (w/v) agarose, 0.1M Tris-HCl, 1mM CaCl2, 0.04% (w/v) rhodamine 6G and 0.5% (v/v) Triton-X100, pH 7.95. Crotalus m. nigrescens crude venom and each fraction collected by RP-HPLC were diluted in 5μl of H2O (~200μg of toxin) and placed into wells in egg yolk plates at 37°C by 24hr. PLA2 activity was detected visually by peripheral clear ring areas surrounding wells and distilled H2O was used as negative control. Comparison of clear halos was measured in millimeters using UV transillumination (Gel Logic 200 Imagin System, Kodak, Rochester, NY, USA) and data were transformed to Phospholipase Units (PhU) in mm/μg.
Nicotinic acetylcholine receptor modulation assay
Electrophysiology was performed in Xenopus laevis oocytes expressing mouse fetal muscle (α1β1γδ) or human neuronal (α7) nicotinic acetylcholine receptor (nACh) subtypes receptors. Two electrode voltage-clamp recordings were performed in a 30μl recording chamber, clamping the oocyte at -70 mV, and gravity perfused with ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5mM HEPES, pH 7.2). Acetylcholine (ACh) gated currents were generated by 1sec pulses/min of 1-5μM ACh for α1β1γδ and 200μM ACh for α7 nAChR subtypes. Crotalus m. nigrescens venom and each fraction obtained by RP-HPLC were dissolved in 5μl (~200μg of toxin) of ND96. Venom fractions were allowed to equilibrate with the receptors in a static bath for 5min before pulsing with ACh. Subsequently, ACh pulses and ND96 perfusion were restarted to permit toxin dissociation from the receptors.
Vasculotoxicity
In vitro proteolysis of basement membrane
This was carried out as described previously (Escalante et al, 2011) using Matrigel™ (BD, United States) as basement membrane-like substrate. Matrigel was incubated with the venom in a venom:substrate ratio of 1:5 (w/w), for 15min, 1hr and 2hr at 37°C. Reactions were stopped by adding 5x SDS-PAGE sample loading buffer, then they were stored at -20°C until its utilization. Matrigel proteolysis was analyzed using a 4-12% (w/v) SDS-PAGE under reducing conditions.
Endothelial cells cytotoxicity and morphology changes
Median cytotoxic concentration (CC50) induced by C. m. nigrescens venom was assessed by Lactate dehydrogenase (LDH) release into the media culture. Porcine aortic endothelial (PAE) cells were kindly donated by Dr. Manuel Miranda-Arango (University of Texas at El Paso). The cells were grown in 5% (v/v) CO2 at 37°C to 80% confluence and were treated with C. m. nigrescens venom (1-20μg/ml) diluted in F-12 media supplemented with 10% (v/v) fetal bovine serum and 100µg/ml gentamicin for 24hr. After venom incubation, media culture was collected and LDH release was measured by CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, United States) according to the instructions of the manufacturer. The maximum LDH release was established by cell lysis using a Sonic Dismembrator 60 (Fisher Scientific, United States). Images of cell morphology were taken in an Axio Vert.A1 Microscope (Carl Zeiss, Germany).
Statistical analysis
For experiments with multiple repeated data sets, means and standard errors were calculated and ANOVA test (P<0.05) was applied.
RESULTS
Hemotoxicity
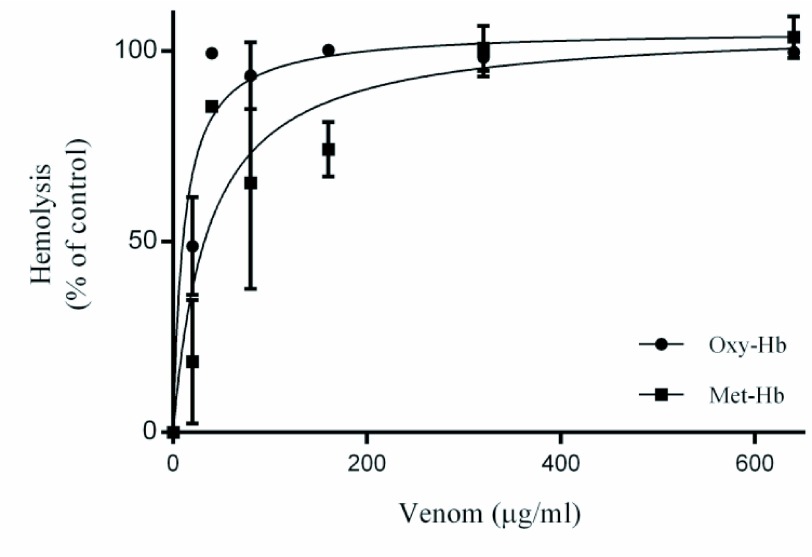
In hemolysis assays we found that C. m. nigrescens venom produces Oxy-Hb release and Met-Hb formation (Figure 1). Oxy-Hb showed a median hemolytic concentration of 11.14±3.24μg/ml and generated a full hemolysis at 40μg/ml of C. m. nigrescens venom; Met-Hb showed a 50% formation at a 36.26±13.30μg/ml of venom concentration and generated a maximum formation at 320μg/ml of C. m. nigrescens venom.
Figure 1.
β- and α-hemolytic activity of C. m. nigrescens venom. Erythrocytes were treated with different concentrations of C. m. nigrescens venom (0-640μg/ml) for 24hr. Data are represented as the mean of three independent experiments with their respective standard error.
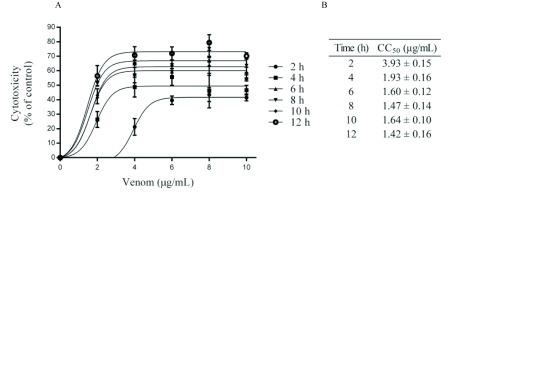
In order to investigate cell damage induced by C. m. nigrescens venom concentration we measured cell viability of human leukocyte cultures exposed in different concentrations of venom (0-10μg/ml) and different time intervals (2-12hr). Our results indicated that leukocyte cytotoxicity increased in time- and concentration-dependent manner. In the first 2hr of venom incubation, the cytotoxicity had the biggest increase with a 40% (Figure 2A) this result can be confirmed with CC50 values, where at 2hr was of 3.93±0.15μg/ml and decreasing through time until CC50 decreased to 1.42±0.16μg/ml in 12hr incubation (Figure 2B).
Figure 2.
C. m. nigrescens venom cytotoxicity on human Leukocytes. A. Leukocytes were treated with different concentrations of C. m. nigrescens venom (0-10μg/ml) at different time intervals (2-12hr). B. CC50 (μg/ml) are shown for every time interval. Data are represented as the mean of three independent experiments with their respective standard error.
α-Neurotoxicity
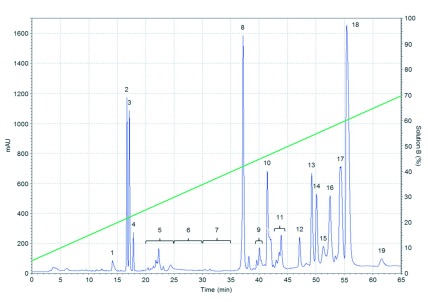
A total of 19 fractions were collected from C. m. nigrescens venom by RP-HPLC, as are shown in Figure 3. Where the fractions 8, 9, 10, 11, 17, and 18 show specific phospholipase activity, and all displayed a higher activity than the C. m. nigrescens crude venom. Fractions 9, 10 and 11 had the highest phospholipase activity with 486.49, 237.30 and 738 PhU respectively. Followed by the fractions 8, 17, and 18 with 71.91, 21.80 and 25.92 PhU respectively. C. m. nigrescens venom had a phospholipase activity of 14.75 PhU.
Figure 3.
Chromatographic fractionation of C. m. nigrescens venom by means of an analytical RP C18 HPLC column. Fractions were eluted using a linear gradient of 5-100% solution B (green line) at a flow rate of 1ml/min.
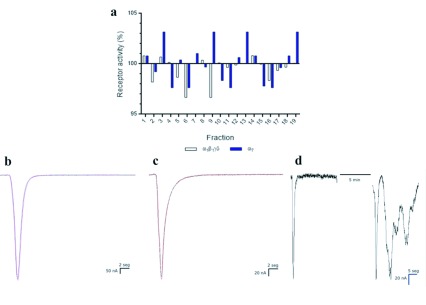
The nAChR subtypes tested in this work (α1β1γδ and α7) presented a minimal or null modulation by C. m. nigrescens venom and the RP-HPLC fractions (Figure 4A). In α1β1γδ nAChR, the fractions 1, 2, and 14 generated less than 1% activity increase of the current (Figure 4B), and the fractions 6 and 9 generated a 3.33% receptor current decrease. In α7 nAChR, the fractions 3, 9, 13 and 19 (Figure 4C) generated 3.16% receptor current increase, and the fractions 4, 6, 11, and 16 generated 2.37% receptor current decrease. The C. m. nigrescens venom did not generate a modulation in both nAChR subtypes currents, but the resting potential of the oocyte after ACh pulses was not reconstituted as control after venom incubation (Figure 4D).
Figure 4.
Modulatory activity of C. m. nigrescens venom and its RP-HPLC fractions on nAChR expressed on Xenopus leavis oocytes. A. Percentage response was defined as current amplitude after application of C. m. nigrescens venom RP-HPLC fractions prior toxin application. B. Representative current trace elicited by one second pulse of ACh in muscle (α1β1γδ) nAChR (black trace) and, in presence of venom fraction 1 (magenta trace). C. Current trace elicited by one second pulse of ACh in neuronal (α7) nAChR (black trace) and, in presence of fraction 19 in neuronal (α7) nAChR (red trace). D. C. m. nigrescens crude venom effect in neuronal (α7) nAChR, left trace represents control current elicited prior venom application. After 5 min equilibration period, ACh pulses resumed and ND96 solution was perfused over the oocyte between ACh pulses, with a loss of membrane potential.
Vasculotoxicity
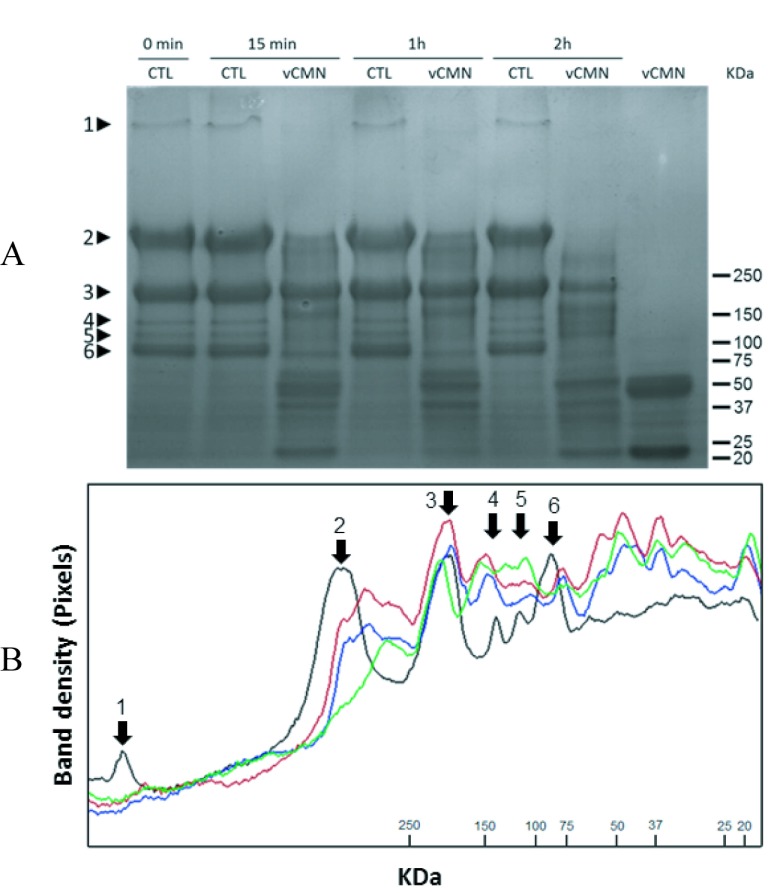
When Matrigel was exposed to C. m. nigrescens venom, we observed degradation of many basement membrane constituents, depending of exposure time (Figure 5, A and B). The molecular masses of the different hydrolysates were calculated directly from the SDS-PAGE through Rf (Retardation factor). As control, six bands were observed in intact Matrigel: perlecan (>1,000 kDa); laminin α1 (420 kDa); laminin β1, laminin γ1, and type IV collagen (190 kDa); and nidogen (130, 110, and 90 kDa). 15min and 1hr incubation with C. m. nigrescens venom resulted in degradation of perlecan, all nidogen bands, and partial degradation of laminin α1, producing hydrolysis products of 370, 144, 100, 61, 54, 49, 40 and 21 kDa. After 2hr incubation all proteins of Matrigel were proteolyzed, except the collagen IV (190 kDa) band, which was partially degraded, producing hydrolysis products of 320, 160, 144, 132, 110, 82, 68, 56, 42, 36, and 21 kDa.
Figure 5.
Matrigel hydrolysis by C. m. nigrescens venom. A. SDS-PAGE of Matrigel incubated either with 0.9% (w/v) saline as control (CTL) or with venom (CMNv) during 0min, 15min, 1hr and 2hr. Lane 9 shows 50μg of crude CMNv. B. Densitogram correspondent to Figure 5A. Incubation times are represented in lines: control (black), 15min (red), 1hr (blue), and 2hr (green). Matrigel proteins are indicated by arrows: perlecan (1), laminin α1 (2), laminin β1, laminin γ1, type IV collagen (3) and nidogens (4, 5, and 6).
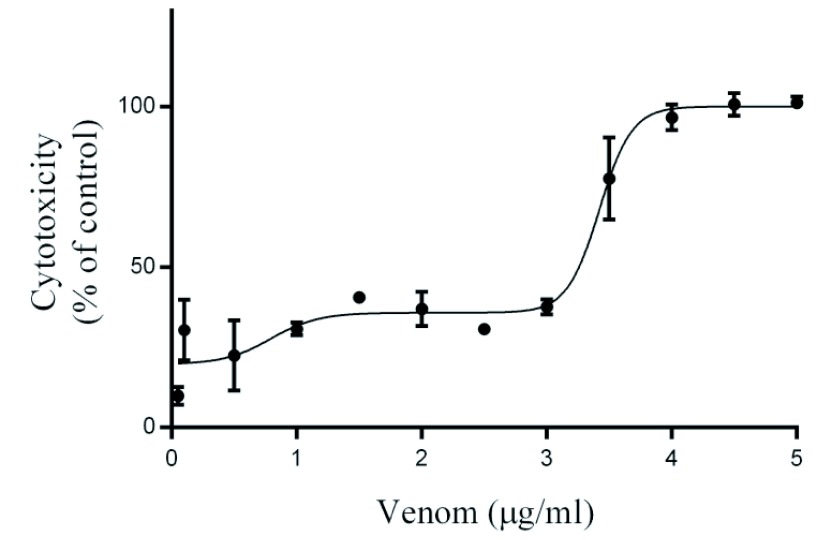
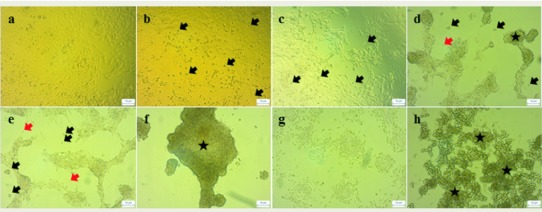
Crotalus m. nigrescens venom produced biphasic cytotoxicity in PAE cells: The first phase CC50 was 0.79±0.29μg/mL (Figure 6) and is related to morphological changes in PAE cells as is shown in Figure 7B, where we observed cell detachment from substrate, starting from 0.1μg/ml and cell thickness is reduced, becoming thinner than the control (Figure 7D and E); the second phase CC50 was 3.42±0.06μg/ml (Figure 6), which was morphologically observed as an aggregation of detached cells and cellular debris (Figure 7F, G and H).
Figure 6.
C. m. nigrescens venom cytotoxicity on PAE cells. PAE cells were treated with different concentrations of C. m. nigrescens venom (0-5μg/ml) during 24hr. Data are represented as the mean of three independent experiments with their respective standard error.
Figure 7.
Morphological changes induced by C. m. nigrescens venom on PAE cells. Optic microscopy of PAE cells treated with different concentrations of C. m. nigrescens venom: a = 0.0 μg/ml, b = 0.1μg/ml, c = 0.5μg/ml, d = 1.0μg/ml, e = 2.0μg/ml, f = 3.0μg/ml, g = 4.0μg/ml, and h = 5.0μg/ml. Symbols: death cells (black arrow), cellular thickness decrease (red arrow), cellular aggregation (star).
DISCUSSION
Hemotoxicity
Hemolysis produced by C. m. nigrescens venom is already described (Macias-Rodríguez et al, 2014); even when the experimental procedures were different, the C. m. nigrescens venom had a concentration-dependent effect on erythrocytes, as we observe in our experiments. In this work, we demonstrate that the C. m. nigrescens venom generate a complex hemolytic process in a concentration-dependent manner, releasing Oxy-Hb release at low venoms concentrations and increasing Met-Hb when venom concentration is increased. There is not enough information about the mechanism of hemolytic process of this venom, but Oxy-Hb could be generated by destabilization and degradation of erythrocyte membrane via PLA2 (Doley et al, 2010), resulting in hemoglobin release. As a consequence, free hemoglobin can react with LAAO-produced H2O2, resulting in oxidation of heme group and generating Met-Hb (Sunitha et al, 2015).
Hemolytic process during envenomation can cause oxygen transportation problems. Methemoglobin, a highly pro-oxidant molecule, can induce serious health problems in bite victims in the form of proinflammatory effect and oxidative stress. This can lead to long-term complications, such as thrombocytopenia, renal abnormalities and persistent local tissue degradation (Sunitha et al, 2015). The leukocyte cytotoxicity could be a consequence of the disruption of the cellular membrane catalyzed by PLA2, as we discussed previously in the hemolytic process. Comparing with other cell types (erythrocytes and PAE cells) used to evaluate the effect of C. m. nigrescens venom, leukocytes had a higher susceptibility to the venom (lower CC50 of 1.42±0.16μg/ml) than in erythrocytes (CC50 of 11.14±3.24 μg/ml) and PAE cells (CC50 of 3.42±0.06μg/ml). Also, leukocytes showed damage starting just after 2hr incubation, whereas in the erythrocytes and PAE cells no damage was observed. Even when the susceptibility is higher, none of the tested time intervals reached 100% of cytotoxicity. This phenomenon could be due to the activity of some isoforms of PLA2 induce adhesion to the endothelium instead of cytotoxicity (Zambelli et al, 2008).
α-Neurotoxicity
Crotalus m. nigrescens venom and its fractions did not modulate the current activity on fetal and α7 nAChR tested in this work. Even the fractions with phospholipase activity that in other species are found to produce α-neurotoxicity (such as crotoxin in Crotalus durissus terrificus venom (Sampaio et al, 2010), thus being similar to PLA2 RP-HPLC fractions of C. m. nigrescens venom) had no neurotoxic effect in nAChR.
C. m. nigrescens venom altered the resting potential of the oocytes via plasmatic membrane destabilization. This destabilization could be mediated by membrane peroxidation due to L-amino acid oxidase H2O2 production (Torii et al, 1997) leading to the cell death process (necrosis or apoptosis) (Suhr and Kim, 1996). Also, PLA2 could contribute to the phenomena via phospholipid degradation (Doley et al, 2010).
Vasculotoxicity
Basement membrane-like components could be hydrolyzed by the catalytic activity of P-I and P-III SVMP. In other snake venoms, it was demonstrated that P-I SVMP has affinity for all the basement membrane previously listed (Escalante et al, 2011). On the other hand, P-III SVMP has an affinity for laminin β1 and laminin γ1 at longer periods of time than P-I SVMP (Escalante et al, 2006). The products of degradation generated by C. m. nigrescens venom could corresponds to laminin γ1 fragments (144 and 56 kDa bands), laminin α1 fragments (56 kDa band), and nidogen fragments (100, 56, 54, 42 and 41 kDa bands). The difference in degradation time of the components of basement membrane suggests that the hemorrhage formation could be generated in two steps: the first step of hemorrhage formation could be led by early degradation of perlecan and nidogens, forming dissociation of the basement membrane complex conformed by laminin and type IV collagen networks (Gutiérrez et al, 2005); the second step could be generated as a consequence of laminin and type IV collagen degradation, which could be related to endothelial cell detachment (Tanjoni et al, 2005). Biphasic cytotoxic behavior of PAE cells, in contrast to morphology, denotes that the cytotoxicity could occur in two steps. The first step appears at low concentration of C. m. nigrescens venom (0.05-3.0μg/ml), where cytotoxicity is minimal but cell detachment occurs. This observation may have been due to SVMP and disintegrins which block the interaction of integrins with basement membrane components (Wu et al, 2003; Suntravat et al, 2015), or in this case, the interaction with the substrate. Also, in this interaction SVMP or disintegrin with integrins cause the cytoskeleton destabilization, generating the cell thickness observed in Figure 7D and E (see Gutiérrez et al, 2005). The second step occurs at higher C. m. nigrescens venom concentrations (>3.0μg/ml) generating cell death; there are two possible scenarios for this: i) oxidative stress via LAAO H2O2 production (Samel et al, 2006), or ii) cellular membrane hydrolysis by PLA2 (Sampaio et al, 2010). Furthermore, other studies suggest that under these conditions the P-I SVMP could produce apoptosis anoikis (Tanjoni et al, 2005; Baldo et al, 2008).
CONCLUSIONS
C. m. nigrescens venom produced both Met-Hb formation and leukocyte cytotoxicity in a concentration-dependent manner. This venom and its HPLC-fractions did not generate α-neurotoxicity in muscle (α1β1γδ) and neuronal (α7) nAChR subtypes. Finally, this venom hydrolyzed basement membrane-like substrate with a higher preference for nidogens and perlecan, and triggered PAE cells cytotoxicity.
ACKNOWLEDMENTS
This project was supported by Programa Integral de Fortalecimiento Institucional, SEP (PIFI) for Academic Groups, Universidad Autónoma de Ciudad Juárez. Electrophysiology experiments were partially supported by Consejo Nacional de Ciencia y Tecnología (CONACYT), Grant 153915. Also we want to acknowledge to Universidad Autónoma de Querétaro Herpetary (INE/CITES/DGVS-CR-IN-0619-QRO-00) for venom donation, Dr Edna Rico from Toxicology Lab of UACJ for donate the Matrigel used, Dr Manuel Miranda-Arango from Border Biomedical Research Center at UTEP for cell PAE cells donation and Dr Angel Diaz for his criticism in this paper.
ABBREVIATIONS
- Ach
Acetylcholine
- ACN
Acetonitrile
- CC50
Median cytotoxic concentration
- DMEM
Dulbecco's Modified Eagle Medium
- LAAO
L-aminoacid oxidase
- LDH
Lactate dehydrogenase
- nAChR
Nicotinic acetylcholine receptor
- PAE
Porcine aortic endothelial cells
- PhU
Phospholipase units
- PLA2
Phospholipase A2
- RBC
Red blood cells
- RP-HPLC
Reverse phase-High Performance Liquid Chromatography
- SVMP
Snake venom metalloproteinases
- TFA
Trifluoroacetic acid
COMPETING INTERESTS
None declared.
References
- 1.BON C., CHANGEUX J.P., JENG T.W., FRAENKEL‐CONRAT H. Postsynaptic Effects of Crotoxin and of Its Isolated Subunits. European Journal of Biochemistry. 1979;99(3):471–482. doi: 10.1111/j.1432-1033.1979.tb13278.x. [DOI] [PubMed] [Google Scholar]
- 2.Braga M. D. M., Martins A. M., Amora D. N., de Menezes D. B., Toyama M. H., Toyama D. O., Marangoni S., Alves C. D., Barbosa P. S. F., de Sousa Alves R., Fonteles M. C., Monteiro H. S. A. Purification and biological effects of l-amino acid oxidase isolated from Bothrops insularis venom. Toxicon. 2008;51(2):199–207. doi: 10.1016/j.toxicon.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Das D., Urs N., Hiremath V., Vishwanath B. S., Doley R. Biochemical and biological characterization of Naja kaouthia venom from North-East India and its neutralization by polyvalent antivenom. J Venom Res. 2013;4:31–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Doley R., Zhou X., Kini M. Snake venom phospholipase A2 enzymes. In: Mackessy SP (Ed) Handbook of venoms and toxins of reptiles. Florida, United States of America: CRC Press, 1st edition; 2009. pp. 174–205. [Google Scholar]
- 5.Escalante T., Shannon J. D., Moura-da-Silva A. M., Gutiérrez J. M., Fox J. W. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Archives of Biochemistry and Biophysics. 2006;455(2):144–153. doi: 10.1016/j.abb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Escalante T., Ortiz N., Rucavado A., Sanchez E. F., Richardson M., Fox J. W., Gutiérrez J. M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez J. M., Rucavado A., Escalante T., Díaz C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon. 2005;45(8):997–1011. doi: 10.1016/j.toxicon.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Hardy D. L., Jeter M., Corrigan J. J., Jr. Envenomation by the northern blacktail rattlesnake (Crotalus molossus molossus): report of two cases and the vitro effects of the venom on fibrinolysis and platelet aggregation. Toxicon. 1982;20(2):487–493. doi: 10.1016/0041-0101(82)90012-5. [DOI] [PubMed] [Google Scholar]
- 9.Kattah L. S., Santoro M. M., Diniz C. R., De Lima M. E. Crotoxin, the major toxin from the rattlesnake Crotalus durissus terrificus, inhibits 3H-choline uptake in guinea pig ileum. Brazilian Journal of Medical and Biological Research. 2000;33(9):1093–1097. doi: 10.1590/s0100-879x2000000900017. [DOI] [PubMed] [Google Scholar]
- 10.Lemos-Espinal J. A., Smith H. M. Anfibios y reptiles del estado de Chihuahua, México, Universidad Nacional Autónoma de México and Comisión Nacional para el Conocimiento y uso de la Biodiversidad, México City, México. 1st edition. 2009:222–223. [Google Scholar]
- 11.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Macías-Rodríguez E. F., Martínez-Martínez A., Gatica-Colima A., Bojórquez-Rangel G., Plenge-Tellechea L. F. Análisis comparativo de la actividad hemolítica entre las subespecies Crotalus molossus molossus y Crotalus molossus nigrescens. Revista Bio Ciencias. 2014;2:302–312. [Google Scholar]
- 13.Mackessy S. P. The field of reptile toxinology: snakes, lizards, and their venoms. In: Mackessy SP (Ed) Handbook of venoms and toxins of reptiles, 1st edition. Florida, United States of America: CRC Press; 2000. pp. 3–23. [Google Scholar]
- 14.Meléndez-Martínez D. L., Macias-Rodríguez E., Vargas-Caraveo A., et al. Capillary damage in the area postrema by venom of the black-tailed rattlesnake (Crotalus molossus molossus) J Venom Res. 2014;5:1–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Ramírez G. A., Fletcher P. L., Jr., Possani L. D. Characterization of the venom from Crotalus molossus nigrescens Gloyd (black tail rattlesnake): Isolation of two proteases. Toxicon. 1990;28(3):285–297. doi: 10.1016/0041-0101(90)90064-e. [DOI] [PubMed] [Google Scholar]
- 16.Samel M., Vija H., Rönnholm G., Siigur J., Kalkkinen N., Siigur E. Isolation and characterization of an apoptotic and platelet aggregation inhibiting l-amino acid oxidase from Vipera berus berus (common viper) venom. Biochimica et Biophysica Acta - Proteins and Proteomics. 2006;1764(4):707–714. doi: 10.1016/j.bbapap.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Sampaio S. C., Hyslop S., Fontes M. R., Prado-Franceschi J., Zambelli V. O., Magro A. J., Brigatte P., Gutierrez V. P., Cury Y. Crotoxin: Novel activities for a classic β-neurotoxin. Toxicon. 2010;55(6):1045–1060. doi: 10.1016/j.toxicon.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Suhr S. M., Kim D. S. Identification of the snake venom substance that induces apoptosis. Biochemical and Biophysical Research Communications. 1996;224(1):134–139. doi: 10.1006/bbrc.1996.0996. [DOI] [PubMed] [Google Scholar]
- 19.Sunitha K., Hemshekhar M., Thushara R. M., Santhosh M. S., Sundaram M. S., Kemparaju K., Girish K. S. Inflammation and oxidative stress in viper bite: An insight within and beyond. Toxicon. 2015;98:89–97. doi: 10.1016/j.toxicon.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Suntravat M., Barret H. S., Jurica C. A., et al. Recombinant disintegrin (r-Cam-dis) from Crotalus adamanteus inhibits adhesion of human pancreatic cancer cell lines to laminin-1 and vitronectin. J Venom Res. 2015;6:1–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanjoni I., Weinlich R., Della-Casa M. S., Clissa P. B., Saldanha-Gama R. F., De Freitas M. S., Barja-Fidalgo C., Amarante-Mendes G. P., Moura-Da-Silva A. M. Jararhagin, a snake venom metalloproteinase, induces a specialized form of apoptosis (anoikis) selective to endothelial cells. Apoptosis. 2005;10(4):851–861. doi: 10.1007/s10495-005-2945-1. [DOI] [PubMed] [Google Scholar]
- 22.Torii S., Naito M., Tsuruo T. Apoxin I, a novel apoptosis-inducing factor with L-amino acid oxidase activity purified from western diamondback rattlesnake venom. Journal of Biological Chemistry. 1997;272(14):9539–9542. doi: 10.1074/jbc.272.14.9539. [DOI] [PubMed] [Google Scholar]
- 23.Valdes J. J., Thompson R. G., Wolff V. L., Menking D. E., Rael E. D., Chambers J. P. Inhibition of calcium channel dihydropyridine receptor binding by purified Mojave toxin. Neurotoxicology and Teratology. 1989;11(2):129–133. doi: 10.1016/0892-0362(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 24.Verma R. S., Babu A. Human Chromosomes Principles and Techniques, 2nd edition. New York, United States of America: McGraw-Hill Inc; 1995. [Google Scholar]
- 25.Wu W. B., Peng H. C., Huang T. F. Disintegrin causes proteolysis of β-catenin and apoptosis of endothelial cells: Involvement of cell - Cell and cell - ECM interactions in regulating cell viability. Experimental Cell Research. 2003;286(1):115–127. doi: 10.1016/s0014-4827(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 26.Yarema M. C., Curry S. C. Envenomation by the Northern Blacktail rattlesnake (Crotalus molossus molossus): Case report. Pediatric Emergency Care. 2005;21(1):40–42. doi: 10.1097/01.pec.0000150989.03981.06. [DOI] [PubMed] [Google Scholar]
- 27.Zambelli V. O., Sampaio S. C., Sudo-Hayashi L. S., Greco K., Britto L. R. G., Alves A. S., Zychar B. C., Gonçalves L. R. C., Spadacci-Morena D. D., Otton R., Della-Casa M. S., Curi R., Cury Y. Crotoxin alters lymphocyte distribution in rats: Involvement of adhesion molecules and lipoxygenase-derived mediators. Toxicon. 2008;51(8):1357–1367. doi: 10.1016/j.toxicon.2008.03.004. [DOI] [PubMed] [Google Scholar]