Abstract
In the past 5 years, immunomodulatory antibodies have revolutionized cancer immunotherapy. CD137, a member of the tumor necrosis factor receptor superfamily, represents a promising target for enhancing antitumor immune responses. CD137 helps regulate the activation of many immune cells, including CD4+ T cells, CD8+ T cells, dendritic cells, and natural killer cells. Recent studies indicate that the antitumor efficacy of therapeutic tumor-targeting antibodies can be augmented by the addition of agonistic antibodies targeting CD137. As ligation of CD137 provides a costimulatory signal in multiple immune cell subsets, combination therapy of CD137 antibody with therapeutic antibodies and/or vaccination has the potential to improve cancer treatment. Recently, clinical trials of combination therapies with agonistic anti-CD137 mAbs have been launched. In this review, we discuss the recent advances and clinical promise of agonistic anti-CD137 monoclonal antibody therapy.
Introduction
Antibody-based strategies for cancer treatment have dramatically advanced in the past 20 years (1). Since rituximab was approved as the first monoclonal antibody (mAb) for the treatment of cancer in 1997 (2, 3), several mAbs have become standard of care for the treatment of both solid tumors and hematologic malignancies (Table 1). Most of the approved mAbs (e.g., rituximab, trastuzumab, and cetuximab) target tumor-associated antigens on the surface of cancer cells and inhibit cell growth. Although several effective antibodies have emerged, long-term, durable responses remain elusive, and resistance and relapse remain major problems (4–6). Immunomodulatory antibodies have revolutionized cancer immunotherapy and helped garner the breakthrough distinction (7–11). In 2011, the FDA approved the cytotoxic T-lymphocyte–associated protein 4 (CTLA-4)–specific mAb, ipilimumab, for the treatment of metastatic melanoma, representing a major milestone in cancer immunotherapy (12). The second FDA-approved immunomodulatory agent, pembrolizumab, is anti–programmed cell death 1 (PD-1, PDCD1 or CD279) mAb, which was approved in 2014 (13). In the same year, blinatumomab, a novel bispecific T-cell engager (BiTE) antibody specific to CD19 and CD3, was approved for patients with acute lymphoblastic leukemia (14). Most cancer immunotherapy strategies stimulate the patient’s immune system to overcome immunosuppression induced by tumor cells and generate an antitumor immune response. The clinical data and recent FDA approvals validate mAb-mediated cancer immunotherapy as a valuable therapeutic strategy.
Table 1.
Therapeutic antibodies approved in the United States
| Approval | Antibody (trade name; company) | Type | Target | Tumor Type |
|---|---|---|---|---|
| 1997 | Rituximab (Rituxan; Genentech) | Chimeric IgG1 | CD20 | NHL, CLL |
| 1998 | Trastuzumab (Herceptin; Genentech) | Humanized IgG1 | HER2 | Breast, gastric |
| 2000 | Gemtuzumab ozogamicin (Mylotarg; Wyeth Pharmaceuticals) | Humanized IgG4, calicheamicin | CD33 | AML |
| 2001 | Alemtuzumab (Campath; Ilex Pharmaceuticals) | Humanized IgG1 | CD52 | CLL |
| 2002 | 90Y-labeled ibritumomab tiuxetan (Zevalin; Spectrum Pharmaceuticals) | Murine IgG1, tiuxetan | CD20 | NHL |
| 2003 | 131I-labeled tositumomab (Bexxar; GlaxoSmithKline) | Murine IgG2, tositumomab | CD20 | NHL |
| 2004 | Cetuximab (Erbitux; Bristol-Myers Squibb) | Chimeric IgG1 | EGFR | Colon, head and neck |
| 2004 | Bevacizumab (Avastin; Genentech) | Humanized IgG1 | VEGF | Colon, NSCLC, glioblastoma, kidney, cervix, ovarian |
| 2006 | Panitumumab (Vectibix; Amgen) | Human IgG2 | EGFR | Colon |
| 2009 | Ofatumumab (Arzerra; Genmab) | Human IgG1 | CD20 | CLL |
| 2011 | Brentuximab vedotin (Adcetris; Seattle Genetics) | Chimeric IgG1, MMAE | CD30 | Hodgkin lymphoma |
| 2011 | Ipilimumab (Yervoy; Bristol-Myers Squibb) | Human IgG1 | CTLA4 | Melanoma |
| 2012 | Pertuzumab (Perjeta; Genentech) | Humanized IgG1 | HER2 | Breast |
| 2013 | Ado-trastuzumab emtansine (Kadcyla; Genentech) | Humanized IgG1, DM1 | HER2 | Breast |
| 2014 | Pembrolizumab (Keytruda; Merck) | Humanized IgG4 | PD-1 | Melanoma |
| 2014 | Ramucirumab (Cyramza; Eli Lilly) | Human IgG1 | VEGFR2 | Gastric, NSCLC |
| 2014 | Blinatumomab (Blincyto; Amgen) | Murine bispecific tandem scFv | CD19, CD3 | ALL |
| 2014 | Nivolumab (OpDivo; Bristol-Myers Squibb) | Human IgG4 | PD-1 | Melanoma, NSCLC |
| 2015 | Dinutuximab (Unituxin; United Therapeutics) | Chimeric IgG1 | GD2 | Neuroblastoma |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia, DM1, derivative of maytansine 1; GD2, glycolipid disialoganglioside 2; MMAE, monomethyl auristatin E; NHL: non-Hodgkin lymphoma; NSCLC, non–small cell lung cancer.
In addition to checkpoint blockade agents such as ipilimumab and pembrolizumab, agents targeting the tumor necrosis factor (TNF) superfamily of costimulatory receptors have entered development (8). CD137 is one of the TNF receptor family targets that have advanced into clinical trials. CD137 regulates many immune cells, including CD4+ and CD8+ T cells, regulatory T cells (Treg), dendritic cells (DC), and natural killer (NK) cells (15, 16). Recent studies indicate that the addition of anti-CD137 mAbs can augment the antitumor efficacy of immunomodulatory antibodies.
CD137
CD137 (4-1BB) or TNF receptor superfamily member 9 (TNFRSF9) is a costimulatory receptor that belongs to the TNF receptor superfamily (9, 16, 17). The cDNA of CD137 was cloned in 1989 as an inducible gene from stimulated T cells (18). Follow-up studies showed that CD137 is also detectable on Tregs, DCs, and NK cells. The functional role of CD137 in enhancing cytotoxic T-cell responses was established in 1997, and soon anti-CD137 mAbs were being explored as cancer therapies (19). Melero and colleagues (20) first reported that the administration of anti-CD137 mAbs could eradicate established large tumors in mice, including the poorly immunogenic Ag104A sarcoma and the highly tumorigenic P815 mastocytoma. The immune response induced by anti-CD137 mAbs was shown to be mediated by CD8+ cells and accompanied by a marked augmentation of tumor-selective cytolytic T-cell activity. CD137 signaling also promotes some CD4+ helper T-cell functions that facilitate a CD8+ CTL response. Interestingly, the efficacy of anti-CD137 mAbs was long lasting and generated memory responses as mice survived rechallenge with the same tumor. The role of CD137 in antitumor responses was also demonstrated in CD137−/− mice in the B16F10 melanoma model (21). The knockout mice displayed increased metastasis in the lungs and shorter survival time compared with wild-type mice. Anti-CD137 mAbs elicit several immune responses on different types of immune cells. The mechanism of anticancer effects mediated by these cells is described below (Fig. 1). In addition, the role of CD137 signaling has been studied in several autoimmune processes (16), including rheumatoid arthritis, experimental autoimmune encephalomyelitis, and systemic lupus erythematosus. These studies showed significant protection against the autoimmune disorders. This dual immunoregulatory activity of CD137 offers the possibility to enhance antitumor activity without autoimmune side effects associated with immunotherapy approaches.
Figure 1.
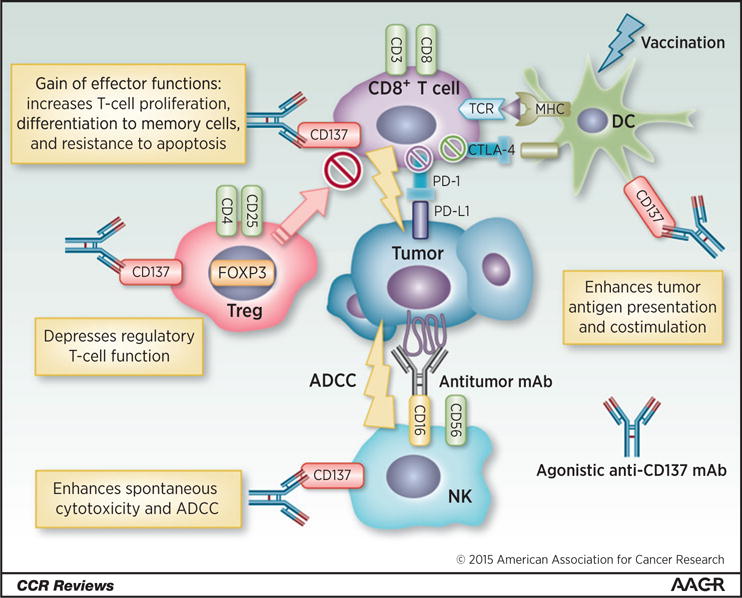
Immunomodulatory mechanisms of CD137. CD137 is expressed in several immune cells. Agonistic anti-CD137 mAb increases T-cell proliferation, differentiation to memory cells, and resistance to apoptosis in CD8+ T cells. In addition, anti-CD137 mAb can depress Treg function. In DCs, anti-CD137 mAb with vaccination enhances tumor antigen presentation and costimulation to increase the functions of antitumor CTLs. One of the primary mechanisms of antitumor activity of mAbs is antibody-dependent cell-mediated cytotoxicity (ADCC). On NK cells, stimulation of CD137 enhances ADCC. Agonistic anti-CD137 mAb stimulates CD8+ T cells, Tregs, DCs, and NK cells to induce potent antitumor immune response. MHC, major histocompatibility complex; TCR, T-cell receptor.
Costimulation through CD137
T cells
The immune response induced by anti-CD137 mAbs is mediated by both CD8+ and CD4+ T cells and is accompanied by a significant increase in tumor-selective cytolytic T-cell activity, including increased T-cell proliferation, resistance to apoptosis, and increased IFNγ secretion (16). Several TNF receptors, including CD137, OX40 (also known as TNFSF4), glucocorticoid-induced TNFR-related protein (GITR or TNFRSF18), and CD30 (TNFRSF8), function primarily as costimulatory molecules for T-cell activation (7, 8). One of the best-characterized costimulatory activities in T cells is mediated by CD137. In vitro studies showed that CD137 agonistic antibody can costimulate both CD4+ and CD8+ T cells and induce IL2 and IL8 secretion by DCs and macrophages, leading to enhanced T-cell proliferation and cytokine secretion (22). Anti-CD137 therapy was ineffective in B6 mouse embryo C3 tumors, TC-1 lung carcinoma, and B16.F10 melanoma models, when CTLs were depleted (23). In melanoma tumor models, anti-CD137 antibodies not only prevented activation-induced cell death but also augmented CD8+ T-cell proliferative potential and enhanced cytolytic activity against tumor cells (24). In addition, costimulation through CD137 and OX40 activates Akt to promote cell cycling through regulation of cyclins and cyclin-dependent kinases (25).
Regulatory T cells
Regulatory T cells (forkhead box P3 (FOXP3)+ or CD4+CD25+) downregulate the functions of T cells to prevent autoimmunity. They also suppress the cytotoxic response of T cells, which leads to immune tolerance to cancer. Recently, we have demonstrated that surface expression of both OX40 and CTLA-4 is limited to the tumor-specific Treg subset (26). Local immunomodulation by the injection of anti-OX40 and anti–CTLA-4 mAbs into one tumor elicited a potent antitumor immune response that led to eradication of distant tumors. Thus, Tregs may control local tumor immunomodulation and also mediate systemic tumor eradication. CD137 is also expressed on Tregs (15). Curran and colleagues (27) and Guo and colleagues (28) reported that anti-CD137 mAb reduced Treg infiltration in tumors. Guo and colleagues (28) asserted that anti-CD137 mAb directly reduced Tregs. Curran and colleagues (27) claimed that Tregs were reduced as a percentage of the tumor T-cell pool that did not necessarily involve any change to the Tregs themselves. It was also reported that only CD137-negative Tregs infiltrated tumor sites and provided protection, while the population of CD137-positive Tregs consisted primarily of activated Tregs (29). Houot and colleagues (30) demonstrated that depletion of Tregs dramatically enhanced anti-CD137 therapy in mice. Based on these reports, suppression or elimination of Tregs may be a valuable component of future therapeutic strategies.
Dendritic cells
DCs represent unique antigen-presenting cells capable of sensitizing T cells to both new and recall antigens. DCs have been shown to play an important role in CD137-mediated antitumor immunity (31); their removal eliminated the efficacy of anti-CD137 in tumor in vivo (32). Anti-CD137 mAbs, when combined with vaccination with tumor cell lysate–pulsed DCs (TP-DC), accelerated tumor regression and enhanced the survival of tumor-bearing mice (33), suggesting a role for vaccinated DCs with upregulated CD137 in enhancing CTL anti-tumor activity. In the presence of human CD137L extracellular domain (exCD137L), antigen-loaded human DCs markedly increased the functions of antitumor CTL as measured by T-lymphocyte proliferation, IL2 and IFNγ secretion, cell viability, and cytotoxicity (34). Recently, DCs were shown to be negatively regulated by immunosuppressive invariant natural killer T cells (iNKT) in 4T1 mouse mammary tumors, and the selective elimination of DCs by iNKT immunosuppressive cells was shown (35). Here, priming of T cells to a tumor-specific CD8+ T-cell epitope in mice treated with radiotherapy and anti–CTLA-4 or anti-CD137 mAbs was markedly enhanced in iNKT−/− compared with wild-type mice. These data suggest DCs play a critical role in the regulation of CD137-mediated CTL activation by enhancing costimulation.
NK cells
NK cells (CD3−CD56+ cells) initiate innate immune responses toward tumor and virus-infected cells (36, 37). One of the primary mechanisms of antitumor activity of mAbs is antibody-dependent cell-mediated cytotoxicity (ADCC), whereby NK cells bearing an Fc receptor (CD16) bind to the antibody-targeted tumor cell and mediate tumor cell lysis. CD137 was also detected on NK cells. It was reported that selective depletion of NK cells in mice by the anti-AsialoGM1 or anti-NK1.1 antibodies completely abrogated the antitumor effect of anti-CD137 mAb, implying an immunoregulatory function of CD137 on NK cells (30, 38). Expression of CD137 on NK cells increases significantly when NK cells encounter mAbs bound to tumor cells (39–41). Anti-CD137 mAbs potentiated the antitumor activity of anti-CD20 and anti-HER2 (also known as ERBB2) mAbs in the mouse models of lymphoma and breast cancer, respectively. We reasoned that the addition of an agonistic mAb against CD137 would further stimulate activated NK cells and result in enhanced ADCC toward a mAb bound to tumor cells (17). Therefore, combination therapy of anti-CD137 mAb with mAbs targeting tumor-associated antigens is an appealing strategy.
Preclinical Studies of CD137 Antibody
Antitumor efficacy was also observed in several tumor models, such as MCA205 sarcoma, MC38 colon carcinoma, GL261 glioma, TC1 carcinoma, J558 myeloma, and A549 human alveolar adenocarcinoma cell lines (16). However, anti-CD137 mAb monotherapy did not eradicate some poorly immunogenic tumors, namely C3 tumor and B16/D5 melanoma (23, 42). To improve the therapeutic efficacy of anti-CD137 mAbs, several combination therapies were investigated.
Combination with other immunomodulators
Overcoming regulatory mechanisms of T cells can enhance antitumor responses. For example, PD-1, CTLA-4, T-cell immunoglobulin mucin-3 (TIM-3; also known as HAVCR2) and lymphocyte activation gene 3 (LAG-3; also known as CD223) negatively regulate T-cell function, whereas CD137, OX40, and CD40 provide costimulation (7). Combination therapies can potentiate T cell–based cancer immunotherapy (Table 2). Agonistic CD137 mAbs with anti–CTLA-4 or anti-CD40 mAbs increased the survival of mice injected with MC38 murine colon cancer cells (43, 44). Uno and colleagues (45) reported that an agonistic mAb to death receptor 5 (DR5; also known as TNFRSF10B), the apoptosis-inducing receptor for TNF-related apoptosis-inducing ligand, combined with agonistic mAbs to the costimulatory molecules CD40 and CD137, rapidly stimulated tumor-specific effector CD8+ T cells that led to eradication of preestablished tumors. This combination was named “trimAb.” In addition to trimAb, the effects of anti–CTLA-4 mAb, anti–glucocorticoid-induced TNF-receptor mAb, or anti–PD-1 mAb were examined (46). Blockade of CTLA-4–mediated signals by an antagonistic mAb substantially increased the tumor rejection rate of trimAb therapy, although the immune responses of draining lymph node cells were not augmented. Anti-DR5 and anti-CD1d mAb with anti-CD137 mAb (1DMab therapy) were also reported to show enhanced effectiveness (47). Interestingly, 1DMab therapy was more effective than trimAb in tumor models regulated by CD1d-restricted type II NKT cells, but less efficacious against tumors where Tregs were critical. Simultaneous dual costimulation through CD137 and OX40 induced a massive burst of CD8 T-cell effector function sufficient to treat established tumors (48). Remarkably, combination of anti–PD-1 mAb also led to the long-term survival of mice with established TC1 lung tumors, B16.F10 murine melanoma, and CT26 cells (49–51). On the other hand, this combination significantly increased some markers for liver toxicity and hematological parameters, compared with the corresponding anti-CD137–alone groups (50). Based on these preclinical studies, several clinical studies of anti-CD137 and anti–PD-1 mAbs have been launched.
Table 2.
Combination therapy with anti-CD137 mAb in mice
| Combination | Materials | Tumor cell | Cell type | Reference |
|---|---|---|---|---|
| Combination with mAb therapy targeting to immune cell | ||||
| Anti–CTLA-4 mAb | 4F10 | MC38 cell | Murine colon cancer cell | (43) |
| Anti-CD40 mAb | FGK-45 | MC38 cell Eμ-Myc cell |
Murine colon cancer cell Murine lymphoma |
(44) |
| Anti-DR5, CD40 mAbs—termed trimAb therapy | MD5-1, FGK45 | 4T1 cell | Murine mammary carcinoma | (45) |
| Anti-DR5, CD40, CTLA-4 mAbs—termed trimAb therapy + anti–CTLA-4 mAb | MD5-1, FGK45, UC10-4F10 | 4T1 cell | Murine mammary carcinoma | (46) |
| Anti-DR5, CD1d mAbs—termed 1DMab | MD5-1, 1B1 | 4T1 cell | Murine mammary carcinoma | (47) |
| Anti–TIM-3 mAb | RMT3-23 | ID8 cell | Murine ovarian carcinoma | (28) |
| Anti-OX40 mAb | MRC OX86 | MethA cell | Murine sarcoma | (48) |
| Anti–PD-1 mAb | RMP1-14 | ID8 cell | Murine ovarian carcinoma | (49) |
| Anti–PD-1, anti–CTLA-4 mAbs | RMP1-14 + 9D9 | TC1 cell | Murine lung epithelial cell | |
| Anti–PD-1 mAb + platinum agent | RMP1-14 + cisplatin | |||
| Anti–PD-1 mAb | RMP1-14 | B16.F10 cell | Murine melanoma cell | (50) |
| Anti–PD-1 mAb | RMP1-14 | CT26 cell | Murine colon adenocarcinoma cells | (51) |
| Combination with vaccination | ||||
| DC vaccine | Tumor lysate–pulsed DCs | MCA205 cell | Murine fibrosarcomas | (33) |
| DC vaccine | Tumor lysate–pulsed DCs | CT26 cell | Murine metastatic colon cancer cell | (52) |
| DC vaccine and anti-OX40 mAb | Tumor lysate–pulsed DCs, OX86 | N202.1A cell | Murine mammary cell line | (53) |
| Adoptive CTL therapy | Tumor-specific CTL | B16.F10 cell | Murine melanoma cell | (69) |
| B16-Flt3L vaccine and anti–CTLA-4 mAb | B16-Flt3 ligand, 9D9 | B16-sFlt3L cell | Murine melanoma cells | (27) |
| CpG vaccine | CpG1826 | Renca cell MC38 cell |
Murine renal cell carcinoma Murine colon tumor cell |
(54) |
| Peptide and CpG vaccine | Trp2 peptides plus CpG | B16 BL6 | Murine melanoma | (55) |
| Oncolytic virus | Oncolytic Vvdd vaccinia virus | AT-3 cell | Murine breast carcinoma | (56) |
| IL12 gene therapy | Adenovirus expressing IL12 | B16.F10 cell | Murine melanoma cell | (70) |
| Virus vaccine and anti–CTLA-4 mAb | Adenovirus with LCMV gene, 9H10 | B16.F10-GP cell | Murine melanoma cell | (57) |
| Combination with mAb therapy targeting to tumor antigen | ||||
| Anti-CD20 mAb | Rituximab | Raji cell | Human CD20+ B cell | (39) |
| Anti-CD20 mAb | MB20-11 | BL3750 cell | Murine CD20+ B cell | |
| Anti-HER2 mAb | Trastuzumab | BT474M1, MCF7 HER18 cells |
Human breast tumor cell | (40) |
| Anti-ErbB-2 mAb and anti–PD-1 mAb | 7.16.4, RMP1-14 | H2N113 cell | Murine ErbB-2+ tumor cell | (59) |
| Anti-EGFR mAb | Cetuximab | SCC6, T84, HCT116 | Human colon cancer cell | (41) |
Abbreviations: CCL2, C–C motif chemokine 2; Trp2, tyrosinase-related protein 2.
Combination with vaccination
DCs can be pulsed with tumor-associated antigens by a variety of methods that result in the ability of DCs to prime naïve T cells, and DCs can mediate regression of established tumor when given as a vaccine in animal models (Table 2). Ito and colleagues (33) and Lee and colleagues (52) examined the role of anti-CD137 administration in modulating the immune responses induced by tumor lysate–pulsed DC (TP-DC) vaccinations. Combined therapy with TP-DC plus anti-CD137 mAb resulted in lower local recurrence rates and improved survival after surgical resection of subcutaneous tumors. Similarly, immunizations in combination with the costimulatory agonistic anti-CD137 mAb significantly enhanced the immune responses in Her-2/neu mice, resulting in complete tumor rejection (53). Using vaccines that stimulate a broad immune response in combination with costimulatory molecules could significantly improve the antitumor immune response in tolerant hosts. CpG vaccination and oncolytic viruses, as well as adoptive transfer of tumor-specific CTLs, were also potentiated by agonistic anti-CD137 mAb (54–57). Thus, agonistic anti-CD137 mAb can modulate immune responses to several vaccinations and enhance antitumor efficacy.
Combination with mAb therapy targeting tumor antigens
One of the primary mechanisms of antitumor activity of mAbs is ADCC (58). Kohrt and colleagues (39–41) demonstrated that an anti-CD137 agonistic mAb enhances the antitumor activity of therapeutic mAbs rituximab, trastuzumab, and cetuximab by enhancing ADCC (Table 2). In addition, human NK cells upregulate CD137 after encountering mAbs and tumor cells in vitro and in patients, and subsequent stimulation of these NK cells with anti-CD137 mAb enhances mAb-dependent cytotoxicity against tumor cells (41). Therefore, sequential administration of therapeutic antibodies and CD137 mAb with a 24-hour gap would be better than concurrent administration. Stagg and colleagues (59) also reported interesting results showing that not only anti-CD137 mAb but also anti–PD-1 mAb enhanced the antitumor activity of a Her2-targeting mAb in mice. In a clinical trial, a combination therapy of anti–PD-1 mAb with rituximab achieved a 66% objective response rate in patients with relapsed follicular lymphoma who were previously treated with rituximab (60). This strongly suggests that combination of immunomodulators, including anti-CD137 mAbs, with tumor targeting mAbs can enhance the clinical efficacy of therapeutic antibodies (61).
Clinical Trials of CD137
Two fully humanized mAbs of CD137, urelumab (BMS-663513) and PF-05082566, have been developed for clinical use. Urelumab is a fully human IgG4 mAb developed by Bristol-Myers Squibb, and PF-05082566 is a fully human IgG2 mAb developed by Pfizer. They are agonistic mAbs, which bind to the extracellular domain of human CD137. Clinical trials of anti-CD137 mAbs are summarized in Table 3.
Table 3.
Clinical trials of anti-CD137 agonistic mAbs
| NCT number | Phase | Condition | Combination | Start year | Status (May 2015) |
|---|---|---|---|---|---|
| Urelumab (BMS-663513): fully human type IgG4, Bristol-Myers Squibb | |||||
| NCT00309023 | I/II | Metastatic or locally advanced solid tumors | – | 2005 | Terminated |
| NCT00351325 | I | Advanced solid malignancies | Chemotherapy | 2007 | Terminated |
| NCT00461110 | I | Non–small cell lung cancer | Chemoradiation | 2008 | Terminated |
| NCT00612664 | II | Melanoma | – | 2008 | Completed |
| NCT00803374 | I | Advanced malignant melanoma | Ipilimumab (anti–CTLA-4 mAb) | 2010 | Withdrawn |
| NCT01471210 | I | Advanced and/or metastatic solid tumors relapsed/refractory B-cell NHL | – | 2012 | Recruiting |
| NCT01775631 | Ib | Relapsed/refractory B-cell NHL | Rituximab (anti-CD20 mAb) | 2013 | Recruiting |
| NCT02110082 | Ib | Colorectal cancer, head and neck cancer | Cetuximab (anti-EGFR mAb) | 2014 | Recruiting |
| NCT02252263 | I | Multiple myeloma | Elotuzumab (anti-CS1 mAb) | 2014 | Recruiting |
| NCT02253992 | I/II | Advanced solid tumors, advanced B-cell NHL | Nivolumab (anti–PD-1 mAb) | 2014 | Recruiting |
| NCT02420938 | II | Relapsed/refractory/high-risk untreated chronic lymphocytic leukemia | Rituximab (anti-CD20 mAb) | 2015 | Not yet recruiting |
| PF-05082566: fully human type IgG2, Pfizer | |||||
| NCT01307267 | I | CD20-positive NHL | Rituximab (anti-CD20 mAb) | 2011 | Recruiting |
| NCT02179918 | Ib | Advanced solid tumors | MK-3475 (anti–PD-1 mAb) | 2014 | Recruiting |
Abbreviation: NHL, non-Hodgkin lymphoma.
Urelumab (BMS-663513)
The NCT00309023 study was a first-in-human open-label, ascending, multidose phase I–II trial conducted in patients with locally advanced or metastatic solid tumors (62). In the dose-escalation phase of the study, patients were sequentially assigned to one of six dose cohorts (0.3–15 mg/kg) to receive urelumab once every 3 weeks. Eighty-three patients (54 melanoma, 15 renal cell carcinoma, 13 ovarian, and 1 prostate) have been treated. Dose-limiting toxicities were reported in the 0.3-mg/kg (grade 3 neutropenia) and 15-mg/kg (grade 4 neutropenia) cohorts. Overall, fatigue (all, 26%; grade 3–4, 3%), reversible grade 3–4 transaminitis (11%), and grade 3–4 neutropenia (5%) were the most common agent-related adverse events. Three partial response and 4 stable disease cases occurred at all three doses tested in expansion cohorts. Preliminary biomarker analysis demonstrated increased expression of IFN-inducible genes in peripheral blood, serum neopterin levels, and percentage of circulating activated CD8+ and CD4+ T cells following a single treatment. These data suggest that urelumab was tolerable across a wide dose range (0.3–15 mg/kg). Based on the phase I study, a randomized, multidose, open-label, phase II study of urelumab as a second-line monotherapy was designed in the patient with metastatic melanoma. However, the study was terminated in May 2009 due to fatal hepatotoxicity. The mechanism of anti-CD137 mAb-induced hepatotoxicity remains unclear, although the relationship between the CD137 pathway and hepatotoxicity was suspected (50, 63, 64). Therefore, careful dosing of anti-CD137 mAb is needed to avoid the risk for severe hepatotoxicity.
Following the first clinical trial, several combination therapies with chemotherapy (NCT00351325), chemoradiation (NCT00461110), ipilimumab (NCT00803374), rituximab (NCT01775631; ref. 65), cetuximab (NCT02110082), and elotuzumab (NCT02252263) have been launched as phase I or I/II studies. We have initiated a biomarker study (NCT01471210) using the novel technology of mass cytometry time of flight (66, 67). Preliminary findings from 4 patients showed an increase in CD8+ T cells and NK cells with a decrease in CD4+ T cells and regulatory CD4+ T cells. These preliminary data are consistent with anti-CD137 agonist. Although the studies (NCT00351325, NCT00461110, and NCT00803374) were terminated or withdrawn, low-dose therapies (<0.1 mg/kg) of urelumab in combination with approved mAbs are worthy of attention.
PF-05082566
Clinical trials of PF-05082566 are also ongoing. NCT01307267 is an open-label, dose-escalation study that was conducted in patients with advanced malignancies, and the preliminary data were reported (68). Cohorts of 3 to 6 patients were enrolled initially using a 3+3 design (0.006–0.3 mg/kg), then a time-to-event continual reassessment method design for higher doses (0.6–5 mg/kg). Patients received PF-05082566 via i.v. infusion every 4 weeks (1 cycle) with an 8-week period for assessment of dose-limiting toxicity (DLT). Twenty-seven patients have been treated with PF-05082566 up to the 0.3-mg/kg dose level, including 11 with colorectal cancer, 6 with Merkel cell carcinoma, and 2 with pancreatic adenocarcinoma. Twenty-five patients completed the DLT assessment period and 7 patients remain on therapy. All discontinuations from treatment were due to disease progression. One patient treated at 0.06 mg/kg had grade 3 elevation in alkaline phosphatase. No additional significant elevations in liver enzymes and no DLTs have occurred to date. The best overall response of stable disease was observed in 22% (6 of 27) patients. These results suggest that PF-05082566 was well tolerated, with evidence of disease stabilization in multiple patients.
Combination with anti–PD-1 mAbs
In 2014, one of the most interesting combination therapies with anti-CD137 mAbs is with nivolumab or MK-3475 (anti–PD-1 mAbs) for patients with advanced solid tumors or advanced B-cell non-Hodgkin lymphoma. The safety and tolerability of urelumab administered in combination with nivolumab is being assessed in a phase I/II dose-escalation and cohort expansion study (NCT02253992). Nivolumab and urelumab were administered every 2 weeks up to 12 cycles and every 4 weeks up to 3 cycles, respectively. NCT02179918 is a phase Ib study of PF-05082566 in combination with MK-3475. Both agents are administered every 3 weeks. A preclinical study indicated that combination of anti-CD137 and anti–PD-1 mAbs enhanced hepatic toxicity, compared with one of the agents alone (50). Although this combination has potential for good efficacy, its toxicity should be evaluated in humans.
Conclusions
More than 20 years have passed since the identification of CD137 as an immune modulator (18). One of the most promising findings is the anticancer efficacy of agonistic anti-CD137 mAb (20). The strong preclinical successes underscore the importance of CD137 in cancer therapy, especially in combination therapy. Several clinical trials of urelumab (BMS-663513) had been terminated or withdrawn, because of hepatitis. However, clinical trials of combination therapies using low-dose urelumab with rituximab, cetuximab, and anti–PD-1 mAbs have been launched. In addition, another anti-CD137 mAb, PF-05082566, has been developed. We believe anti-CD137 mAbs hold great clinical promise. Their clinical potential should be tested in conjunction with other FDA-approved immunomodulators and antibody therapeutics. It is anticipated that combination cancer immunotherapy with CD137 will make significant contributions to the field of cancer immunotherapy.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 2.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:3266–74. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 3.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 4.Brufsky AM. Current approaches and emerging directions in HER2-resistant breast cancer. Breast Cancer. 2014;8:109–18. doi: 10.4137/BCBCR.S9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, Siena S, Bardelli A. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol. 2014;8:1084–94. doi: 10.1016/j.molonc.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–46. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li SY, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol. 2013;5:47–53. doi: 10.2147/CPAA.S46199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNutt M. Cancer immunotherapy. Science. 2013;342:1417. doi: 10.1126/science.1249481. [DOI] [PubMed] [Google Scholar]
- 11.Areas to watch in 2015. Science. 2014;346:1450. doi: 10.1126/science.346.6216.1450-a. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–40. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 15.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multilayered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–90. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062–70. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 17.Houot R, Kohrt H, Levy R. Boosting antibody-dependent cellular cytotoxicity against tumor cells with a CD137 stimulatory antibody. Oncoimmunology. 2012;1:957–8. doi: 10.4161/onci.19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci U S A. 1989;86:1963–7. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 21.Ju SA, Lee SC, Kwon TH, Heo SK, Park SM, Paek HN, et al. Immunity to melanoma mediated by 4-1BB is associated with enhanced activity of tumour-infiltrating lymphocytes. Immunol Cell Biol. 2005;83:344–51. doi: 10.1111/j.1440-1711.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 22.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox RA, Flies DB, Zhu G, Johnson AJ, Tamada K, Chapoval AI, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–9. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34:236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–63. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Z, Cheng D, Xia Z, Luan M, Wu L, Wang G, et al. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J Transl Med. 2013;11:215. doi: 10.1186/1479-5876-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein MJ, Kohrt HE, Houot R, Varghese B, Lin JT, Swanson E, et al. Adoptive cell therapy for lymphoma with CD4 T cells depleted of CD137-expressing regulatory T cells. Cancer Res. 2012;72:1239–47. doi: 10.1158/0008-5472.CAN-11-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–8. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 32.Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–36. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 33.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–9. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Guo H, Wang Y, Gao Y, Zhu Z, Du Z. Extracellular domain of human 4-1BBL enhanced the function of cytotoxic T-lymphocyte induced by dendritic cell. Cell Immunol. 2011;271:118–23. doi: 10.1016/j.cellimm.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Pilones KA, Aryankalayil J, Babb JS, Demaria S. Invariant natural killer T cells regulate anti-tumor immunity by controlling the population of dendritic cells in tumor and draining lymph nodes. J Immunother Cancer. 2014;2:37. doi: 10.1186/s40425-014-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 2013;4:499. doi: 10.3389/fimmu.2013.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 39.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–32. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–75. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124:2668–82. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Kim JA, Averbook BJ, Chambers K, Rothchild K, Kjaergaard J, Papay R, et al. Divergent effects of 4-1BB antibodies on antitumor immunity and on tumor-reactive T-cell generation. Cancer Res. 2001;61:2031–7. [PubMed] [Google Scholar]
- 43.Kocak E, Lute K, Chang X, May KF, Jr, Exten KR, Zhang H, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–84. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 44.Westwood JA, Matthews GM, Shortt J, Faulkner D, Pegram HJ, Duong CP, et al. Combination anti-CD137 and anti-CD40 antibody therapy in murine myc-driven hematological cancers. Leuk Res. 2014;38:948–54. doi: 10.1016/j.leukres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–8. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 46.Takeda K, Kojima Y, Uno T, Hayakawa Y, Teng MW, Yoshizawa H, et al. Combination therapy of established tumors by antibodies targeting immune activating and suppressing molecules. J Immunol. 2010;184:5493–501. doi: 10.4049/jimmunol.0903033. [DOI] [PubMed] [Google Scholar]
- 47.Teng MW, Sharkey J, McLaughlin NM, Exley MA, Smyth MJ. CD1d-based combination therapy eradicates established tumors in mice. J Immunol. 2009;183:1911–20. doi: 10.4049/jimmunol.0900796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–12. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 49.Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS One. 2013;8:e84927. doi: 10.1371/journal.pone.0084927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3:149–60. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 51.Shindo Y, Yoshimura K, Kuramasu A, Watanabe Y, Ito H, Kondo T, et al. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35:129–36. [PubMed] [Google Scholar]
- 52.Lee H, Park HJ, Sohn HJ, Kim JM, Kim SJ. Combinatorial therapy for liver metastatic colon cancer: dendritic cell vaccine and low-dose agonistic anti-4-1BB antibody co-stimulatory signal. J Surg Res. 2011;169:e43–50. doi: 10.1016/j.jss.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 53.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–43. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 54.Westwood JA, Potdevin Hunnam TC, Pegram HJ, Hicks RJ, Darcy PK, Kershaw MH. Routes of delivery for CpG and anti-CD137 for the treatment of orthotopic kidney tumors in mice. PLoS One. 2014;9:e95847. doi: 10.1371/journal.pone.0095847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sin JI, Kim H, Ahn E, Jeon YH, Park WS, Lee SY, et al. Combined stimulation of TLR9 and 4.1BB augments Trp2 peptide vaccine-mediated melanoma rejection by increasing Ag-specific CTL activity and infiltration into tumor sites. Cancer Lett. 2013;330:190–9. doi: 10.1016/j.canlet.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 56.John LB, Howland LJ, Flynn JK, West AC, Devaud C, Duong CP, et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 2012;72:1651–60. doi: 10.1158/0008-5472.CAN-11-2788. [DOI] [PubMed] [Google Scholar]
- 57.Jensen BA, Pedersen SR, Christensen JP, Thomsen AR. The availability of a functional tumor targeting T-cell repertoire determines the anti-tumor efficiency of combination therapy with anti-CTLA-4 and anti-4-1BB antibodies. PLoS One. 2013;8:e66081. doi: 10.1371/journal.pone.0066081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, et al. Combination strategies to enhance antitumor ADCC. Immunotherapy. 2012;4:511–27. doi: 10.2217/imt.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chester C, Marabelle A, Houot R, Kohrt HE. Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumors. Curr Opin Immunol. 2015;33C:1–8. doi: 10.1016/j.coi.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Sznol M, Hodi FS, Margolin K, McDermott DF, Ernstoff MS, Kirkwood JM, et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA) J Clin Oncol. 2008;26(15 suppl 3007) [Google Scholar]
- 63.Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol. 2007;178:4194–213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vinay DS, Choi BK, Bae JS, Kim WY, Gebhardt BM, Kwon BS. CD137-deficient mice have reduced NK/NKT cell numbers and function, are resistant to lipopolysaccharide-induced shock syndromes, and have lower IL-4 responses. J Immunol. 2004;173:4218–29. doi: 10.4049/jimmunol.173.6.4218. [DOI] [PubMed] [Google Scholar]
- 65.Kohrt HE, Godwin JE, Lossos IS, Williams ME, Timmerman J, Link BK, et al. A phase Ib, open-label, multicenter study of urelumab (BMS-663513) in combination with rituximab in subjects with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31(suppl) abstr TPS3108. [Google Scholar]
- 66.Chester C, Chang S, Kurland JF, Sagiv-Barfi I, Czerwinski D, Rajapaksa A, et al. Biomarker characterization using mass cytometry in a phase 1 trial of urelumab (BMS-663513) in subjects with advanced solid tumors and relapsed/refractory B-cell non-Hodgkin lymphoma. J Clin Oncol. 2014;32(suppl):5s. abstr 3017. [Google Scholar]
- 67.Melero I, Gangadhar TC, Kohrt HE, Segal NH, Logan T, Urba WJ, et al. A phase I study of the safety, tolerability, pharmacokinetics, and immunoregulatory activity of urelumab (BMS-663513) in subjects with advanced and/or metastatic solid tumors and relapsed/refractory B-cell non-Hodgkin’s lymphoma (B-NHL) J Clin Oncol. 2013;31(suppl) abstr TPS3107. [Google Scholar]
- 68.Segal NH, Gopal AK, Bhatia S, Kohrt HE, Levy R, Pishvaian MJ, et al. A phase 1 study of PF-05082566 (anti-41BB) in patients with advanced cancer. J Clin Oncol. 2014;32(suppl):5s. abstr 3007. [Google Scholar]
- 69.Noji S, Hosoi A, Takeda K, Matsushita H, Morishita Y, Seto Y, et al. Targeting spatiotemporal expression of CD137 on tumor-infiltrating cytotoxic T lymphocytes as a novel strategy for agonistic antibody therapy. J Immunother. 2012;35:460–72. doi: 10.1097/CJI.0b013e31826092db. [DOI] [PubMed] [Google Scholar]
- 70.Xu D, Gu P, Pan PY, Li Q, Sato AI, Chen SH. NK and CD8+ T cell-mediated eradication of poorly immunogenic B16-F10 melanoma by the combined action of IL-12 gene therapy and 4-1BB costimulation. Int J Cancer. 2004;109:499–506. doi: 10.1002/ijc.11696. [DOI] [PubMed] [Google Scholar]


