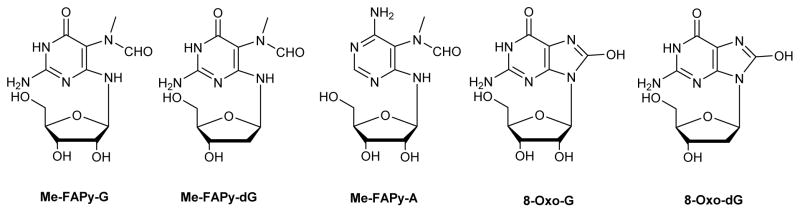
Abstract
DNA nucleobases are the prime targets for chemical modifications by endogenous and exogenous electrophiles. Alkylation of the N7 position of guanine and adenine in DNA triggers base-catalyzed imidazole ring opening and the formation of N5-substituted formamidopyrimidine (N5-R-FAPy) lesions. Me-FAPy-dG adducts induced by exposure to methylating agents and AFB-FAPy-dG lesions formed by aflatoxin B1 have been shown to persist in cells and to contribute to toxicity and mutagenicity. In contrast, the biological outcomes of other N5-substituted FAPy lesions have not been fully elucidated. To enable their structural and biological evaluation, N5-R-FAPy adducts must be site-specifically incorporated into synthetic DNA strands using phosphoramidite building blocks, which can be complicated by their unusual structural complexity. N5-R-FAPy exist as a mixture of rotamers and can undergo isomerization between α, β anomers and furanose-pyranose forms. In this Perspective, we will discuss the main types of N5-R-FAPy adducts and summarize the strategies for their synthesis and structural elucidation. We will also summarize the chemical biology studies conducted with N5-R-FAPy-containing DNA to elucidate their effects on DNA replication and to identify the mechanisms of N5-R-FAPy repair.
Graphical Abstract
1. Introduction
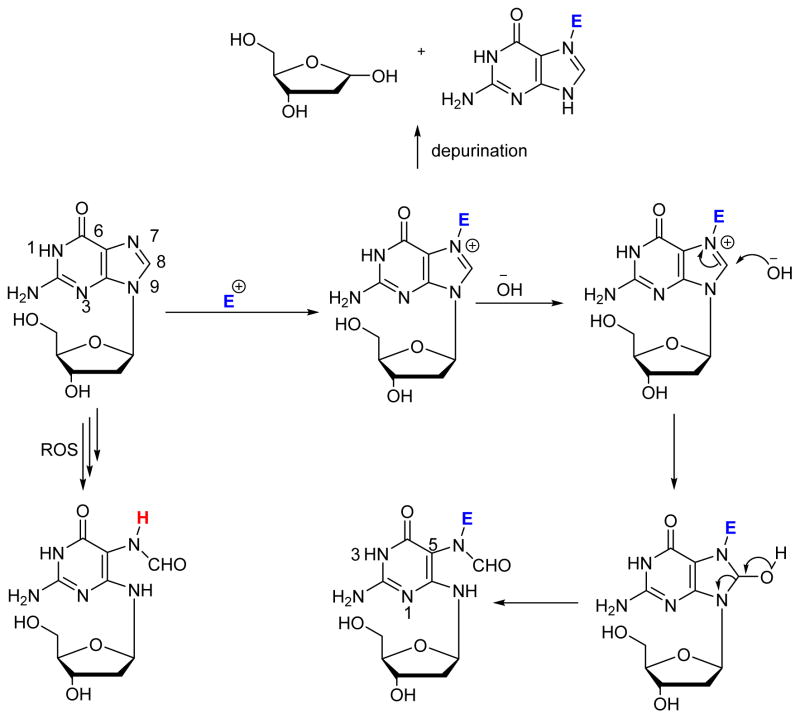
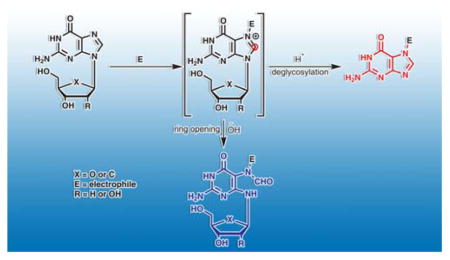
N5-Alkyl-formamidopyrimidines (N5-R-FAPy) are ring open DNA adducts that form upon imidazole ring opening of the corresponding N7-alkylpurine lesions.1–8 N7 positions of guanine and adenine in DNA are susceptible to electrophilic attack by a variety of alkylating agents. The resulting N7-substituted purines are destabilized due to the presence of positive charge at the N7 position9 and can undergo two competing reactions: depurination to form apurinic sites and imidazole ring opening to give N5-R-FAPy.1, 2, 10–13 While depurination is accelerated at low pH, N5-R-FAPy formation is preferred under basic conditions (Scheme 1). Although under physiological conditions, N5-R-FAPy adducts are formed in much lower yields than the corresponding depurinated adducts, they may have a significant biological impact because of their persistence in cells and their ability to induce mutations.
Scheme 1.
Mechanisms leading to the formation of FAPy adducts in DNA.
Many simple alkylating agents including epoxides, nitrogen mustards, and alkyl halides preferentially alkylate the nucleophilic N7 position of guanine in DNA.14–21 However, not all of the resulting N7-dG adducts form the corresponding FAPy adducts under physiological conditions. Imidazole ring opening of N7-alkyl-dG is favored by electron withdrawing groups on the N7 substituent, which makes the C7-C8 bond more susceptible towards attack by hydroxyl anions.22, 23 Interestingly, imidazole ring opening of N7-alkyl-G adducts in RNA is 2–3 times faster than of their DNA counterparts, presumably due to the electron withdrawing effect of the 2′-hydroxyl group.24 Aflatoxin B1 epoxide,25–27 N-methylnitrosamines,28–32 dimethyl sulfate,33, 34 tobacco carcinogen 4-(methylnitrosamino)-1-(pyridyl)-1-butanone (NNK),35 N-methylnitrosourea,36 1, 2-dimethylhydrazine, N, N-dimethylnitrosamine,28–32, 37 cyclophosphamide,38, 39 mitomycin C,40, 41 and ethyleneimine42 are some examples of alkylating agents that give rise to N5 substituted FAPy adducts. N5-substituted FAPy adducts are also induced by leinamycin,20 pluramycins,43 azinomycin,44, 45 and S-(2-haloethyl)glutathione.46, 47 Structurally related unsubstituted FAPy adducts can be formed by a radical mechanism upon exposure to reactive oxygen species (ROS)48–50 but are beyond the scope of this review.
Imidazole ring opening drastically changes the molecular shape and the hydrogen bonding characteristics of the parent purine nucleobase. As a result, N5-substituted FAPy lesions are likely to induce DNA polymerase stalling, toxicity, and mutations. For example, N5-AFB1-FAPy adducts induced by Aflatoxin B1 are thought to play a major role in its hepatocarcinogenicity.51, 52 However, our understanding of the cellular formation and biological outcomes of N5-R-FAPy adducts induced by other DNA modifying agents is incomplete. Chemical synthesis of N5-R-FAPy nucleosides and N5-R-FAPy-containing DNA strands represents a special challenge due to the structural complexity of these unusual ring open lesions and their propensity to undergo isomerization. The goal of this Perspective is to summarize the current understanding of the mechanisms of formation, synthesis, isomerism, and biological consequences of N5-R-FAPy adducts. For additional information on N7-guanine alkylation and the chemistry of unsubstituted FAPy adducts induced by ROS, readers are referred to several recent reviews.7, 48–50, 53,54
2. Structural Identification and Synthesis of N5-Substituted FAPy Lesions (N5-R-FAPy)
Since their discovery in the early 1960s, N5-substituted FAPy adducts have been a subject of intense investigation. In addition to simple DNA alkylating agents, a number of antitumor drugs and natural products have been shown to induce N5-R-FAPy adducts under physiological conditions, generating significant interest in these ring open structures. In this section, we will describe the discovery, mechanisms of formation, and synthesis of the major types of N5-R-FAPy adducts.
2.1. Methyl-FAPy-dG
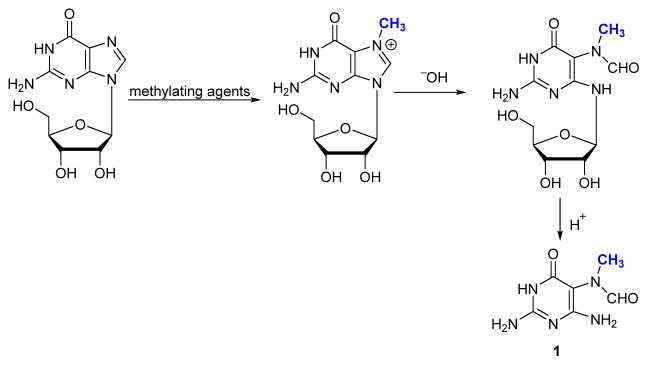
DNA can be methylated upon exposure to exogenous agents such as dimethyl sulfate,17, 33, 34 N-methylnitrosamines,28–32 and tobacco specific nitrosamine 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK).35 DNA methylation can also occur from the reactions with endogenous enzyme cofactor, S-adenosyl-L-methionine (SAM).55 The most reactive site in DNA towards methylating agents is the N7 position of guanine.9, 16 Haines et al. described imidazole ring opening of N7-methylguanosine in the presence of ammonia or sodium hydroxide at room temperature.2 The ring open adduct was cleaved with acid, and the resulting aglycone was assigned the structure of 2,4-diamino-6-hydroxy-5N-methylformamidine (1 in Scheme 2) based on its spectroscopic properties.2
Scheme 2.
Formation of methyl-FAPy from dG.
The biological significance of this finding remained unclear until 1983, when Beranek et al. isolated a novel nucleobase adduct from liver DNA of rats treated with the methylating agents, N, N,-dimethylnitrosoamine and 1,2-dimethyl hydrazine.37 The same adducts were subsequently found in bladder epithelial DNA of rats treated with N-methylnitrosourea.36 The unknown lesions were chromatographically identical to the ring opened derivative of 7-methylguanine prepared by alkaline hydrolysis of N7-methyl-Guo, followed by cleavage of the glycosidic bond with acid (Scheme 2).36 Two isomers of the adduct were isolated by HPLC. Further experiments revealed that following isolation as two separate peaks, these two isomers interconverted with each other to give a 1:1 mixture.36, 37 Thermal desorption mass spectra of the two isomers were identical, giving a molecular ion peak m/z 183 and major fragments at m/z 155 and 140, corresponding to the loss of CO and CO+CH3, respectively.37 1H-NMR spectra of the two products were also identical, both exhibiting two distinct sets of resonances (Figure 1).36 NMR spectra of these isomers were consistent with cis and trans isomers around the C5-N5 amide bond (Figure 2). NMR spectra revealed two sets of resonances, each corresponding to two different forms of N5-methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine, which interconverted with each other.36 The relative abundances of the two rotamers were 1:9, 1:4, and 1:2 when spectra were taken in dimethylsulfoxide-d6, methanol-d4, and dimethylsulfoxide-d6/D2O, respectively (Figure 1).36 These results indicated that ring-open N5-methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine adducts are found in vivo (and thus may not require strongly basic conditions to be formed) and exist as at least two interconverting forms (1 and 2 in Figure 2).
Figure 1.
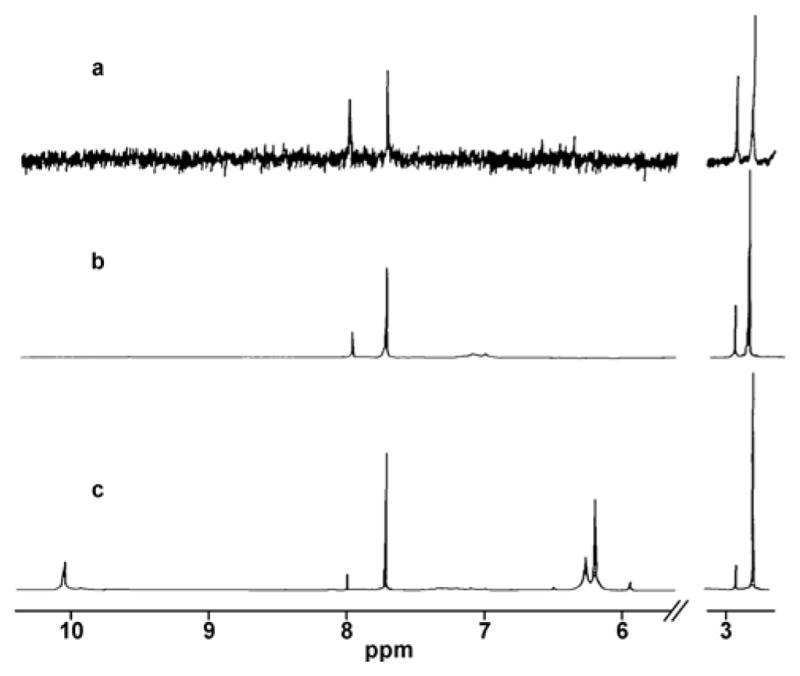
500 MHz NMR spectra of N5-methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine. Spectra were obtained in DMSO-d6/D2O (1:1) (a), in MeOH-d4 (b); and in DMSO-d6 (c).36 Used by permission of Oxford University Press.
Figure 2.

Rotational isomers of Methyl-FAPy-Gua adducts identified by Beranek et al.36, 37
The structural identities of the interconverting isomers of N5-methyl-FAPy adducts were further examined by proton NMR by Boiteux et al.10 Two methyl group signals were observed at 2.7 and 2.81 ppm, with the relative intensities of 88% and 12%, while the corresponding formamido signals were observed at 7.61 ppm (88%) and 7.88 ppm (12%) (Figure 3). Nuclear Overhauser Enhancement (NOE) was observed on the formamido proton at 7.61 ppm by irradiation of the methyl signal at 2.7 ppm, whereas no NOE effect was observed for formamido proton when methyl signal at 2.81 ppm was irradiated. This indicated that the protons observed as resonances at 2.7 ppm and 7.61 ppm are in a close proximity to each other and therefore belong to the Z conformer of the N-methyl-formamido bond, while the other isomers giving rise to resonances at 2.8 and 7.88 ppm are the E rotamer (3 and 4 in Figure 2).
Figure 3.
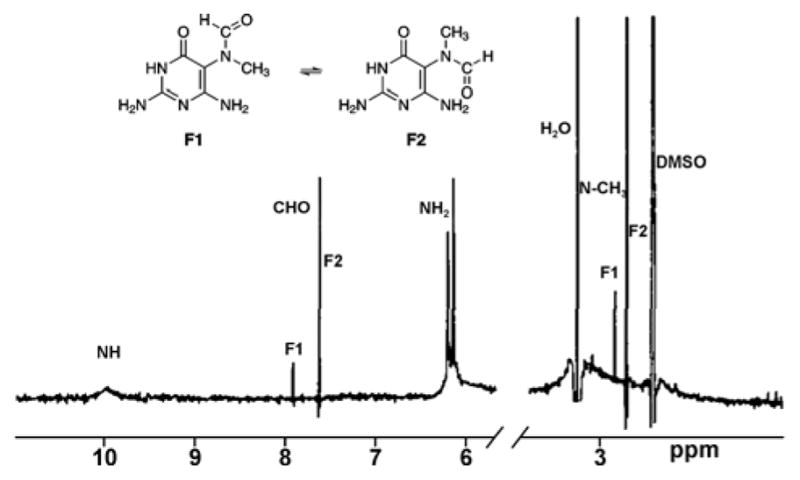
Proton NMR spectra showing formamido signals with methylene protons of Methyl-Fapy isomers. Spectra were taken in DMSO-d6.10 Used by permission of Oxford University Press.
In summary, these early studies have revealed that, ring open N7-methyl-G adducts can form under physiological conditions. These adducts can exist as a mixture of four rotational isomers due to a hindered rotation about the C5-N5 and N-methyl-formamido bonds (1–4 in Figure 2). In the free nucleoside form these conformers rapidly interconvert with an estimated half-life of 8 min at 37 °C.10 Subsequently, these results were unambiguously supported by NMR studies of 15N-labeled adducts.56 As discussed below, the distribution of conformational isomers of N5-methyl-FAPy and other FAPy lesions is altered in double stranded DNA due to steric factors and hydrogen bonding interactions.
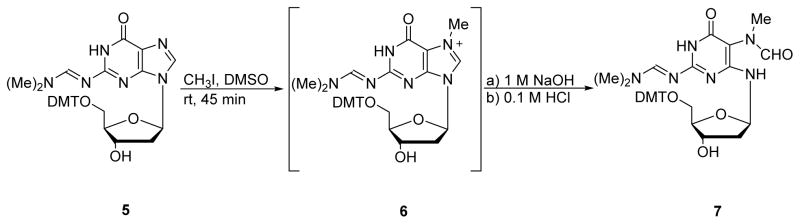
The first synthesis of Me-FAPy-dG nucleoside was reported by Christov et. al. in 2008.57 In their approach, 2′-deoxyguanosine was protected at the exocyclic amine with N,N-dimethylformamide dimethylacetal and at the 5′-OH with DMT to give the doubly protected nucleoside 5 (Scheme 3). N7-methylation of 5 was carried out using CH3I in DMSO to give the N7-methyl-dG intermediate 6, which was not isolated (Scheme 3). Subsequent treatment of 6 with 1M NaOH and immediate neutralization yielded protected Me-FAPy-dG 7, which was characterized by NMR spectroscopy and high resolution mass spectrometry.57 The corresponding Me-FAPy-G ribonucleoside was synthesized using a similar route.58 N7-methylation of guanosine was achieved by reaction of unprotected guanosine with diazomethane, which was obtained from nitrosomethylurea as reported by Farmer et. al.59 Next, N7-methylguanosine was incubated with 0.15M ammonia for 30 min at 25 °C, yielding the Me-FAPy-G in 60% yield. The availability of Me-FAPy-dG and Me-FAPy-Guo has made it possible to incorporate these structures in nucleic acids chains using the phosphoramidite approach (Section 3 below).
Scheme 3.
Synthesis of Me-FAPy-dG from N2, 5′-OH -protected dG.57
2.2. Ethyl FAPy Adducts
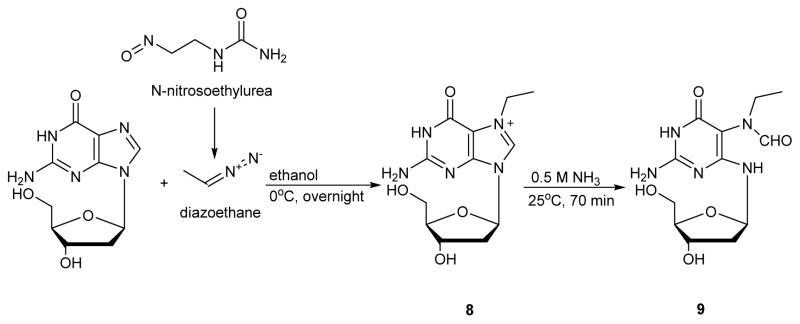
N7-ethylguanine is the main DNA lesion formed upon exposure to ethylating agents, and it can be converted to the corresponding Et-FAPy adducts.19, 58 van Delft et al. reported the synthesis of Ethyl-FAPy-dG (9 in Scheme 4) from N7-ethyl-dG 8 by basic treatment in 0.5 M ammonia for 70 min at 25 °C, followed by cooling to −80 °C and lyophilization (Scheme 4).58 The conversion yield was reported as 95%.58 The corresponding ribonucleoside (Et-FAPy-Guo) was prepared analogously by treating N7-Et-Guo with 0.1 N KOH at ambient temperature.19 A characteristic change in UV spectra was observed when N7-Et-Guo (λmax, 243 and 272 nm) was converted to Et-FAPy-Guo (λmax 265 nm in acid and 247 nm under basic conditions (pH 13, Figure 4).19
Scheme 4.
Synthetic scheme for Ethyl-FAPy-dG.58
Figure 4.
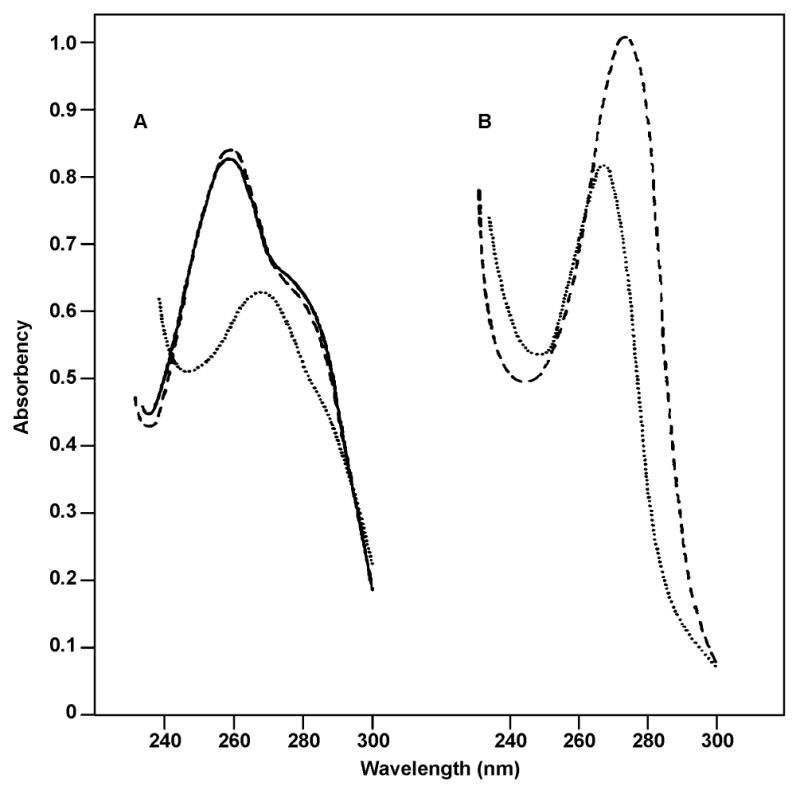
UV absorption spectra of (A) 7-Et-Guo in water (—), 0.1 N HCl (--) and 0.1N KOH (….). Spectrum shown for 0.1N KOH is for Et-FAPy-G. (B) Ethyl-FAPy-Guo in 0.1 N KOH (….), 0.1N HCl (--).19
2.3. 2-Hydroxyethyl FAPy Adducts
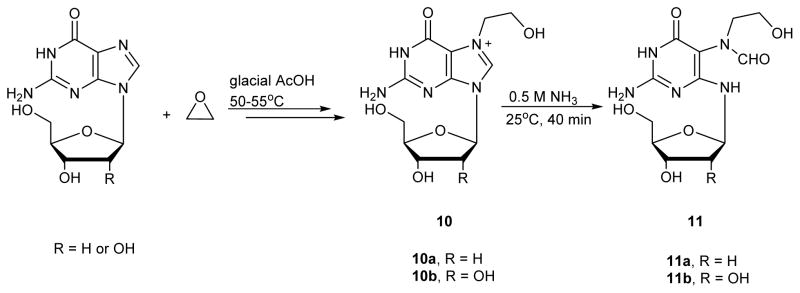
N7-(2-hydroxyethyl)guanine (N7-heG, structure 10a in Scheme 5) represents the major adduct produced upon exposure of DNA to ethylene oxide, which is commonly used as an intermediate in chemical industry. Ethylene oxide can also be formed endogenously through P450-mediated metabolism of ethylene.60, 61 As a result, N7-heG adducts are ubiquitously present in tissues of mice exposed to ethylene oxide and are among the most abundant endogenous DNA lesions measured, with > 30,000 N7-heG lesions per cell.53, 62
Scheme 5.
Synthesis of N7-(2-hydroxyethyl)-FAPy-dG.63
Roe et. al.63 reported the first synthesis of he-FAPy adducts. Guanosine was treated with ethylene oxide in glacial acetic acid at 50–55 °C to give N7-he-Guo (10b in Scheme 5).58 This intermediate was incubated with 0.5 M ammonia for 40 min at 25 °C, followed by lyophilization to give N7-(2-hydroxyethyl)-FAPy-Guo (compound 11b).63 The 2′-deoxy counterparts 10a and 11a were prepared in a similar fashion.63
2.4. 2-Oxoethyl-FAPy
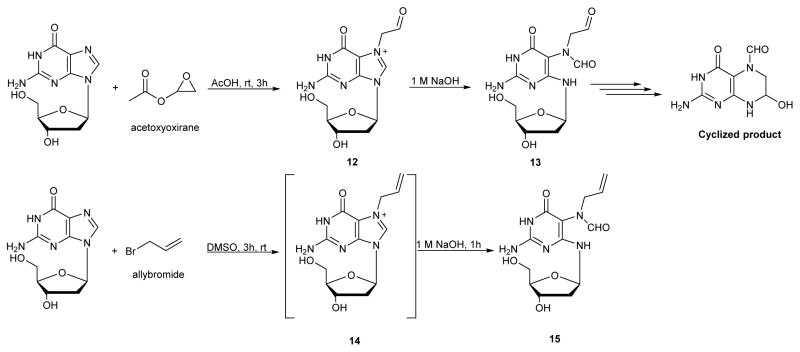
Vinyl chloride is an important industrial chemical classified as a known carcinogen.64–67 It is epoxidized by cytochrome P450 2E1 to chlorooxirane, which reacts extensively with DNA.68 Acetoxyoxirane, an acetylated analogue of chlorooxirane, was used in many studies of DNA alkylation because it is more chemically stable than chlorooxirane, but generates the same types of DNA adducts.69 Acetoxyoxirane can be readily prepared from vinyl acetate and dimethyldioxirane.69
Similar to other simple epoxides, chlorooxirane preferentially alkylates N7G in DNA, and the resulting adducts can undergo imidazole ring opening.69 Christov et. al. reported the first synthesis of oxoethyl-FAPy-dG (Scheme 6).69 In brief, dG was reacted with acetoxyoxirane in acetic acid at room temperature for 3 h to give N7-oxyethyl-dG 12. At pH 7.8, compound 12 was found to undergo two competing reactions: deglycosylation to give an abasic site (major pathway) and imidazole ring opening to give compound 13 in 10 % yield (Scheme 6, top). Compound 13, which was accounted for 10% of total reaction mixture, was highly unstable and underwent spontaneous cyclization to give a ring closed compound (cyclized product, Scheme 6). In a similar way the allyl-FAPy-dG was obtained by the alkylation of dG with allyl bromide to give compound 14 which was further treated with 1M NaOH to give the corresponding FAPy adduct 15 (Scheme 6, bottom).
Scheme 6.
Synthesis of 2-oxyethyl-FAPy-dG.69
2.5. Ethylamine-FAPy
Ethyleneimine (aziridine) is an industrial chemical widely used in the production of polymers, coatings, adhesives, drugs, dyes, cosmetics, and antineoplastic agents.70, 71 Carboxylic acid derivatives of aziridine have been reported as immunomodulators.72 Ethyleneimine is an extremely reactive alkylating agent which targets the N7-purine positions in DNA. In 1984, Hemminki et. al.42 reported that ethyleneimine reactions with guanosine and 2′-deoxyguanosine at pH 6.5 in 0.2 M ammonium formate for 6 h gave rise to N7-dG adducts 16a or 16b (Scheme 7).42 The corresponding ring opened adducts 17a and 17b were formed in 80% yield. Unlike N7 adducts of simple alkylating agents, the conversion of aziridine adducts to the corresponding FAPy structures took place under mild conditions, probably due to protonation of the amino side chain, which facilitates hydroxyl ion attack at C-8 position of N7-alkylated nucleoside (Scheme 7). As described below, a similar mechanism accelerates FAPy adduct formation from antitumor nitrogen mustards.
Scheme 7.
Synthesis of Ethylamine-FAPy-dG.42
2.6. FAPy Adducts of Nitrogen Mustards
Nitrogen mustards (NM) are bis-electrophiles capable of cross-linking DNA to form toxic interstrand and intrastrand cross-links (Figure 5). Nitrogen mustard drugs such as cyclophosphamide, chlorambucil, and mechlorethamine (18–21) are widely used in the treatment of immune diseases, lymphoma, leukemia, multiple myeloma, and ovarian carcinoma (Figure 5).73–76 The antitumor activity of NMs has been attributed to their ability to cross-link the twin strands of DNA.77 The resulting bifunctional lesions, if not repaired, can inhibit DNA replication and transcription, eventually leading to cell cycle arrest, apoptosis, and the inhibition of tumor growth. In 1982, Chetsanga et. al. first reported imidazole ring opening of N7-guanine adducts generated by phosphoramide mustard in DNA.78 Alkylated DNA was treated with 0.2 N NaOH for 30 min at 37 °C to obtain the corresponding FAPy adducts. The reaction mixture contained five isomers of phosphoramide mustard-imidazole ring-opened dG complexes.78 Recently, the Turesky group for the first time detected NM-FAPy adduct in mustard-treated DNA and in human cell culture.79
Figure 5.
Structures of nitrogen mustards.
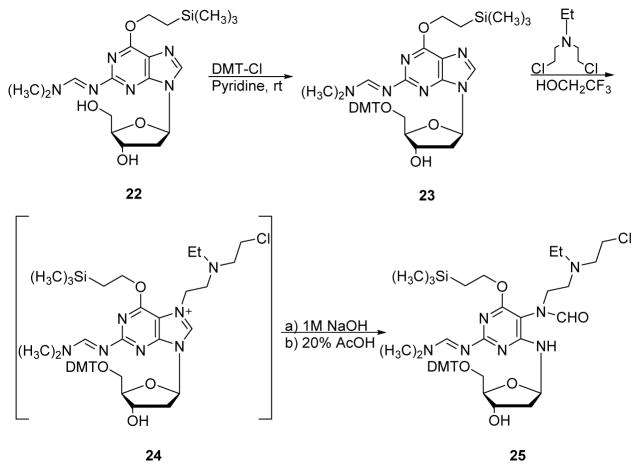
Christov et al. recently reported the first synthesis of NM-FAPy adducts, which were subsequently incorporated into DNA strands by phosphoramidite chemistry (Section 3 below).80 The synthesis began with N2 – formamidine protected compound 22, which was further protected at 5′OH treated with DMT-Cl to give DMT protected dG (23, Scheme 8). Compound 23 was reacted with ethyl nitrogen mustard in trifluoroethanol to give N7-dG intermediate 24, which was not isolated. Further imidazole ring opening of 24 was performed in the presence of 1M NaOH to give NM-FAPy-dG 25 (85% yield).80
Scheme 8.
Synthesis of NM-FAPy-dG by Christov et. al.80
2.7. Aflatoxin B1 (AFB 1)-FAPy-dG
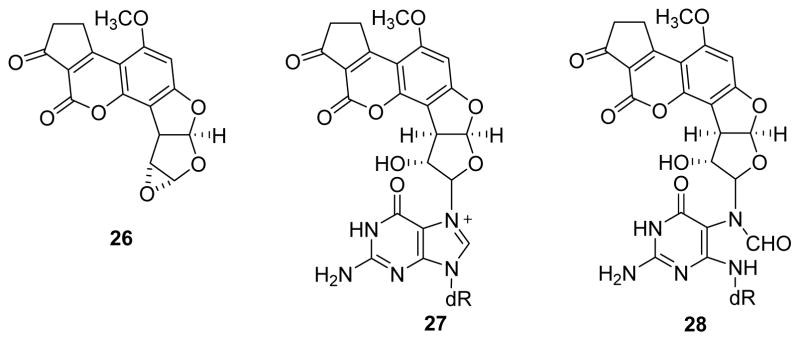
Aflatoxins are carcinogenic mycotoxins produced by certain molds that can contaminate agricultural products such as peanuts and corn.81, 82 Specifically, Aflatoxin B1 is produced by Aspergillus flavus and A. parasiticus and has been implicated in liver cancer in populations consuming contaminated grains.83 Aflatoxin B1 is metabolically activated to epoxide 26 (Figure 6), which is capable of alkylating guanine nucleobases of DNA to give N7-guanine adducts 27, which spontaneously depurinate to give abasic sites.84
Figure 6.
Structures of aflatoxin B1 epoxide 26, its cationic N7-dG adduct 27, and AFB-FAPy-dG 28.
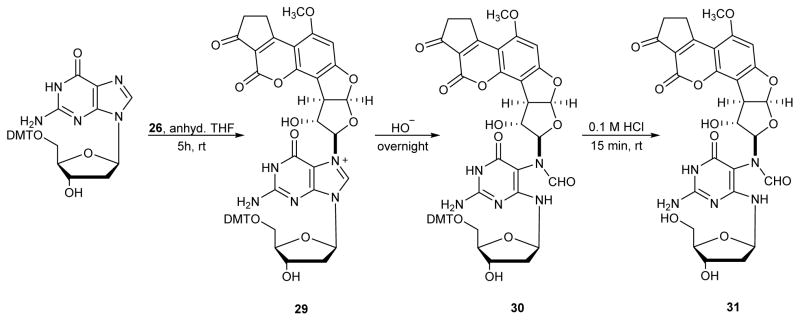
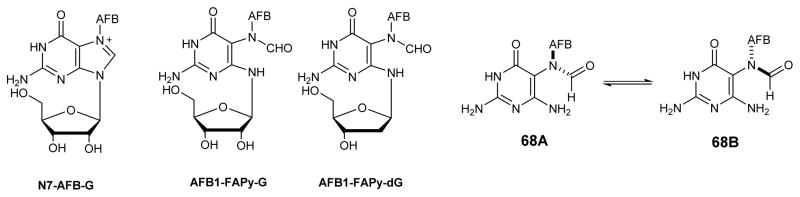
Aflatoxin exposure induces high levels of G→T transversions.85–88 AFB-N7-dG adducts were initially hypothesized to be responsible for these genetic changes.83, 89, 90 However, site-specific mutagenesis experiments have revealed that N7-dG adducts 27 are only weakly mutagenic in E. coli. (4%).91 It was then proposed that apurinic (AP) sites arising from depurination of N7-guanine adducts may be the source for AFB genotoxicity.14, 92 However, Essigmann and coworkers have shown that AFB 1 epoxide treated cells exhibit a unique mutagenic signature distinct from that of AP sites. It was subsequently shown that N7-AFB-dG undergoes imidazole ring opening at physiological pH to give the corresponding AFB-FAPy-dG adduct 28.89, 90 AFB-FAPy-dG formation occurs readily at physiological pH,83 and the resulting lesions are highly mutagenic84, 90, 93–95 and persist in vivo.84, 93, 94 It is now generally accepted that ring open adducts AFB-FAPy-dG (28 in Figure 6) are largely responsible for the key G to T mutations that lead to hepatocarcinoma development following exposure to Aflatoxin B1.96
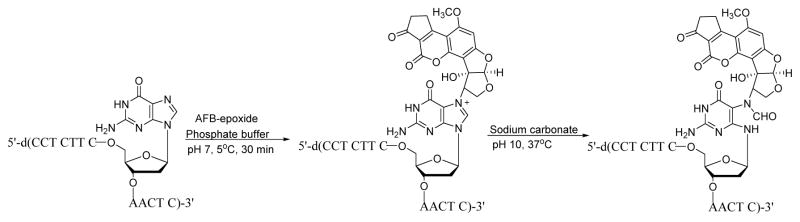
The first synthesis of AFB-FAPy-dG adducts was reported by Harris and coworkers (Scheme 9).82 5′-DMT-protected-2′-deoxyguanosine was treated with AFB epoxide 26 in THF to give the corresponding N7-dG adduct 29. Protected AFB-FAPy-dG 30 was obtained by incubating compound 29 at pH 9.5 overnight (15 mM Na2CO3/30 mM NaHCO3 buffer at ambient temperature). Subsequent detritylation of compound 30 in 0.1 M HCl for 15 min gave AFB-FAPy-dG nucleoside 31.82 The use of 5′-protected dG was critical to prevent anomerization of the sugar (see below Section 3).82
Scheme 9.
Synthesis of AFB-FAPy-dG.82
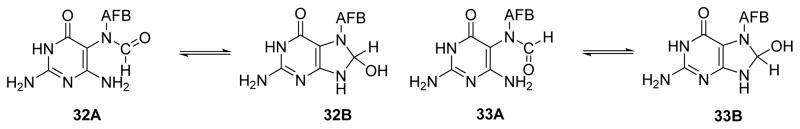
The presence of multiple isomeric forms of AFB-FAPy-dG has made it challenging to deduce the exact chemical structure of AFB-FAPy-dG. Initial NMR studies by Miller and Garner groups have revealed that AFB-FAPy-dG existed as a mixture of at least four isomers.89, 90 These four isomers were isolated by HPLC as two separate peaks, with each fraction containing a pair of inseparable species. Miller and Garner initially hypothesized that the structures of the AFB-FAPy isomers involved ring closed forms 32B and 33B, along with the corresponding ring open structures 32A and 33A (Figure 7).89, 90 Subsequent structural studies by Hertzog et. al.90 have reassigned the structures of AFB-FAPy isomers as two pairs of geometrical isomers 34 and 35, which were in equilibrium with the rotamers 36 and 37 (Figure 8). UV absorption spectra of 34 and 35 were reported to have maxima at 265, 340 and 364 nm, with pH having only a minor effect on the spectra.71 However, the presence of structural isomers 34 and 35, with different position of the formyl group (N5 vs N6), due to different direction of imidazole ring opening following hydroxyl ion attack, was in contrast with previous studies of FAPy adducts generated by methylating agents, sterigmatocystin, and mitomycin, in which the formyl group was always placed at the N5 atom as in 34 and 36.10, 36, 41, 97
Figure 7.
Proposed structures of AFB-FAPy-dG isomers put forward by Miller and others.89
Figure 8.

Structures of AFB-FAPy isomers proposed by Hertzog et. al.90
Most recently, detailed NMR experiments were conducted by the Harris group82 to elucidate the correct structure of AFB-FAPy adducts (Figure 9). Two sets of stereoisomers are possible for AFB-FAPy adducts; (a) geometrical isomers around the formamide group and (b) atropisomers at the pyrimidine C5–N5 bond (38–41 in Figure 9). The first pair of isomers (38/40 and 39/41) forms as a result of rotation about the formamide bond (highlighted in blue, Figure 9). Since aflatoxin is a chiral molecule, this leads to a pair of diastereomers separable by HPLC. Additional isomers (38/39 and 40/41) are produced due to hindered rotation about the C5–N5 bond (highlighted in red). The four isomers (38–41) in Figure 9 are separable by HPLC, but interconvert with each other.82 The rotational barrier for this interconversion is relatively high due to the steric bulk of the AFB substituent at the N5.82 1H NMR spectra of nucleobases 38 and 39 are very similar. The pair of formyl signals are observed at 8.29 and 7.59 ppm (compound 38) and 8.22 and 7.52 ppm (compound 39). These four formyl signals are split into doublets with coupling constants ~17 Hz, confirming the attachment of the formyl group to the N5 position of pyrimidine ring. Similar observations were made for the isomers of FAPy nucleoside 31 (AFB-FAPy-dG). The complete NMR studies of AFB-FAPy-dG included COSY, TOCSY, HMQC, HMBC, NOESY, and ROESY.82
Figure 9.
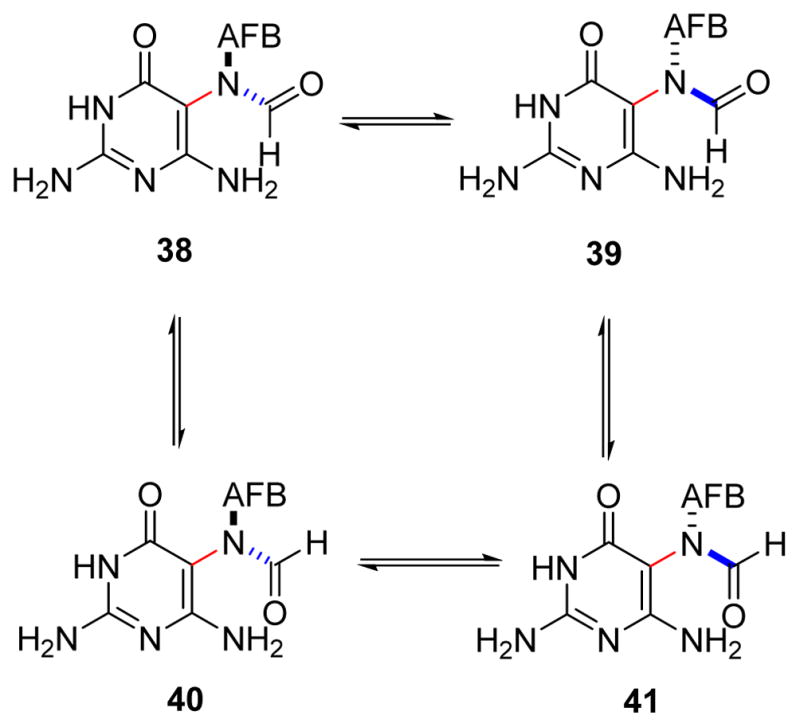
Structures of AFB-FAPy isomers elucidated by Harris et al.82
Structurally related FAPy adducts of sterigmatocystin, a mycotoxin that can form in moldy grain, green coffee, and cheese, have been reported by Baertschi et. al.98 Sterigmatocystin is produced by some strains of Aspergillus, Penicillium, and Bipolaris sp.99, 100 Sterigmatocystin 1,2 epoxide 42 (Scheme 10) was prepared from sterigmatocystin and incubated with DNA for 7 days at 5 °C to form the corresponding N7-alkylated adduct 43. Imidazole ring opening of this adduct was performed by incubating compound 43 at pH 9.8 at 25 C for 2 hr to obtain sterigmatocystin-FAPy 44 (Scheme 10).97, 99, 100
Scheme 10.
FAPy formation of sterigmatocystin 1 epoxide.97
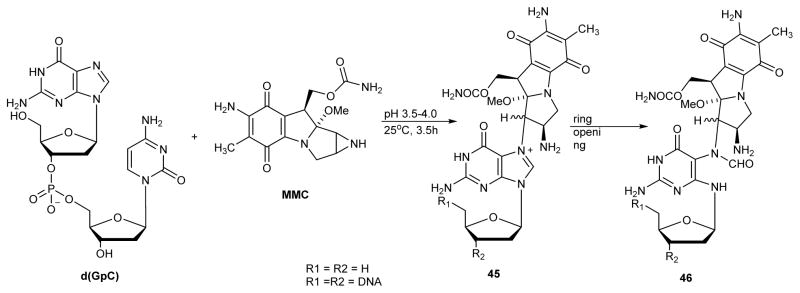
2.8. Mitomycin C-FAPy Adducts
Mitomycin C (MMC, in Scheme 11) is an antitumor antibiotic widely used in cancer chemotherapy.101 It is a bifunctional alkylating agent capable of cross-linking DNA, leading to cell cycle arrest and apoptosis. MMC alkylates N7-G position in DNA to give the corresponding guanine adduct.40 In 1987, Tomasz et. al. reported that the mitomycin C forms FAPy-dG lesions under basic conditions.41 d(GpC) dinucleotide was treated with MMC at pH 3.5–4 to give the corresponding N7-alkylguanine adduct 45. Subsequent imidazole ring opening under basic condition yielded MMC-FAPy-dG 46 (Scheme 11).
Scheme 11.
Synthesis of MMC-FAPy-dG.41
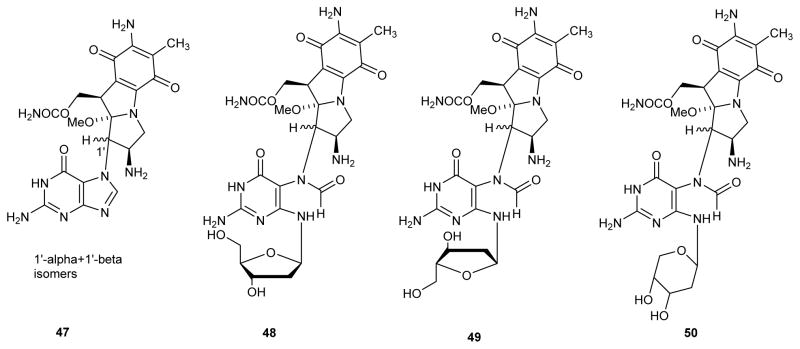
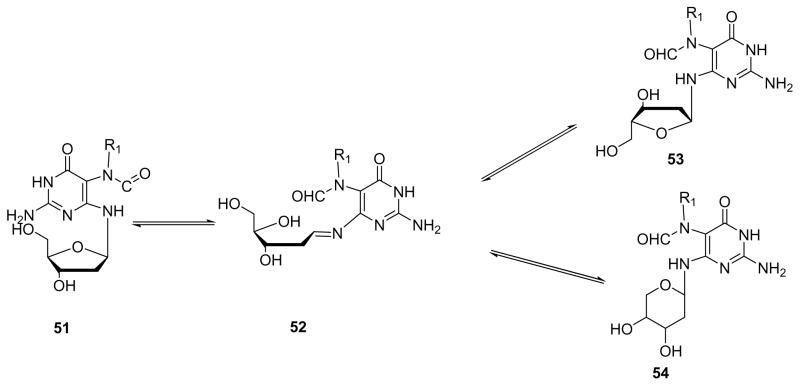
Five isomers of MMC-FAPy-dG (47–50) were identified, including α and β anomers and furanose/ pyranose nucleosides (Figure 10).41 The α anomer 53 may form through imino intermediate 52 (Scheme 12), a rearrangement that can take place in double stranded DNA, but is much faster for free nucleosides. The pyranose isomers of FAPy-dG adducts (54 in Scheme 12) may form by intramolecular attack of C5′-hydroxyl on the imine functionality of intermediate 52.41, 102 It should be noted that the rearrangement to pyranose form is not possible in the absence of a free C5′-hydroxyl group (as in DNA strands).7, 103 Therefore, during nucleoside and phosphoramidite synthesis of FAPy adducts, the formation of pyranose isomers can be minimized by protecting the 5′position of the sugar, typically using the DMT group.57
Figure 10.
Structural isomers of MMC-FAPy-dG.
Scheme 12.
Isomerization of N5-R-FAPy adducts to α, β anomers 51, 53 and pyranose derivatives 54 via an imine intermediate.
3. Synthesis of DNA Oligodeoxynucleotides Containing N5-R-FAPy Adducts
In order to establish the role of N5-substituted FAPy adducts in mutagenicity and to uncover their possible contributions to the therapeutic effects of DNA modifying agents, it is necessary to establish the chemical structures, stability, and mispairing characteristics of N5-R-FAPy adducts. This requires chemical synthesis of DNA molecules containing structurally defined, site-specific N5-R-FAPy adducts. Such a synthesis can be a challenging task due to the unusual structural complexity of this class of adducts and their ability to undergo isomerization.82 Standard solid phase synthesis coupling conditions can result in a mixture of DNA strands containing α and β anomers, as well as both furanose and pyranose forms, and special precautions must be taken to minimize this structural complexity.
Previous attempts to prepare N5-R-FAPy containing DNA strands can be broadly divided into three general approaches; (i) direct treatment of DNA with alkylating agents and base to introduce FAPy, (ii) chemical synthesis of alkylated-FAPy-phosphoramidite building blocks and their incorporation into DNA via solid phase synthesis (SPS), and (iii) solid phase synthesis employing carbocyclic nucleoside analogues.
3.1. Direct Alkylation of DNA to Generate N5-R-FAPy
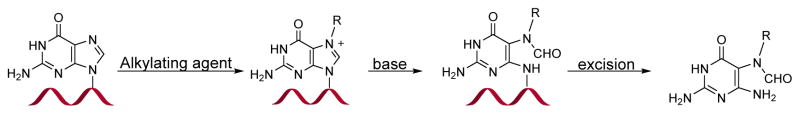
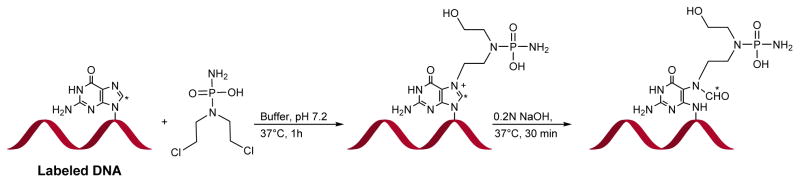
In the most straightforward approach, DNA strands containing a FAPy adduct can be prepared by treating oligodeoxynucleotides containing a single guanine base with alkylating agents, followed by basic treatment to introduce N5-R-FAPy (Scheme 13). This methodology was employed by Brown et al. to generate DNA containing AFB-FAPy-dG.82 Synthetic DNA 13-mer containing a single dG residue was treated with AFB-epoxide in 100 μL phosphate buffer (10 mM sodium phosphate, 100 mM NaCl, pH 7.0) for 30 min (Scheme 14). Further, the alkylated DNA was dissolved in sodium carbonate solution (pH 10) to open the imidazole ring, and the resulting AFB-FAPy-dG containing oligodeoxynucleotide was purified by HPLC. The main limitations of this approach are that it is limited to DNA sequences containing a single guanine and that a mixture of isomers can be generated.
Scheme 13.
Direct treatment of DNA containing a single dG residue to introduce N5-R-FAPy-residue.
Scheme 14.
Synthesis of AFB-FAPy-dG oligonucleotide.82
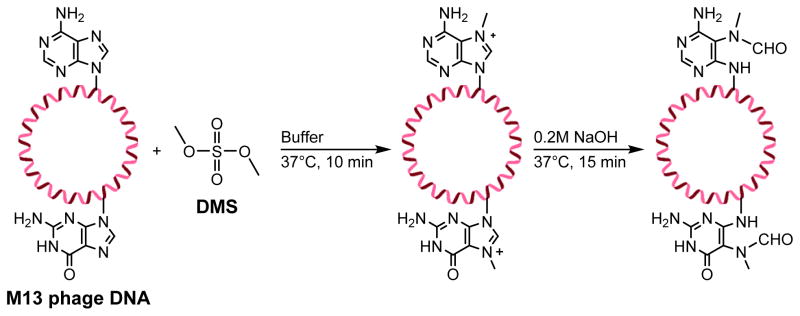
Tudek et al.104 employed a similar direct alkylation approach to study the mutagenic specificity of Me-FAPy-purines in M13mp18 phage DNA. Single stranded M13 phage DNA was incubated with dimethylsulfate (DMS) to obtain DNA containing N7-methyl-dG (83%), and N7-methyl-dA (2.2%) (Scheme 15). This DNA was further incubated in 0.2M NaOH for 15 min at 37 °C to obtain DNA containing the corresponding FAPy adducts.104 It should be noted that this approach induced Methyl-FAPy-dG and Methyl-FAPy-dA adducts at random sites within the plasmid.
Scheme 15.
Direct methylation of M13 phage DNA to form Me-FAPy adducts.111
Chetsanga et. al. treated DNA containing [3H]-dG with phosphoramide mustard. To obtain labelled DNA, a guanine requiring Bacilius Subtilis strain was grown in cell culture supplemented with deoxy[8-3H]guanosine, and cells were lysed by lysosome treatment.78 The purified DNA consisting [3H]guanosine was treated with phosphoramide mustard to obtain alkylated DNA and with 0.2N NaOH to produce PM-FAPy containing DNA (Scheme 16). As in the paper by Tudek et al., this approach does not produce site-specific adducts.104
Scheme 16.
Preparation of DNA containing radilabaled phosphoramide mustard-FAPy adducts.104
3.2. Incorporation of Alkylated-FAPy Adducts into DNA via Phosphoramidite Chemistry
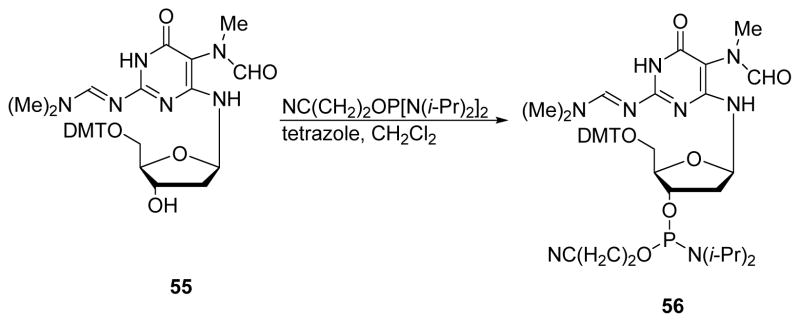
To enable the preparation of DNA strands containing site specific, structurally defined N5-R-FAPy adducts, solid phase synthesis starting with nucleoside phosphoramidites can be employed. In 2008, the Rizzo group reported the synthesis of Me-FAPy-dG phosphoramidite (Scheme 17). Compound 55 (generated as shown in Scheme 3 above) was treated with phosphoramidite reagent in the presence of tetrazole in anhydrous dichloromethane for 2 h at room temperature to obtain Me-FAPy-dG phosphoramidite 56 in 78% yield (Scheme 17).57 This phosphoramidite building block was employed in solid phase synthesis (SPS) experiments in order to incorporate Me-FAPy-dG into short DNA sequence 5′-d(TT-Me-FAPy-dG-TTC)-3′.
Scheme 17.
Synthesis of Me-FAPy-dG phosphoramidite.57
The critical step in the solid phase synthesis of Me-FAPy-dG containing ODNs is the deprotection of the 5′-OH group of Me-FAPy-dG, since the unprotected nucleoside undergoes rapid rearrangement to the pyranose form (Scheme 12 and discussion above).57 During solid phase synthesis, a “short” deprotection step was employed rather than the traditional deprotection step.57 Upon HPLC analysis of enzymatic digests, five Me-FAPy-dG peaks were observed (Figure 11), of which one peak was the pyranose form (Peak 1) whereas other four peaks corresponded to furanose nucleosides in an α and β anomeric forms.57
Figure 11.
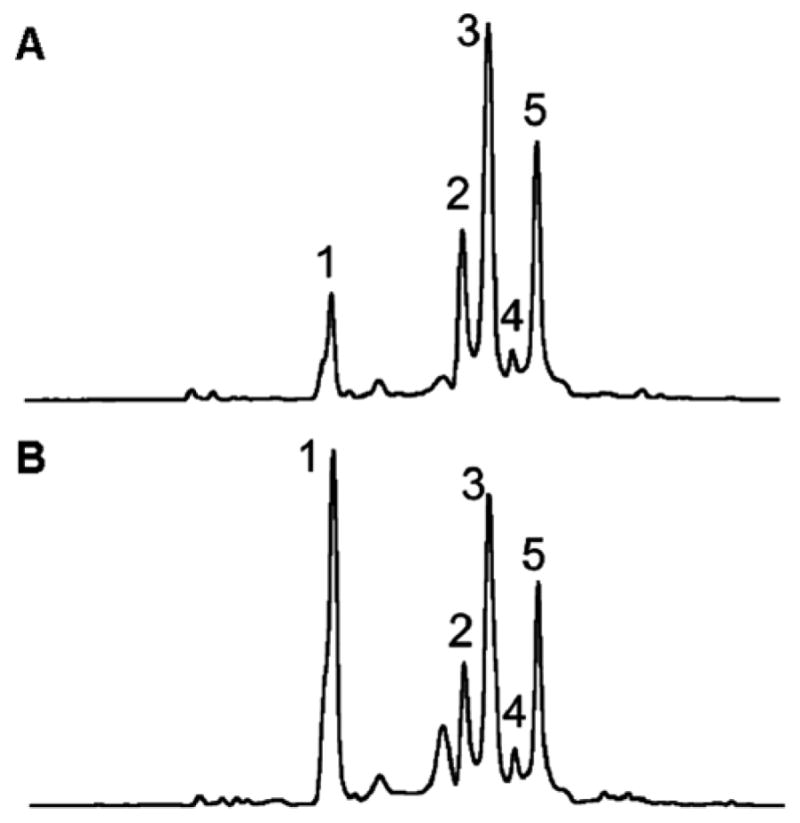
HPLC analysis of ODN 5′-d(TT-Me-FAPy-dG)-TTC-3′. (A) Analysis of ODN synthesis with a short deprotection cycle. (B) Analysis of ODN synthesis with a long deprotection cycle. In both figures, peak 1 represents the formation of pyranose adduct, whereas other peaks are mixture of α and β isomers.57
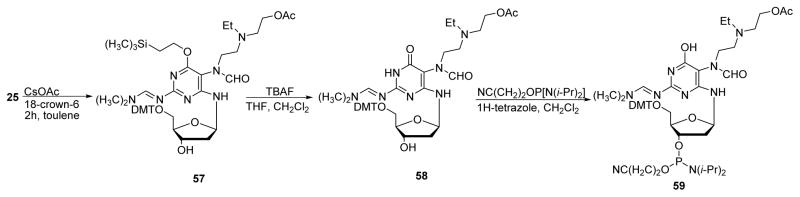
A similar strategy was employed to prepare NM-FAPy-dG building block (59 in Scheme 18).80 The protected NM-FAPy-dG (compound 25) was prepared as shown in Scheme 8. Compound 25 was then treated with cesium acetate in 18-crown-6 ether to replace chloro group of NM with an acetyl group (57). The 6-oxo group was deprotected with TBAF in THF to give compound 58, which upon phosphitylation in dichloromethane in the presence of tetrazole gave phosphoramidite building block 59 in 50% yield. This nucleoside was incorporated into 12 and 24-mer oligodeoxynucleotides by solid phase synthesis.80
Scheme 18.
Preparation of NM-FAPy-dG phosphoramidite building block for solid phase synthesis.80
During automated solid-phase synthesis, a short deprotection cycle was employed in order to minimize the rearrangement of NM-FAPy-dG to the pyranose form of the nucleoside. However, HPLC analysis of the resulting DNA strands revealed two peaks, both having the expected molecular weight and representing furanose and pyranose forms of the adduct. When the standard DNA synthesis protocol (normal deprotection time) was employed, a 1:1 ratio of furanose to pyranose ring containing product formation was observed. The thermal melting profile of the NM-FAPy-containing 12 mers gave inconsistent results due to the presence of α and β anomers (1:1).80
3.3. Carbocyclic Nucleoside Analogues of FAPy
As described above, a major obstacle in solid phase synthesis of FAPy-dG containing DNA strands is that they readily undergo rapid isomerization to give α anomers and pyranose forms under standard DNA synthesis conditions.105, 106 Due to this rapid anomerization, it has only been possible to incorporate α/β anomeric mixtures of FAPy adducts into DNA. Hydrolysis of glycosidic bond of FAPy-dG at elevated temperatures further complicates their synthesis.
To circumvent these problems, Carell et al. have developed the nonhydrolizable and non-epimerizable β analogues of the FAPy-dG lesions.105–107 In this approach, the oxygen atom of the deoxyribose moiety was replaced with a methylene group to give the corresponding carbocyclic nucleoside. This replacement had a minor effect on base pairing.59
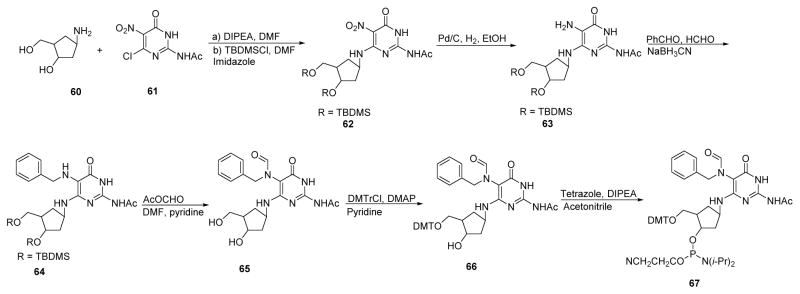
The synthesis of Bz-cFAPy-dG (Scheme 19)105–107 started with enantiomerically pure cyclopentylamine 60, which was synthesized as described by Cullis and Dominguez.108 Coupling of compound 60 with protected 2-amino-6-chloro-5-nitro-4-oxopyrimidine (61) furnished the nitro pyrimidine derivative 62 (86%), which was subsequently subjected to reduction to give the corresponding aminopyrimidine compound 63 (58%). Further, the primary amine of compound 63 was coupled with benzaldehyde and subjected to reduction with sodium cyanoborohydride to give the N5-benzylated compound 64. The formyl group was introduced at the C-5 position by reaction of compound 64 with acetic formic anhydride to give Bz-cFAPy-dG 65 (89%) (Scheme 19).
Scheme 19.
To prepare the nucleoside phosphoramidite of 65, compound 65 was protected at 5′-OH by DMTCl in pyridine at room temperature to give the DMT derivative 66 in 55% yield (Scheme 19). Phosphitylation of 66 was carried out under argon in acetonitrile in the presence of tetrazole and DIPEA to give phosphoramidite 67 in 54% yield, which was subsequently incorporated into DNA. These authors reported that since the standard capping procedure during DNA synthesis was not compatible with Bz-cFAPy-dG, a solution of 2,6-lutidine and pivaloylic anhydride in THF (v/v/v 1:1:8) was used in place of phenoxyacetyl anhydride for capping, and 4,5-dicyanoimidazole (0.25 M) was used as the coupling reagent. The coupling time for the Bz-cFAPy-dG phosphoramidite was much longer compared to standard phosphoramidite methods (10 min vs 144 s).106
DNA strands containing Bz-cFAPy-dG were purified by HPLC and analyzed by MALDI-TOF. LC-MS of enzymatic digests showed no structural alteration of Bz-cFAPy-dG during ODN synthesis and purification. Thermodynamic studies of c-FAPy (no substitution on the formamide group) oligodeoxynucleotides revealed that c-FAPy conferred significant duplex destabilization.105, 106 Interestingly, the base excision repair enzyme Fpg recognized the unnatural N7-benzyl-cFAPy-dG lesion via an unproductive binding mode, leading to enzyme inhibition.107
4. Effects of N5-R-FAPy Adducts on DNA Replication
4.1. Methyl-FAPy-dG
Structurally, ring open N5-R-FAPy adducts are substituted pyrimidines, and are expected to mispair with purines during DNA replication. However, initial studies with bacterial DNA polymerases (e.g. Klenow fragment of E. coli. DNA polymerase I) have shown that Methyl-FAPy-dG blocked bacterial DNA replication in vitro, but did not induce any mutations.11, 53, 109, 110 Similarly, O’Connor and others reported that E. coli. DNA polymerase I exo and T4 DNA polymerases were inhibited one nucleotide before Me-FAPy-G.11 Inhibition of DNA synthesis by Me-FAPy-G is stronger than ROS-induced lesion 8-oxo-dG, but is weaker than that of apurinic sites and FAPy-A (FAPy-Ade ≈AP site>FAPy-7Me-G>8-oxoG).54, 111
Rizzo et al. examined in vitro bypass of Me-FAPy-dG in the presence of eukaryotic DNA polymerases α, β, and hPol δ/PCNA.112 Me-Fapy-dG blocked high fidelity polymerases at either the insertion or the extension step. Translesion synthesis was observed for hPols η, κ, and hRev1/Pol ζ. These polymerases primarily inserted the correct base (C) opposite the lesion, however hPols η and κ also catalyzed the misinsertion of Thy, Gua, and Ade opposite Me-Fapy-dG, and generated a single nucleotide deletion product. These authors concluded that although the amounts of Me-FAPy-dG lesions in cells are relatively low, their miscoding potential could contribute to genomic instability.112
Tudek et. al.111, 113 investigated the biological properties of Me-Fapy-dG and Me-Fapy-dA in M13mp18 phage DNA. Lesions containing plasmids were generated as described above in section 3.1 (Scheme 15), and were transfected into E. coli. The presence of Me-FAPy adducts led to a significant decrease in transfection efficiency and increased mutational frequency in the lacZ gene following SOS induction.111, 113 However, sequencing analyses have revealed primarily A →G transitions, while mutations at GC base pairs were only slightly elevated. These results suggest that Me-FAPy-G is primarily a lethal lesion in E. coli. In contrast, the corresponding Me-FAPy-A adducts are a more miscoding, causing A→G transitions.54, 104 Me-FAPy-A (Figure 12) was two orders of magnitude more mutagenic than Me-FAPy-G.54, 111
Figure 12.
Structures of N5-R-FAPy lesions investigated in biological studies.
4.2. AFB-FAPy-G
The biological outcomes of AFB1-FAPy-dG adducts (Figure 13) have been examined in detail due to their proposed roles in aflatoxin-mediated liver cancer. In contrast to N7-AFB-G, AFB1-FAPy-G is highly persistent in rat liver DNA, reaching maximum adduct amounts 2 weeks after exposure.89 One of the AFB1-FAPy rotamers (68A in Figure 13) has been shown to be a potent block to DNA synthesis, even when DNA polymerase of lowered replication fidelity was used (MucAB).96 Both AFB1-FAPy-G and N7-AFB1-G caused G→T transversions, which is consistent with the observed G→ T mutations in codon 249 of the p53 tumor suppressor gene in 50% of hepatocellular carcinomas and in AFB1-treated human hepatocytes cultures.51, 52 In addition, AFB1-induced G→ T mutations in the ras oncogene appear to be important for liver tumor progression.86, 87 Taken together, these results indicate that AFB1-FAPy adducts may be the ultimate lesions responsible for mutagenesis and genotoxicity of aflatoxin.96, 114
Figure 13.
Structures of N7-AFB1-G and AFB1-FAPy lesions investigated in biological studies.82
5. Cellular Repair of N5-R-FAPy Adducts
Many of the previous studies of FAPy adduct repair have been limited to unsubstituted FAPy induced by ROS.49, 50, 53 Repair studies of N5-substituted FAPy-adducts are less extensive, and several examples are given below.
FPG glycosylase: Formamidopyrimidine DNA glycosylase (Fpg) was first identified in 1978–1979 as a DNA glycosylase that removes Me-FAPy-G from DNA.13 Along with Me-FAPy-G, this glycosylase also excises ROS-induced unsubstituted FAPy-G, unsubstituted FAPy-A, as well as damaged pyrimidines and 8-oxo-dG.13,115–119 Substituent size on the N5 position of -the adduct has been shown to influence enzyme activity. For example, Tudek et. al. have shown that Me-FAPy-G was excised by Fpg 7-times faster than Et-FAPy-G.120 It is not known whether other N5-R-FAPY adducts are also substrates for this repair pathway.
yOgg 1: 8-oxo-G DNA glycosylase (yOgg 1) excises Me-FAPy-G, but does not remove Me-FAPy-A.121, 122 Furthermore, Fpg and its eukaryotic homolog Ogg1 have been reported to recognize unsubstituted FAPy-dG and the carbocyclic analog of Bz-FAPy-dG (Bz-cFAPy-dG, Figure 14) with high affinity.
hNEIL and mNEIL 1: Both hNEIL 1 (human NEIL 1) and mNEIL 1 (mouse NEIL 1) excised Me-FAPy-G, along with a number of pyrimidine-derived nucleobase lesions. However, hNEIL is the only human enzyme that excises FAPy-Ade (unsubstituted).123–127
E. coli. Endonuclease IV: E. coli. Endonucleoase IV (Endo IV) is an AP endonuclease specific for double stranded DNA . It also removes the 3′-blocking phosphate groups128–130 and possess the 3′ →5′ endonuclease activity.131, 132 ODNs containing α-dA, α-dT, α-dC, α-FAPy-dG, α-FAPy-dA lesions are substrates to Endo IV.133–137 Asagoshi et. al. reported that oligodeoxynucleotides containing α-Me-FAPy-dG are not substrates for Endo IV.110, 138 However, in 2000 Christov and others showed that α-Me-FAPy-dG is indeed a substrate for Endo IV. 139 The Me-FAPy-dG lesion is a substrate for Fpg/Nei family of glycosylases as well. It is possible that Endo IV and Fpg glycosylases play specialized roles in FAPy adduct repair, with Endo IV recognizing only the α-anomer of N5-alkyl-FAPy lesions and Fpg glycosylase recognizing the β-anomer form of the adduct.
Nucleotide excision repair Alekseyev and Essigmann reported that Aflatoxin B1 formamidopyrimidine adducts (AFB1-FAPy-dG, Figure 13) are preferentially repaired by the nucleotide excision repair pathway in vivo.140 These authors transfected plasmids containing site-specific AFB1-FAPy-dG lesions into E.coli cells and employed the host cell reactivation assay to monitor lesion repair in wild type cells and their repair deficient clones. Cells deficient in nucleotide excision repair (uvrA) were unable to remove the damage, while BER mutants (mutM) were affected to a lesser extent.140 This was confirmed by in vitro experiments with site-specifically modified oligodeoxynucleotides and purified MutM protein, which revealed excision products characteristic of NER.140
Figure 14.
Rotamers of Bz-cFAPy-dG.107
6. Future Prospects and Outlook
Although the chemistry and biology of the N5-substituted FAPy lesions have been a subject of interest for several decades, the majority of the published studies have focused on two types of FAPy lesions (Me-FAPy and AFB-FAPy). The chemical biology of other N5-R-FAPy adducts is incompletely understood, and their roles in chemical carcinogenesis remain unknown. Based on the published studies of Me-FAPy and AFB-FAPy, such lesions may be extremely important for the biological mechanisms of many DNA damaging agents, if formed in vivo. However, with a few exceptions, it is not known whether significant amounts of N5-R-FAPy adducts form in cells treated with DNA alkylating drugs and environmental agents. Future mass spectrometry based studies are urgently needed to establish the concentrations of these secondary adducts in cells and tissues. Chemical synthesis of DNA strands containing structurally defined N5-R-FAPy represents a significant challenge due to their propensity to undergo isomerization. Furthermore, FAPy lesions exist as a mixture of rotamers which present a range of possibilities for base pairing due to changes in hydrogen bond donor acceptor patterns. Future studies employing novel solid phase synthesis methodologies are needed in order to establish the relationship between N5-R-FAPy adduct structures and their biological outcomes, as well as to elucidate their effects on DNA structure.
Acknowledgments
Funding information:
Funding for this research was provided by grants from the National Cancer Institute (CA1006700), the National Institute of Environmental Health Sciences (ES023350), and University of Minnesota College of Pharmacy.
We thank Arnold Groehler IV (University of Minnesota) for helpful discussions and Bob Carlson (Masonic Cancer Center, University of Minnesota) for preparing figures for this manuscript and editorial assistance.
Abbreviations
- FAPy
formamidopyridines
- dG
2′-deoxyguanosine
- dA
2′-deoxyadenosine
- DNA
deoxynucleic acid
- N7-heG
N7-(2-hydroxy)ethyl guanine
- PM
phosphoramide
- NM
nitrogen mustard
- MMC
mitomycin C
- AFB
aflatoxin
- THF
tetrahydofuran
- DCM
dichloromethane
- CH3I
methyliodide
- HCl
hydrochloric acid
- DMSO
dimethylsulfoxide
- NaOH
sodium hydroxide
- AcOH
acetic acid
- EI
ethyleneimine
- DMTCl
dimethoxytrityl chloride
- HPLC
high performance liquid chromatography
- UV
ultraviolet
- NMR
nuclear magnetic resonance
- ppm
parts per million
- Me
methyl
- ROS
reactive oxygen species
- ODN
oligodeoxynucleotides
- DMS
dimethylsulfate
- Na2CO3
sodium carbonate
- NaHCO3
sodium bicarbonate
- AFB-FAPy
aflatoxin formamidopyrimidine
- Me-FAPy
methyl formamidopyrimidine
- Et-FAPy
ethyl formamidopyrimidine
- MMC-FAPy
mitomycin C formamidopyridine
- NM-FAPy
nitrogen mustard formamidopyridine
- PM-FAPy
phosphoramide formamidopyridine
- Bz-cFAPy
benzyl carbocyclic formamidopyridine
Biographies

Dr. Suresh S. Pujari received his M.Sc. from Karnatak University, Dharwad, India and worked as a project assistant at National Chemical Laboratory, Pune, India. He pursued his Ph.D. from the University of Osnabrueck, Germany (Dr. Frank Seela) in Chemistry. After Ph.D, he worked as a postdoctoral fellow at Clemson University, Clemson, USA. Currently, he is working as a postdoctoral associate in Dr. Tretyakova’s group at the Department of Medicinal Chemistry, Masonic Cancer Center and the College of Pharmacy, University of Minnesota, USA.

Dr. Natalia Tretyakova received her M.S. degree from Moscow State University in Russia and her Ph.D. from the University of North Carolina-Chapel Hill, where she worked with James A. Swenberg. She did her postdoctoral work with Steven R. Tannenbaum at the Massachusetts Institute of Technology. She is a Professor of Medicinal Chemistry at the Masonic Cancer Center and the College of Pharmacy, University of Minnesota.
Footnotes
Final version of this manuscript is available at http://pubs.acs.org/doi/abs/10.1021/acs.chemrestox.6b00392
References
- 1.Townsend LB, Robins RK. Ring Cleavage of Purine Nucleosides to Yield Possible Biogenetic Precursors of Pteridines and Riboflavin. J Am Chem Soc. 1963;85:242–243. [Google Scholar]
- 2.Haines JA, Reese CB, Todd L. 1009. The methylation of guanosine and related compounds with diazomethane. J Chem Soc (Resumed) 1962:5281–5288. [Google Scholar]
- 3.Chetsanga CJ, Bearie B, Makaroff C. Alkaline opening of imidazole ring of 7-methylguanosine. 1 Analysis of the resulting pyrimidine derivatives. Chem Biol Interact. 1982;41:217–233. doi: 10.1016/0009-2797(82)90091-6. [DOI] [PubMed] [Google Scholar]
- 4.Chetsanga CJ, Makaroff C. Alkaline opening of imidazole ring of 7-methylguanosine. 2 Further studies on reaction mechanisms and products. Chem Biol Interact. 1982;41:235–249. doi: 10.1016/0009-2797(82)90092-8. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SM, Adams BL, Kozarich JW. Chemical transformations of 7,9-disubstituted purines and related heterocycles. Selective reduction of imines and immonium salts. J Org Chem. 1976;41:2303–2311. doi: 10.1021/jo00875a019. [DOI] [PubMed] [Google Scholar]
- 6.Darzynkiewicz E, Labadi I, Haber D, Burger K, Lonnberg H. 7-Methylguanine nucleotides and their structural analogues; protolytic equilibria, complexing with magnesium (II) ion and kinetics for alkaline opening of the imidazole ring. Acta Chem Scand B. 1988;42:86–92. doi: 10.3891/acta.chem.scand.42b-0086. [DOI] [PubMed] [Google Scholar]
- 7.Gates KS, Nooner T, Dutta S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem Res Toxicol. 2004;17:839–856. doi: 10.1021/tx049965c. [DOI] [PubMed] [Google Scholar]
- 8.Gates KS. An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brookes P, Lawley PD. 766. The alkylation of guanosine and guanylic acid. J Chem Soc (Resumed) 1961:3923–3928. [Google Scholar]
- 10.Boiteux S, Belleney J, Roques BP, Laval J. Two rotameric forms of open ring 7-methylguanine are present in alkylated polnucleotides. Nucleic Acids Res. 1984;12:5429–5439. doi: 10.1093/nar/12.13.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor TR, Boiteux S, Laval J. Ring-opened 7-methylguanine residues in DNA are a block to in vitro DNA synthesis. Nucleic Acids Res. 1988;16:5879–5894. doi: 10.1093/nar/16.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawley PD, Brookes P. Acidic Dissociation of 7 : 9-Dialkylguanines and its Possible Relation to Mutagenic Properties of Alkylating Agents. Nature. 1961;192:1081–1082. doi: 10.1038/1921081b0. [DOI] [PubMed] [Google Scholar]
- 13.Chetsanga CJ, Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979;6:3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 15.Sowers LC, Sedwick WD, Shaw BR. Hydrolysis of N3-methyl-2′-deoxycytidine: model compound for reactivity of protonated cytosine residues in DNA. Mutat Res. 1989;215:131–138. doi: 10.1016/0027-5107(89)90225-x. [DOI] [PubMed] [Google Scholar]
- 16.Lawley PD, Brookes P. Further studies on the alkylation of nucleic acids and their constituent nucleotides. Biochem J. 1963;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawley PD, Warren W. Removal of minor methylation products 7-methyladenine and 3-methylguanine from DNA of Escherichia coli treated with dimethyl sulphate. Chem Biol Interact. 1976;12:211–220. doi: 10.1016/0009-2797(76)90100-9. [DOI] [PubMed] [Google Scholar]
- 18.Boiteux S, Huisman O, Laval J. 3-Methyladenine residues in DNA induce the SOS function sfiA in Escherichia coli. EMBO J. 1984;3:2569–2573. doi: 10.1002/j.1460-2075.1984.tb02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer B. Reaction of guanosine with ethylating agents. Biochemistry. 1972;11:3939–3947. doi: 10.1021/bi00771a017. [DOI] [PubMed] [Google Scholar]
- 20.Nooner T, Dutta S, Gates KS. Chemical properties of the leinamycin-guanine adduct in DNA. Chem Res Toxicol. 2004;17:942–949. doi: 10.1021/tx049964k. [DOI] [PubMed] [Google Scholar]
- 21.Guy A, Molko D, Wagrez L, Téoule R. Chemical Synthesis of Oligonucleotides Containing N6-Methyladenine Residues in the GATC Site. Helv Chim Acta. 1986;69:1034–1040. [Google Scholar]
- 22.Moschel RC, Hudgins WR, Dipple A. Substituent-induced effects on the stability of benzylated guanosines: model systems for the factors influencing the stability of carcinogen-modified nucleic acids. J Org Chem. 1984;49:363–372. [Google Scholar]
- 23.Müller N, Eisenbrand G. The influence of N7 substituents on the stability of N7-alkylated guanosines. Chem-Biol Interact. 1985;53:173–181. doi: 10.1016/s0009-2797(85)80094-6. [DOI] [PubMed] [Google Scholar]
- 24.Hendler SS, Fuerer E, Srinivasan PR. Synthesis and chemical properties of monomers and polymers containing 7-methylguanine and an investigation of their substrate or template properties for bacterial deoxyribonucleic acid or ribonucleic acid polymerases. Biochemistry. 1970;9:4141–4153. doi: 10.1021/bi00823a017. [DOI] [PubMed] [Google Scholar]
- 25.D’Andrea AD, Haseltine WA. Modification of DNA by aflatoxin B1 creates alkali-labile lesions in DNA at positions of guanine and adenine. Proc Natl Acad Sci U S A. 1978;75:4120–4124. doi: 10.1073/pnas.75.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coles BF, Welch AM, Hertzog PJ, Lindsay SJ, Garner RC. Biological and chemical studies on 8,9-dihydroxy-8,9-dihydro-aflatoxin B1 and some of its esters. Carcinogenesis. 1980;1:79–90. doi: 10.1093/carcin/1.1.79. [DOI] [PubMed] [Google Scholar]
- 27.Johnston DS, Stone MP. Replication of a site-specific trans-8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B(1) adduct by the exonuclease deficient klenow fragment of DNA polymerase I. Chem Res Toxicol. 2000;13:1158–1164. doi: 10.1021/tx000129m. [DOI] [PubMed] [Google Scholar]
- 28.Craddock VM, Henderson AR. Effect of N-nitrosamines carcinogenic for oesophagus on O6-alkyl-guanine-DNA-methyl transferase in rat oesophagus and liver. J Cancer Res Clin Oncol. 1986;111:229–236. doi: 10.1007/BF00389238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margison GP, O’Connor PJ. Nucleic acid modification by N-nitroso compounds. In: Grover PL, editor. Chemical carcinogens and DNA. CRC Press; Boca Raton, FL: 1979. pp. 11–159. [Google Scholar]
- 30.Lawley PD. Carcinogenesis by alkylating agents. In: SCE, editor. Chemical Carcinogens. Vol. 173. American Chemical Society Monograph; Washington, DC: 1976. pp. 83–224. [Google Scholar]
- 31.Miller EC, Miller JA. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981;47:2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Singer B, Kusmierek JT. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- 33.Hsiung H, Lown JW. Effects of alkylation by dimethyl sulfate, nitrogen mustard, and mitomycin C on DNA structure as studied by the ethidium binding assay. Can J Biochem. 1976;54:1047–1054. doi: 10.1139/o76-153. [DOI] [PubMed] [Google Scholar]
- 34.McGhee JD, Felsenfeld G. Reaction of nucleosome DNA with dimethyl sulfate. Proc Natl Acad Sci U S A. 1979;76:2133–2137. doi: 10.1073/pnas.76.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyrtopoulos SA. DNA adducts in humans after exposure to methylating agents. Mutat Res. 1998;405:135–143. doi: 10.1016/s0027-5107(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 36.Kadlubar FF, Beranek DT, Weis CC, Evans FE, Cox R, Irving CC. Characterization of the purine ring-opened 7-methylguanine and its persistence in rat bladder epithelial DNA after treatment with the carcinogen N-methylnitrosourea. Carcinogenesis. 1984;5:587–592. doi: 10.1093/carcin/5.5.587. [DOI] [PubMed] [Google Scholar]
- 37.Beranek DT, Weis CC, Evans FE, Chetsanga CJ, Kadlubar FF. Identification of N5-methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine as a major adduct in rat liver DNA after treatment with the carcinogens, N,N-dimethylnitrosamine or 1,2-dimethylhydrazine. Biochem Biophys Res Commun. 1983;110:625–631. doi: 10.1016/0006-291x(83)91195-6. [DOI] [PubMed] [Google Scholar]
- 38.Maccubbin AE, Caballes L, Riordan JM, Huang DH, Gurtoo HL. A cyclophosphamide/DNA phosphoester adduct formed in vitro and in vivo. Cancer Res. 1991;51:886–892. [PubMed] [Google Scholar]
- 39.Johnson LA, Malayappan B, Tretyakova N, Campbell C, MacMillan ML, Wagner JE, Jacobson PA. Formation of cyclophosphamide specific DNA adducts in hematological diseases. Pediatr Blood Cancer. 2012;58:708–714. doi: 10.1002/pbc.23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore HW. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977;197:527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- 41.Tomasz M, Lipman R, Lee MS, Verdine GL, Nakanishi K. Reaction of acid-activated mitomycin C with calf thymus DNA and model guanines: elucidation of the base-catalyzed degradation of N7-alkylguanine nucleosides. Biochemistry. 1987;26:2010–2027. doi: 10.1021/bi00381a034. [DOI] [PubMed] [Google Scholar]
- 42.Hemminki K. Reactions of ethyleneimine with guanosine and deoxyguanosine. Chem Biol Interact. 1984;48:249–260. doi: 10.1016/0009-2797(84)90138-8. [DOI] [PubMed] [Google Scholar]
- 43.Hansen MR, Hurley LH. Pluramycins. Old Drugs Having Modern Friends in Structural Biology. Acc Chem Res. 1996;29:249–258. [Google Scholar]
- 44.Coleman RS, Burk CH, Navarro A, Brueggemeier RW, Diaz-Cruz ES. Role of the azinomycin naphthoate and central amide in sequence-dependent DNA alkylation and cytotoxicity of epoxide-bearing substructures. Org Lett. 2002;4:3545–3548. doi: 10.1021/ol0267275. [DOI] [PubMed] [Google Scholar]
- 45.Hartley JA, Hazrati A, Kelland LR, Khanim R, Shipman M, Suzenet F, Walker LF. A Synthetic Azinomycin Analogue with Demonstrated DNA Cross-Linking Activity: Insights into the Mechanism of Action of this Class of Antitumor Agent. Angew Chem Int Ed. 2000;39:3467–3470. [PubMed] [Google Scholar]
- 46.Kim MS, Guengerich FP. Polymerase blockage and misincorporation of dNTPs opposite the ethylene dibromide-derived DNA adducts S-[2-(N7-guanyl)ethyl]glutathione, S-[2-(N2-guanyl)ethyl]glutathione, and S-[2-(O6-guanyl)ethyl]glutathione. Chem Res Toxicol. 1998;11:311–316. doi: 10.1021/tx970206m. [DOI] [PubMed] [Google Scholar]
- 47.Valadez JG, Guengerich FP. S-(2-chloroethyl)glutathione-generated p53 mutation spectra are influenced by differential repair rates more than sites of initial dna damage. J Biol Chem. 2004;279:13435–13446. doi: 10.1074/jbc.M312358200. [DOI] [PubMed] [Google Scholar]
- 48.Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg MM. The formamidopyrimidines: purine lesions formed in competition with 8-oxopurines from oxidative stress. Acc Chem Res. 2012;45:588–597. doi: 10.1021/ar2002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dizdaroglu M, Kirkali G, Jaruga P. Formamidopyrimidines in DNA: mechanisms of formation, repair, and biological effects. Free Radic Biol Med. 2008;45:1610–1621. doi: 10.1016/j.freeradbiomed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hot spot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 52.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 53.Boysen G, Pachkowski BF, Nakamura J, Swenberg JA. The formation and biological significance of N7-guanine adducts. Mutat Res. 2009;678:76–94. doi: 10.1016/j.mrgentox.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tudek B. Imidazole ring-opened DNA purines and their biological significance. J Biochem Mol Biol. 2003;36:12–19. doi: 10.5483/bmbrep.2003.36.1.012. [DOI] [PubMed] [Google Scholar]
- 55.Rydberg B, Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J. 1982;1:211–216. doi: 10.1002/j.1460-2075.1982.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humphreys WG, Guengerich FP. Structure of formamidopyrimidine adducts as determined by NMR using specifically 15N-labeled guanosine. Chem Res Toxicol. 1991;4:632–636. doi: 10.1021/tx00024a005. [DOI] [PubMed] [Google Scholar]
- 57.Christov PP, Brown KL, Kozekov ID, Stone MP, Harris TM, Rizzo CJ. Site-specific synthesis and characterization of oligonucleotides containing an N6-(2-deoxy-D-erythro-pentofuranosyl)-2,6-diamino-3,4-dihydro-4-oxo-5-N-methylfor mamidopyrimidine lesion, the ring-opened product from N7-methylation of deoxyguanosine. Chem Res Toxicol. 2008;21:2324–2333. doi: 10.1021/tx800352a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Delft JH, van Weert EJ, Schellekens MM, Claassen E, Baan RA. The isolation of monoclonal antibodies selected for the detection of imidazole ring-opened N7-ethylguanine in purified DNA and in cells in situ. Crossreaction with methyl, 2-hydroxyethyl and sulphur mustard adducts. Carcinogenesis. 1991;12:1041–1049. doi: 10.1093/carcin/12.6.1041. [DOI] [PubMed] [Google Scholar]
- 59.Farmer PB, Foster AB, Jarman M, Tisdale MJ. The alkylation of 2′-deoxyguanosine and of thymidine with diazoalkanes. Some observations on o-alkylation. The Biochemical journal. 1973;135:203–213. doi: 10.1042/bj1350203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segerback D. Alkylation of DNA and hemoglobin in the mouse following exposure to ethene and ethene oxide. Chem Biol Interact. 1983;45:139–151. doi: 10.1016/0009-2797(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 61.Filser JG, Denk B, Tornqvist M, Kessler W, Ehrenberg L. Pharmacokinetics of ethylene in man; body burden with ethylene oxide and hydroxyethylation of hemoglobin due to endogenous and environmental ethylene. Arch Toxicol. 1992;66:157–163. doi: 10.1007/BF01974008. [DOI] [PubMed] [Google Scholar]
- 62.Walker VE, Fennell TR, Boucheron JA, Fedtke N, Ciroussel F, Swenberg JA. Macromolecular adducts of ethylene oxide: a literature review and a time-course study on the formation of 7-(2-hydroxyethyl)guanine following exposures of rats by inhalation. Mutat Res. 1990;233:151–164. doi: 10.1016/0027-5107(90)90159-2. [DOI] [PubMed] [Google Scholar]
- 63.Roe R, Paul JS, Montgomery POB. Synthesis and PMR spectra of 7-hydroxyalkylguanosinium acetates. J Heterocycl Chem. 1973;10:849–857. [Google Scholar]
- 64.Lee FI, Harry DS. Angiosarcoma of the liver in a vinyl-chloride worker. Lancet. 1974;303:1316–1318. doi: 10.1016/s0140-6736(74)90683-7. [DOI] [PubMed] [Google Scholar]
- 65.Marsteller HJ, Lelbach WK, Muler R, Gedigk P. Unusual splenomegalic liver disease as evidenced by peritoneoscopy and guided liver biopsy among polyvinyl chloride production workers. Ann N Y Acad Sci. 1975;246:95–134. doi: 10.1111/j.1749-6632.1975.tb51085.x. [DOI] [PubMed] [Google Scholar]
- 66.Nicholson WJ, Hammond EC, Seidman H, Selikoff IJ. Mortality experience of a cohort of vinyl chloride-polyvinyl chloride workers. Ann N Y Acad Sci. 1975;246:225–230. doi: 10.1111/j.1749-6632.1975.tb51096.x. [DOI] [PubMed] [Google Scholar]
- 67.Creech JL, Jr, Johnson MN. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Med. 1974;16:150–151. [PubMed] [Google Scholar]
- 68.Guengerich FP, Crawford WM, Watanabe PG. Activation of vinyl chloride to covalently bound metabolites: roles of 2-chloroethylene oxide and 2-chloroacetaldehyde. Biochemistry. 1979;18:5177–5182. doi: 10.1021/bi00590a023. [DOI] [PubMed] [Google Scholar]
- 69.Christov PP, Kozekov ID, Rizzo CJ, Harris TM. The formamidopyrimidine derivative of 7-(2-oxoethyl)-2′-deoxyguanosine. Chem Res Toxicol. 2008;21:1777–1786. doi: 10.1021/tx800142m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hata Y, Watanabe M, Tonda K, Hirata M. Aziridine biotransformation by microsomes and lethality to hepatocytes isolated from rat. Chem Biol Interact. 1987;63:171–184. doi: 10.1016/0009-2797(87)90096-2. [DOI] [PubMed] [Google Scholar]
- 71.Verschaeve L, Kirsch-Volders M. Mutagenicity of ethyleneimine. Mutat Res. 1990;238:39–55. doi: 10.1016/0165-1110(90)90038-d. [DOI] [PubMed] [Google Scholar]
- 72.Bosies E, Endele R, Pahlke W. United States Patent. Boehringer Mannheim; GmbH, USA: 1989. N-Substituted aziridine-2-carboxylic acid derivatives, processes for the preparation thereof and pharmaceutical compositions containing them. [Google Scholar]
- 73.Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 74.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–647. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 75.Loeber RL, Michaelson-Richie ED, Codreanu SG, Liebler DC, Campbell CR, Tretyakova NY. Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards. Chem Res Toxicol. 2009;22:1151–1162. doi: 10.1021/tx900078y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yule SM, Foreman NK, Mitchell C, Gouldon N, May P, McDowell HP. High-dose cyclophosphamide for poor-prognosis and recurrent pediatric brain tumors: a dose-escalation study. J Clin Oncol. 1997;15:3258–3265. doi: 10.1200/JCO.1997.15.10.3258. [DOI] [PubMed] [Google Scholar]
- 77.Kohn KW, Spears CL, Doty P. Inter-strand crosslinking of DNA by nitrogen mustard. J Mol Biol. 1966;19:266–288. doi: 10.1016/s0022-2836(66)80004-9. [DOI] [PubMed] [Google Scholar]
- 78.Chetsanga CJ, Polidori G, Mainwaring M. Analysis and excision of ring-opened phosphoramide mustard-deoxyguanine adducts in DNA. Cancer Res. 1982;42:2616–2621. [PubMed] [Google Scholar]
- 79.Gruppi F, Hejazi L, Christov PP, Krishnamachari S, Turesky RJ, Rizzo CJ. Characterization of nitrogen mustard formamidopyrimidine adduct formation of bis(2-chloroethyl)ethylamine with calf thymus DNA and a human mammary cancer cell line. Chem Res Toxicol. 2015;28:1850–1860. doi: 10.1021/acs.chemrestox.5b00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christov PP, Son KJ, Rizzo CJ. Synthesis and characterization of oligonucleotides containing a nitrogen mustard formamidopyrimidine monoadduct of deoxyguanosine. Chem Res Toxicol. 2014;27:1610–1618. doi: 10.1021/tx5002354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Busby WFJ, Wogan GN. In: Chemical Carcinogens. Searle C, editor. American Chemical Society; Washington, DC: 1984. pp. 945–1136. [Google Scholar]
- 82.Brown KL, Deng JZ, Iyer RS, Iyer LG, Voehler MW, Stone MP, Harris CM, Harris TM. Unraveling the Aflatoxin–FAPY Conundrum: Structural Basis for Differential Replicative Processing of Isomeric Forms of the Formamidopyrimidine-Type DNA Adduct of Aflatoxin B1. J Am Chem Soc. 2006;128:15188–15199. doi: 10.1021/ja063781y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Croy RG, Wogan GN. Temporal Patterns of Covalent DNA Adducts in Rat Liver after Single and Multiple Doses of Aflatoxin B1. Cancer Res. 2008;41:197–203. [PubMed] [Google Scholar]
- 84.Essigmann JM, Croy RG, Bennett RA, Wogan GN. Metabolic Activation of Aflatoxin B1: Patterns of DNA Adduct Formation, Removal, and Excretion in Relation to Carcinogenesis. Drug Metab Rev. 1982;13:581–602. doi: 10.3109/03602538209011088. [DOI] [PubMed] [Google Scholar]
- 85.Foster PL, Eisenstadt E, Miller JH. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983;80:2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMahon G, Davis EF, Huber LJ, Kim Y, Wogan GN. Characterization of c-Ki-ras and N-ras oncogenes in aflatoxin B1-induced rat liver tumors. Proc Natl Acad Sci U S A. 1990;87:1104–1108. doi: 10.1073/pnas.87.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang YJ, Mathews C, Mangold K, Marien K, Hendricks J, Bailey G. Analysis of ras gene mutations in rainbow trout liver tumors initiated by aflatoxin B1. Mol Carcinog. 1991;4:112–119. doi: 10.1002/mc.2940040206. [DOI] [PubMed] [Google Scholar]
- 88.Trottier Y, Waithe WI, Anderson A. Kinds of mutations induced by aflatoxin B1 in a shuttle vector replicating in human cells transiently expressing cytochrome P4501A2 cDNA. Mol Carcinog. 1992;6:140–147. doi: 10.1002/mc.2940060209. [DOI] [PubMed] [Google Scholar]
- 89.Lin JK, Miller JA, Miller EC. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977;37:4430–4438. [PubMed] [Google Scholar]
- 90.Hertzog PJ, Smith JRL, Garner RC. Characterisation of the imidazole ring-opened forms of trans-8,9-di-hydro-8-(7-guanyl)9-hydroxy aflatoxin B1. Carcinogenesis. 1982;3:723–725. doi: 10.1093/carcin/3.6.723. [DOI] [PubMed] [Google Scholar]
- 91.Bailey EA, Iyer RS, Stone MP, Harris TM, Essigmann JM. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc Natl Acad Sci U S A. 1996;93:1535–1539. doi: 10.1073/pnas.93.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lawrence CW, Borden A, Banerjee SK, LeClerc JE. Mutation frequency and spectrum resulting from a single abasic site in a single-stranded vector. Nucleic Acids Res. 1990;18:2153–2157. doi: 10.1093/nar/18.8.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leadon SA, Snowden MM. Differential repair of DNA damage in the human metallothionein gene family. Mol Cell Biol. 1988;8:5331–5338. doi: 10.1128/mcb.8.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaden DA, Call KM, Leong PM, Komives EA, Thilly WG. Killing and mutation of human lymphoblast cells by aflatoxin B1: evidence for an inducible repair response. Cancer Res. 1987;47:1993–2001. [PubMed] [Google Scholar]
- 95.Ball RW, Wilson DW, Coulombe RA., Jr Comparative formation and removal of aflatoxin B1-DNA adducts in cultured mammalian tracheal epithelium. Cancer Res. 1990;50:4918–4922. [PubMed] [Google Scholar]
- 96.Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gopalakrishnan S, Patel DJ. Formation and structural features of a sterigmatocystin-formamidopyrimidine adduct at the DNA duplex level. J Am Chem Soc. 1993;115:9321–9322. [Google Scholar]
- 98.Baertschi SW, Raney KD, Stone MP, Harris TM. Preparation of the 8,9-epoxide of the mycotoxin aflatoxin B1: the ultimate carcinogenic species. J Am Chem Soc. 1988;110:7929–7931. [Google Scholar]
- 99.Gopalakrishnan S, Stone MP, Harris TM. Preparation and characterization of an aflatoxin B1 adduct with the oligodeoxynucleotide d(ATCGAT)2. J Am Chem Soc. 1989;111:7232–7239. [Google Scholar]
- 100.Gopalakrishnan S, Harris TM, Stone MP. Intercalation of aflatoxin B1 in two oligodeoxynucleotide adducts: comparative 1H NMR analysis of d(ATCAFBGAT). d(ATCGAT) and d(ATAFBGCAT)2. Biochemistry. 1990;29:10438–10448. doi: 10.1021/bi00498a002. [DOI] [PubMed] [Google Scholar]
- 101.Szybalski W, Iyer VN. Mechanisms of Action. In: Gottleib D, Shaw PD, editors. Antibiotics I. Springer-Verlag; New York: 1967. p. 230. [Google Scholar]
- 102.Berger M, Cadet J. Isolation and Characterization of the Radiation-Induced Degradation Products of 2′-Deoxyguanosine in Oxygen-Free Aqueous Solutions. Z Naturforsch B. 1985:1519. [Google Scholar]
- 103.Haraguchi K, Greenberg MM. Synthesis of Oligonucleotides Containing Fapy·dG (N6-(2-Deoxy-α,β-d-erythro-pentofuranosyl)-2,6-diamino-4-hydroxy-5-formamidopyrimidine) J Am Chem Soc. 2001;123:8636–8637. doi: 10.1021/ja0160952. [DOI] [PubMed] [Google Scholar]
- 104.Tudek B, Graziewicz M, Kazanova O, Zastawny TH, Obtulowicz T, JL Mutagenic specificity of imidazole ring-opened 7-metbylpurines in M13mp18 phage DNA. Acta Biochim Polo. 1999;46:785–799. [PubMed] [Google Scholar]
- 105.Ober M, Linne U, Gierlich J, Carell T. The two main DNA lesions 8-Oxo-7,8-dihydroguanine and 2,6-diamino-5-formamido-4-hydroxypyrimidine exhibit strongly different pairing properties. Angew Chem Int Ed. 2003;42:4947–4951. doi: 10.1002/anie.200351287. [DOI] [PubMed] [Google Scholar]
- 106.Ober M, Muller H, Pieck C, Gierlich J, Carell T. Base pairing and replicative processing of the formamidopyrimidine-dG DNA lesion. J Am Chem Soc. 2005;127:18143–18149. doi: 10.1021/ja0549188. [DOI] [PubMed] [Google Scholar]
- 107.Coste F, Ober M, Le Bihan YV, Izquierdo MA, Hervouet N, Mueller H, Carell T, Castaing B. Bacterial base excision repair enzyme Fpg recognizes bulky N7-substituted-FapydG lesion via unproductive binding mode. Chem Biol. 2008;15:706–717. doi: 10.1016/j.chembiol.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Domínguez BM, Cullis PM. 2-Azabicyclo[2.2.1]hept-5-en-3-one epoxide: A versatile intermediate for the synthesis of cyclopentyl carbocyclic 2-deoxy-, 3-deoxy-& ara-ribonucleoside analogues. Tetrahedron Lett. 1999;40:5783–5786. [Google Scholar]
- 109.Boiteux S, Laval J. Imidazole open ring 7-methylguanine: an inhibitor of DNA synthesis. Biochem Biophys Res Commun. 1983;110:552–558. doi: 10.1016/0006-291x(83)91185-3. [DOI] [PubMed] [Google Scholar]
- 110.Asagoshi K, Terato H, Ohyama Y, Ide H. Effects of a guanine-derived formamidopyrimidine lesion on DNA replication: translesion DNA synthesis, nucleotide insertion, and extension kinetics. J Biol Chem. 2002;277:14589–14597. doi: 10.1074/jbc.M200316200. [DOI] [PubMed] [Google Scholar]
- 111.Tudek B, Boiteux S, Laval J. Biological properties of imidazole ring-opened N7-methylguanine in M13mp18 phage DNA. Nucleic Acids Res. 1992;20:3079–3084. doi: 10.1093/nar/20.12.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christov PP, Yamanaka K, Choi JY, Takata K-i, Wood RD, Guengerich FP, Lloyd RS, Rizzo CJ. Replication of the 2,6-Diamino-4-hydroxy-N5-(methyl)-formamidopyrimidine (MeFapy-dGuo) Adduct by Eukaryotic DNA Polymerases. Chem Res Toxicol. 2012;25:1652–1661. doi: 10.1021/tx300113e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grziewicz MA, Zastawny TH, Oliński R, Tudek B. SOS-dependent A→G transitions induced by hydroxyl radical generating system hypoxanthine/xanthine oxidase/Fe3+/EDTA are accompanied by the increase of Fapy-adenine content in M13 mp18 phage DNA. Mutat Res/DNA Repair. 1999;434:41–52. doi: 10.1016/s0921-8777(99)00012-9. [DOI] [PubMed] [Google Scholar]
- 114.Smela ME, Currier SS, Bailey EA, Essigmann JM. The chemistry and biology of aflatoxin B(1): from mutational spectrometry to carcinogenesis. Carcinogenesis. 2001;22:535–545. doi: 10.1093/carcin/22.4.535. [DOI] [PubMed] [Google Scholar]
- 115.Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry. 1992;31:106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- 116.Breimer LH. Enzymatic excision from γ-irradiated polydeoxyribonucleotids of adenine residues whose imidazole rings have been rypture. Nucleic Acids Res. 1984;12:6359–6367. doi: 10.1093/nar/12.16.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boiteux S, O’Connor TR, Laval J. Formamidopyrimidine-DNA glycosylase of Escherichia coli: cloning and sequencing of the fpg structural gene and overproduction of the protein. Embo J. 1987;6:3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laval J, Boiteux S, O’Connor TR. Physiological properties and repair of apurinic/apyrimidinic sites and imidazole ring-opened guanines in DNA. Mutat Res. 1990;233:73–79. doi: 10.1016/0027-5107(90)90152-t. [DOI] [PubMed] [Google Scholar]
- 119.Boiteux S, O’Connor TR, Lederer F, Gouyette A, Laval J. Homogeneous Escherichia coli FPG protein. A DNA glycosylase which excises imidazole ring-opened purines and nicks DNA at apurinic/apyrimidinic sites. J Biol Chem. 1990;265:3916–3922. [PubMed] [Google Scholar]
- 120.Tudek B, Van Zeeland AA, Kusmierek JT, Laval J. Activity of Escherichia coli DNA-glycosylases on DNA damaged by methylating and ethylating agents and influence of 3-substituted adenine derivatives. Mutat Res. 1998;407:169–176. doi: 10.1016/s0921-8777(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 121.van der Kemp PA, Thomas D, Barbey R, de Oliveira R, Boiteux S. Cloning and expression in Escherichia coli of the OGG1 gene of Saccharomyces cerevisiae, which codes for a DNA glycosylase that excises 7,8-dihydro-8-oxoguanine and 2,6-diamino-4-hydroxy-5-N-methylformamidopyrimidine. Proc Natl Acad Sci U S A. 1996;93:5197–5202. doi: 10.1073/pnas.93.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karahalil B, Girard PM, Boiteux S, Dizdaroglu M. Substrate specificity of the Ogg1 protein of Saccharomyces cerevisiae: excision of guanine lesions produced in DNA by ionizing radiation- or hydrogen peroxide/metal ion-generated free radicals. Nucleic Acids Res. 1998;26:1228–1233. doi: 10.1093/nar/26.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hazra TK, Kow YW, Hatahet Z, Imhoff B, Boldogh I, Mokkapati SK, Mitra S, Izumi T. Identification and characterization of a novel human DNA glycosylase for repair of cytosine-derived lesions. J Biol Chem. 2002;277:30417–30420. doi: 10.1074/jbc.C200355200. [DOI] [PubMed] [Google Scholar]
- 124.Hazra TK, Izumi T, Boldogh I, Imhoff B, Kow YW, Jaruga P, Dizdaroglu M, Mitra S. Identification and characterization of a human DNA glycosylase for repair of modified bases in oxidatively damaged DNA. Proc Natl Acad Sci U S A. 2002;99:3523–3528. doi: 10.1073/pnas.062053799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bandaru V, Sunkara S, Wallace SS, Bond JP. A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair (Amst) 2002;1:517–529. doi: 10.1016/s1568-7864(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 126.Takao M, Kanno S, Kobayashi K, Zhang QM, Yonei S, van der Horst GT, Yasui A. A back-up glycosylase in Nth1 knock-out mice is a functional Nei (endonuclease VIII) homologue. J Biol Chem. 2002;277:42205–42213. doi: 10.1074/jbc.M206884200. [DOI] [PubMed] [Google Scholar]
- 127.Izumi T, Wiederhold LR, Roy G, Roy R, Jaiswal A, Bhakat KK, Mitra S, Hazra TK. Mammalian DNA base excision repair proteins: their interactions and role in repair of oxidative DNA damage. Toxicology. 2003;193:43–65. doi: 10.1016/s0300-483x(03)00289-0. [DOI] [PubMed] [Google Scholar]
- 128.Doetsch PW, Cunningham RP. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res/DNA Repair. 1990;236:173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- 129.Ramotar D. The apurinic-apyrimidinic endonuclease IV family of DNA repair enzymes. Biochem Cell Biol. 75:327–336. [PubMed] [Google Scholar]
- 130.Ljungquist S, Lindahl T, Howard-Flanders P. Methyl methane sulfonate-sensitive mutant of Escherichia coli deficient in an endonuclease specific for apurinic sites in deoxyribonucleic acid. J Bacteriol. 1976;126:646–653. doi: 10.1128/jb.126.2.646-653.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kerins SM, Collins R, McCarthy TV. Characterization of an Endonuclease IV 3′-5′ Exonuclease Activity. J Biol Chem. 2003;278:3048–3054. doi: 10.1074/jbc.M210750200. [DOI] [PubMed] [Google Scholar]
- 132.Golan G, Ishchenko AA, Khassenov B, Shoham G, Saparbaev MK. Coupling of the nucleotide incision and 3′ → 5′ exonuclease activities in Escherichia coli endonuclease IV: Structural and genetic evidences. Mutat Res Fund Mol Mech Mut. 2010;685:70–79. doi: 10.1016/j.mrfmmm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 133.Ide H, Tedzuka K, Shimzu H, Kimura Y, Purmal AA, Wallace SS, Kow YW. alpha.-Deoxyadenosine, a Major Anoxic Radiolysis Product of Adenine in DNA, Is a Substrate for Escherichia coli Endonuclease IV. Biochemistry. 1994;33:7842–7847. doi: 10.1021/bi00191a011. [DOI] [PubMed] [Google Scholar]
- 134.Aramini JM, Cleaver SH, Pon RT, Cunningham RP, Germann MW. Solution Structure of a DNA Duplex Containing an α-Anomeric Adenosine: Insights into Substrate Recognition by Endonuclease IV. J Mol Biol. 2004;338:77–91. doi: 10.1016/j.jmb.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 135.Gros L, Ishchenko AA, Ide H, Elder RH, Saparbaev MK. The major human AP endonuclease (Ape1) is involved in the nucleotide incision repair pathway. Nucleic Acids Res. 2004;32:73–81. doi: 10.1093/nar/gkh165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Daviet S, Couvé-Privat S, Gros L, Shinozuka K, Ide H, Saparbaev M, Ishchenko AA. Major oxidative products of cytosine are substrates for the nucleotide incision repair pathway. DNA Repair. 2007;6:8–18. doi: 10.1016/j.dnarep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 137.Patro JN, Haraguchi K, Delaney MO, Greenberg MM. Probing the Configurations of Formamidopyrimidine Lesions Fapy·dA and Fapy·dG in DNA Using Endonuclease IV. Biochemistry. 2004;43:13397–13403. doi: 10.1021/bi049035s. [DOI] [PubMed] [Google Scholar]
- 138.Asagoshi K, Yamada T, Terato H, Ohyama Y, Monden Y, Arai T, Nishimura S, Aburatani H, Lindahl T, Ide H. Distinct Repair Activities of Human 7,8-Dihydro-8-oxoguanine DNA Glycosylase and Formamidopyrimidine DNA Glycosylase for Formamidopyrimidine and 7,8-Dihydro-8-oxoguanine. J Biol Chem. 2000;275:4956–4964. doi: 10.1074/jbc.275.7.4956. [DOI] [PubMed] [Google Scholar]
- 139.Christov PP, Banerjee S, Stone MP, Rizzo CJ. Selective Incision of the α-N(5)-Methyl-Formamidopyrimidine Anomer by Escherichia coli Endonuclease IV. J Nucleic Acids. 2010;2010:850234. doi: 10.4061/2010/850234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alekseyev YO, Hamm ML, Essigmann JM. Aflatoxin B1 formamidopyrimidine adducts are preferentially repaired by the nucleotide excision repair pathway in vivo. Carcinogenesis. 2004;25:1045–1051. doi: 10.1093/carcin/bgh098. [DOI] [PubMed] [Google Scholar]