Abstract
A body of evidence has implicated dietary deficiency in omega-3 polyunsaturated fatty acids (n-3 PUFA), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in the pathophysiology and etiology of recurrent mood disorders including major depressive disorder (MDD) and bipolar disorder. Cross-national and cross-sectional evidence suggests that greater habitual intake of n-3 PUFA is associated with reduced risk for developing mood symptoms. Meta-analyses provide strong evidence that patients with mood disorders exhibit low blood n-3 PUFA levels which are associated with increased risk for the initial development of mood symptoms in response to inflammation. While the etiology of this n-3 PUFA deficit may be multifactorial, n-3 PUFA supplementation is sufficient to correct this deficit and may also have antidepressant effects. Rodent studies suggest that n-3 PUFA deficiency during perinatal development can recapitulate key neuropathological, neurochemical, and behavioral features associated with mood disorders. Clinical neuroimaging studies suggest that low n-3 PUFA biostatus is associated with abnormalities in cortical structure and function also observed in mood disorders. Collectively, these findings implicate dietary n-3 PUFA insufficiency, particularly during development, in the pathophysiology of mood dysregulation, and support implementation of routine screening for and treatment of n-3 PUFA deficiency in patients with mood disorders.
Keywords: Major depressive disorder, Bipolar disorder, Omega-3 fatty acids, Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA)
1. Introduction
Major mood disorders including major depressive disorder (MDD) and bipolar I disorder are characterized by a dysregulation in emotional homeostasis. Specifically, MDD is typically associated with recurrent and protracted episodes of depression and bipolar I disorder is characterized by recurrent episodes of both mania as well as depression. The initial onset of MDD most frequently occurs during adolescence and young adulthood [1], and the initial onset of mania, and by definition bipolar I disorder, most frequently occurs during childhood and adolescence [2]. Depressive symptoms frequently precede the initial onset of mania [3,4] and are associated with increased risk for developing mania in the offspring of bipolar parents [5]. Moreover, the high rate of attention deficit hyperactivity disorder (ADHD) in youth with bipolar disorder, and lower age at onset of mania in patients with co-occurring ADHD, are consistent with ADHD being risk factor for mania [6]. In addition to significant psychosocial impairment, MDD and bipolar I disorder are associated with elevated rates of cardiometabolic risk factors [7,8] and excess premature mortality [9,10] compared with general population estimates.
Over the past three decades there has been extensive research devoted to identifying genetic risk factors associated with mood disorders. However, to-date a robust and consistent pattern has yet to emerge suggesting that the etiology is polygenic and multifactorial. Indeed, subtotal heritability estimates and monozygotic twin discordance rates indicate that non-genetic factors also confer significant vulnerability [11,12]. For example, a meta-analysis of community-based twin studies of MDD yielded a heritability estimate of 0.37, indicating that approximately two thirds of the liability is attributable to environmental factors [13]. While there is strong evidence for familial transmission of bipolar disorder [14–16], non-genetic factors shared within families may also contribute to risk transmission [17]. There is also growing evidence that early environmental factors can impact gene expression patterns through epigenetic modifications (i.e., DNA methylation)[18]. Because environmental risk factors are amenable to modification, developing a clearer understanding of their role in the etiology of mood disorders may provide new insights to guide and inform early intervention strategies.
One candidate environmental risk factor that is amenable to modification is the habitual diet. Evidence has emerged over the last three decades which suggests that the fatty acid composition of the habitual diet may be relevant to the pathophysiology and potentially etiology of mood disorders. Cross-national and cross-sectional epidemiological surveys, longitudinal prospective cohort studies, prospective intervention studies, and basic science research have provided converging evidence implicating omega-3 (n-3) polyunsaturated fatty acids (PUFA) insufficiency, and associated increases in the n-6/n-3 PUFA ratio, in the pathophysiology of mood disorders. The goals of this review are to provide an overview of epidemiological and clinical research investigating the role of these PUFAs in mood disorders, discuss plausible mechanisms mediating dietary PUFA composition and mood dysregulation, and then consider strategies to translate this evidence into clinical practice.
2. PUFA Biochemistry
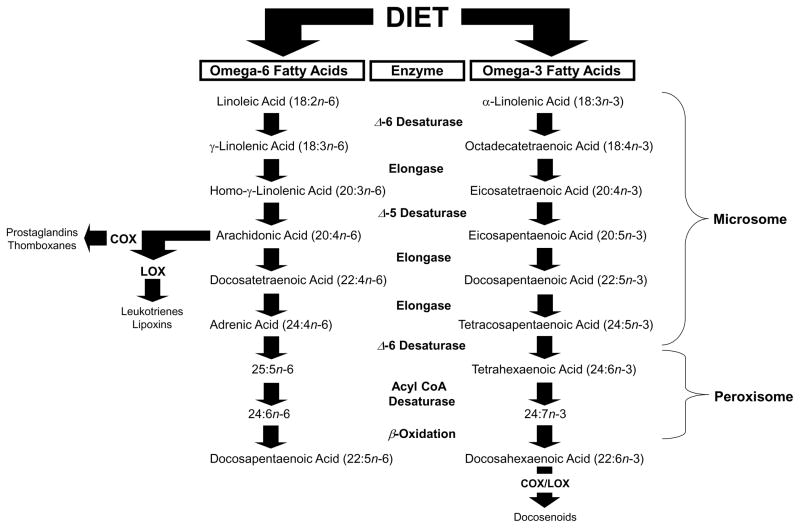
As background, the PUFA family includes n-3 and n-6 fatty acids. Ubiquitous dietary sources of the plant-derived n-3 PUFA precursor α-linolenic acid (18:3n-3) include flaxseed, linseed, canola, soy, and perilla oils, and sources of the plant-derived n-6 PUFA precursor linoleic acid (18:2n-6) include safflower, soy, and corn oils. These plant-derived PUFAs are considered ‘essential’ because they cannot be formed endogenously and therefore require procurement through the diet. The biosynthesis of n-3 PUFAs, including eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), and n-6 PUFAs including arachidonic acid (20:4n-6), from their plant-derived precursors requires a series of common and competitive microsomal desaturation and elongation reactions [19](Fig. 1). Enzymes regulating PUFA biosynthesis include delta-6 desaturase (FADS2) and delta-5 desaturase (FADS1) and elongases (e.g., ELOVL5). Desaturase enzymes are regulated by multiple factors including gonadal hormones [20–24], insulin [25], single nucleotide polymorphisms [26], as well as epigenetic (i.e., DNA methylation) modifications induced by dietary fatty acids [27,28]. The final synthesis of DHA is catalyzed by multiple enzymes within peroxisomes [29], and heritable defects in peroxisome biogenesis genes are associated with impaired DHA synthesis as well as other lipid and neurological abnormalities [30]. Nevertheless, it has been estimated that only 24 percent of the variability in levels of EPA and DHA arise from heritable factors [31]. Therefore, n-3 and n-6 PUFA biosynthesis is complex and involves both heritable genetic as well as non-genetic factors.
Figure 1.
Diagram illustrating the biosynthetic pathways of n-3 and n-6 PUFAs from plant-derived dietary precursors. The biosynthesis of docosahexaenoic acid (DHA, 22:6n-3) from α-linolenic acid (18:3n-3), and arachidonic acid (20:4n-6) from linolenic acid (18:2n-6), requires a series of common and competitive elongation and desaturation reactions mediated by microsomal enzymes. The final synthesis of DHA requires additional modifications including β-oxidation within peroxisomes. Unesterified arachidonic acid is a substrate for cyclooxygenase (COX)-mediated biosynthesis of prostaglandins and thromboxanes, as well as lipoxygenase (LOX)-mediated biosynthesis of leukotrienes and lipoxins. COX and LOX metabolites of unesterified DHA (i.e., docosanoids) as well as EPA have inflammation-resolving properties.
Extant evidence from human studies suggest that the biosynthesis of n-3 and n-6 PUFAs from plant-derived fatty acid precursors is extremely inefficient [32–35]. This may be due in part to the fact that n-3 and n-6 PUFA biosynthesis are mediated by common and competitive enzymatic reactions. Indeed, translational evidence indicates that the balance of linolenic acid to α-linolenic acid in the diet is an important determinant of n-3 and n-6 PUFA biosynthesis [36–39]. Dietary intake of preformed n-3 or n-6 PUFAs is more effective for increasing their levels in peripheral or central tissues than is enzyme-mediated biosynthesis from short-chain precursors [40–44]. Preformed n-6 PUFAs including arachidonic acid can be obtained directly from animal-based foods including beef, chicken, and eggs, and preformed n-3 PUFAs can be obtained directly from fatty cold water fish, including salmon, trout, tuna, as well as fish oil and algal-derived supplements. Therefore, both n-3 and n-6 PUFA content as well as the ratio of linolenic acid to α-linolenic acid in the habitual diet are important determinants of PUFA biostatus.
In addition to being incorporated into cellular phospholipid membranes, the n-6 PUFAs including arachidonic acid and n-3 PUFAs including EPA and DHA also serve as precursors for immune-inflammatory signaling modulators (Fig. 1). Phospholipid-bound arachidonic acid can be mobilized via calcium-dependent cytosolic isoform of phospholipase A2 (cPLA2), and free arachidonic acid is a substrate for cyclooxygenase (COX)-mediated biosynthesis of prostaglandins (i.e., PGH2) and thromboxanes, as well as lipoxygenase (LOX)-mediated biosynthesis of leukotrienes and lipoxins. COX-generated PGH2 is converted to PGE2 via PGE synthase, and PGE2 stimulates the biosynthesis of down-stream pro-inflammatory cytokines including interleukin-6 (IL-6) at the level of transcription [45–48]. In contrast, EPA competes with arachidonic acid for metabolism by COX enzymes, and COX and LOX metabolites of DHA and EPA (i.e., D- and E-series resolvins) have potent inflammation-resolving properties [48–52]. Therefore, EPA+DHA and arachidonic acid have opposing effects on immune-inflammatory signaling cascades, and a shift in their ratio may contribute to dysregulated inflammatory signaling homeostasis [53].
3. Associations with mood disorders
3.1. Observational studies
Cross-national evidence indicates that habitual fish intake is correlated with breastmilk [54] and blood [55] n-3 PUFA biostatus. For example, in the U.S., where annual seafood consumption is approximately 2-fold lower than in Japan [56], erythrocyte EPA+DHA levels are approximately fifty percent lower [57,58] and breastmilk DHA levels approximately 5-fold lower [54] compared with Japanese levels. Cross-national epidemiological surveys have observed a significant inverse correlation between per capita fish or seafood consumption and lifetime prevalence rates of MDD [59,60], postpartum depression [61], and bipolar spectrum disorders [62]. A retrospective study found that shifts away from fish-based to Western diets in Arctic communities were associated with increased rates of seasonal affective disorder, depression, suicide, and cardiovascular disease [63]. Moreover, a longitudinal prospective study of 4,856 adults residing in the U.S. found that greater linoleic acid intake was associated with increased risk of depression in men but not women during the 10.6 year follow-up period [64]. Within the U.S., it has been estimated that over the last century there has been a gradual increase in the consumption of linoleic acid and a corresponding decline in α-linolenic acid and n-3 PUFAs [65]. It will therefore be of interest to retrospectively evaluate whether this increase in n-6/n-3 PUFA ratio was associated with increased prevalence rates of mood disorders in the U.S. during this period.
Cross-sectional studies further suggest that higher intake of fish (as well as fruit, vegetables, and whole grains) is associated with a reduced depression risk [66]. For example, a cross-sectional survey of 21,835 adult and elderly subjects from Norway found that subjects who ingested cod liver oil on a daily basis (EPA: ~300–600 mg/d; DHA: ~300–600 mg/d) were 30 percent less likely to have depressive symptoms than non-users after adjusting for multiple possible confounding factors [67]. In view of evidence that the initial onset of mood disorders frequently occurs during the peri-adolescent period [1,2], it is notable that surveys have found that a large percentage of adolescents residing in Western countries consume low quantities of n-3 PUFA in their habitual diet which may be associated with depressive symptoms and cardiometabolic risk factors [68–77]. While findings from dietary surveys provide general support for an inverse association between n-3 PUFA intake and the prevalence of depressive symptoms in the general population, collinear cultural, socioeconomic, and/or genetic variables may mediate or moderate this association [78].
3.2. PUFA biostatus
Because estimating dietary fatty acid intake based on retrospective recall may be prone to errors and biases, and the n-3 PUFA composition in different types of fish varies widely, an alternative and more objective approach is to investigate fatty acid levels in blood and tissues. For example, erythrocyte (red blood cell) phospholipid membrane EPA+DHA levels are positively correlated with habitual dietary fish intake frequency [58], and increase in a linear dose-dependent manner following long-term fish oil supplementation [79]. Using this approach, several cross-sectional studies conducted in different countries have investigated blood fatty acid levels in patients with mood disorders. A meta-analysis of fourteen cross-sectional studies comprising n=648 depressed patients and n=2,670 healthy control subjects observed significant blood (plasma, erythrocyte) deficits in EPA and DHA, but not arachidonic acid or total n-6 PUFA, in patients with depressive symptoms [80]. Similarly, a meta-analysis of 6 cross-sectional studies comprising n=118 bipolar I disorder patients and n=147 healthy controls found significant erythrocyte deficits in DHA, and to a lesser extent EPA, in patients with bipolar I disorder [81]. In the latter study levels of linoleic acid and arachidonic acid were not different, and extant evidence also suggests that saturated and monounsaturated (i.e., oleic acid) fatty acid levels are not abnormal in patients with mood disorders. While the majority of these studies were conducted in adults, other studies have similarly found that pediatric and adolescent patients with MDD [82,83] or bipolar I disorder [84,85] also exhibit erythrocyte EPA and/or DHA deficits compared with healthy youth. Together, these findings provide strong evidence for an association between mood disorders and low EPA and/or DHA biostatus, as well as associated increases in the n-6/n-3 PUFA ratio.
Recent evidence further suggests that n-3 PUFA deficits coincide with, and may precede, the initial onset of mood symptoms. For example, robust erythrocyte DHA deficits were observed in mediation-naïve first-episode manic patients that were diagnosed with bipolar I disorder [86]. Moreover, asymptomatic adolescents who are at increased risk for developing mood disorders i.e., they have with a biological parent with bipolar I disorder [15], exhibit erythrocyte EPA+DHA levels that are intermediate between first-episode manic patients and offspring of parents with no family history of psychiatric illness [85]. Furthermore, adolescent offspring of bipolar parents with depressive symptoms or MDD, i.e., at ultra-high risk for developing bipolar I disorder [5], exhibit erythrocyte EPA+DHA deficits that are significantly lower than adolescents with no personal or family history of psychiatric illness [85]. These findings suggest that low EPA+DHA biostatus coincides with the initial onset of mood symptoms and may be associated with symptom progression in high-risk youth.
Some fatty acid composition studies [84,87,88] but not others [83,85,86,89] have observed an inverse correlation between blood n-3 PUFA levels, or positive correlations with the n-3/n-6 PUFA ratio (i.e., arachidonic acid/EPA), and depression or manic symptom severity within groups of patients. The latter discrepancies may be due in part to uniformity in mood symptom severity scores and more robust inverse correlations between manic and depressive symptom severity and blood n-3 PUFA levels are observed when both healthy controls and patients with a wider range of symptom severity are included [85]. Moreover, a recent study found that the inverse association between EPA+DHA levels and depressive symptoms was only observed in subjects with elevated oxidative stress biomarkers [90]. It is also notable that a large percentage of bipolar patients have a history of psychotic symptoms [91] and a meta-analysis of case-control studies observed significant erythrocyte DHA as well as arachidonic acid deficits in first-episode psychosis patients [92]. The latter finding suggests that arachidonic acid deficits may distinguish risk for psychosis versus MDD or bipolar disorder. Furthermore, there is a high rate of ADHD in youth with bipolar disorder [6], and a meta-analysis found that youth with ADHD also exhibit robust EPA+DHA deficits [93]. These findings suggest that n-3 PUFA deficits are not uniquely associated with mood symptoms, and are also associated with a broader range of psychiatric symptoms frequently exhibited by patients with mood disorders.
While prospective longitudinal studies are required to evaluate whether low EPA+DHA biostatus can serve as a reliable prognostic indicator of risk for ‘endogenous’ mood dysregulation, recent evidence suggests that low n-3 PUFA biostatus increases risk for the initial onset of mood symptoms elicited by ‘exogenous’ pro-inflammatory signaling cascades [94]. Specifically, prospective studies have found that lower baseline DHA levels, or a higher ratio of arachidonic acid to EPA+DHA, are a significant predictor of depression development in initially non-depressed hepatitis C patients during treatment with interferon-α (IFN-α)[95–97]. Additionally, during IFN-α treatment hepatitis C patients with a higher baseline ratio of arachidonic acid to EPA+DHA are also at increased risk for developing core symptoms of bipolar I disorder including anger and irritability [98]. In view of evidence that a subset of patients with mood disorders exhibit elevated biomarkers of inflammation [53], these prospective findings suggest that low EPA+DHA biostatus may also increase risk for ‘endogenous’ mood dysregulation in response to a natural pro-inflammatory challenge (e.g., viral infection). While there is currently nothing known about DHA and EPA metabolite levels (i.e., D- and E-series resolvins) in patients with mood disorders, research is warranted to evaluate whether impaired biosynthesis of D- and E-series resolvins secondary to DHA+EPA deficits contribute to mood dysregulation by creating a chronic low-grade pro-inflammatory state.
Other cross-sectional studies have investigated fatty acid levels in postmortem brain tissue. The most abundant n-3 PUFA found in mammalian brain gray matter is DHA, which comprises approximately 12% of total fatty acid composition, whereas EPA is rapidly oxidized and consequently comprises <1% of total brain fatty acid composition [99]. Preliminary evidence suggests that mammalian erythrocyte and cortical gray matter DHA levels are positively correlated under steady state dietary conditions [41,100]. Although several case-control studies have investigated the fatty acid composition of regional postmortem gray matter from patients with mood disorders, some studies but not others have observed DHA deficits in patients with MDD or bipolar I disorder [101–113]. These equivocal results may be due in part to the challenges and limitations associated with the postmortem approach [114], and additional research is needed to understand the relationship between blood and cortical DHA levels in patients with mood disorders. An alternative approach is to investigate relationships between n-3 PUFA intake and biostatus and neurophysiological variables in living patients using neuroimaging [115]. As discussed in great detail below, emerging evidence suggests that DHA biostatus is associated with different measures of cortical structural and functional integrity in brain regions repeatedly implicated in the pathophysiology of mood disorders.
In view of evidence that mood disorders are associated with elevated rates of cardiometabolic risk factors [7,8] and excess premature mortality attributable in part to cardiovascular-related disorders [9,10], it is also relevant that low erythrocyte EPA+DHA biostatus is associated with cardiometabolic risk factors including elevated triacylglycerol and C-reactive protein levels [116,117] as well as risk for sudden cardiac death [118–121]. Moreover, suicide is a primary cause of excess premature mortality in patients with mood disorders [9,10], and low erythrocyte DHA biostatus was found to be a significant predictor of future suicidal attempts in medication-free MDD patients [122], and blood n-3 PUFA deficits are observed in suicidal patients [123,124]. Therefore, n-3 PUFA deficits exhibited by patients with mood disorders may also contribute risk for premature mortality secondary to suicide and cardiometabolic disorders.
4. Etiological mechanisms
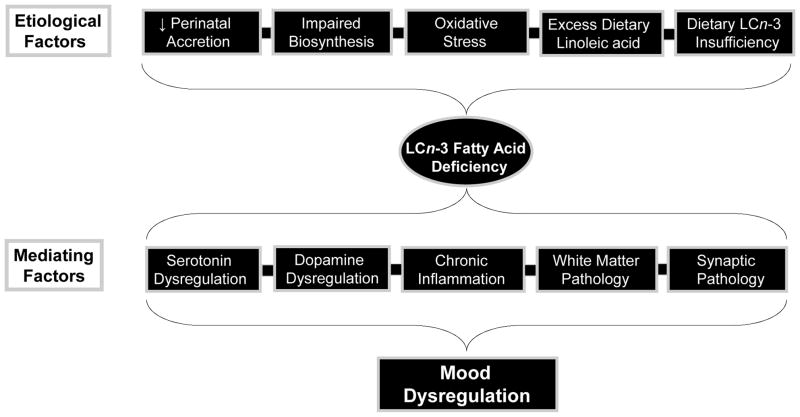
While extant evidence suggests that mood disorders are associated with blood deficits in n-3 PUFA, but not n-6 PUFA, understanding the etiological variables that contribute to blood n-3 PUFA deficits may have implications for treatment and prevention. Candidate etiological mechanisms including impaired biosynthesis, elevated lipid peroxidation, psychotropic medication effects, and dietary n-3 PUFA insufficiency, are discussed in greater detail below (Fig. 2).
Figure 2.
Diagram illustrating candidate etiological factors that may contribute to n-3 PUFA deficiency in patients with mood disorders, and candidate pathogenic mechanisms that may mediate n-3 PUFA deficiency and mood dysregulation.
4.1. Impaired biosynthesis
Reductions in the expression or function of the microsomal and peroxisomal enzymes that mediate EPA and/or DHA biosynthesis from α-linolenic acid would be anticipated to lead to robust deficits in blood EPA and DHA levels [29,125,126]. However, extant evidence suggests that mood disorders are not associated with deficits in other major n-3 PUFAs including docosapentaenoic acid (DPA, 22:5n-3) or n-6 PUFA including arachidonic acid [80,81]. This fatty acid signature would suggest that EPA+DHA deficits cannot be attributed to impaired microsomal desaturase and elongase activity. This is also supported by a recent genotyping study which did not observe an association between common single-nucleotide polymorphisms in FADS1 or FASD2 genes and depression or suicidality in MDD patients [127]. However, DNA methylation in the Elovl5 gene was found to be associated with depression and suicidality [128], and abnormalities in FADS1 and FADS2 mRNA expression have been observed in the postmortem prefrontal cortex of patients with mood disorders [129–131]. Recent evidence further suggests that patients with mood disorders do not exhibit impaired peroxisomal function despite exhibiting robust DHA deficits [132]. While additional research is needed to better characterize the role of genetic and epigenetic factors in the n-3 PUFA deficits observed in patients with mood disorders, existing evidence suggests that impaired biosynthesis does not represent a major etiological mechanism.
4.2. Lipid peroxidation
Mood disorders are associated with elevated indices of oxidative stress [133,134] which may also contribute to erythrocyte membrane EPA and DHA deficits. For example, incubation of erythrocytes from healthy control subjects with hydrogen peroxide decreased EPA and DHA composition to levels observed in MDD patients [135]. Moreover, deficiencies in oxidative defenses (i.e., vitamin E, alpha-tocopherol) are associated with increased susceptibility of erythrocyte n-3 PUFA to oxidative degradation [136], and erythrocyte EPA+DHA composition is positively correlated with plasma vitamin E concentrations [137]. Different case-control studies have separately found that MDD patients exhibit significantly lower serum vitamin E concentrations [138] and elevated indices of oxidative stress [133]. However, other evidence suggests that erythrocyte EPA+DHA deficits exhibited by patients with mood disorders are dissociable from biomarkers of lipid peroxidation [139]. For example, first-episode manic patients exhibit lower indices of lipid peroxidation [140] and lower erythrocyte EPA+DHA levels [85,86] compared with healthy controls. Nevertheless, additional studies are warranted to investigate whether antioxidant supplementation can increase EPA+DHA levels in patients with mood disorders to evaluate causality.
In view of the high rate of cigarette smoking among patients with mood disorders [141,142], it is also relevant that studies have found that cigarette smoking is associated with elevated indices of erythrocyte lipid peroxidation [143], and is also inversely correlated with plasma EPA+DHA and arachidonic acid concentrations [144]. However, other studies have found that cigarette smoking is not correlated with erythrocyte EPA+DHA levels among healthy male and female adults [57,58], and cross-sectional studies that specifically investigated the contribution of cigarette smoking to erythrocyte EPA+DHA status in patients with mood disorders did not observe an association [86,145]. Furthermore, robust erythrocyte EPA+DHA deficits have been observed in patients that reported to have never smoked cigarettes [86]. These findings suggest that elevated lipid peroxidation secondary to cigarette smoking cannot uniformly account for the EPA+DHA deficits observed in patients with mood disorders.
4.3. Psychotropic medications
Another potential etiological factor that may contribute to n-3 PUFA deficits in mood disorder patients is chronic exposure to psychotropic medications, including mood-stabilizers, antidepressants, or antipsychotics. Indeed, many of the case-control studies observing n-3 PUFA deficits in patients with mood disorders employed medicated patients. However, EPA+DHA deficits have been observed in chronically medicated, medication-withdrawn, and medication-naïve patients [85,86,89]. Moreover, in a prospective study it was found that erythrocyte DHA deficits observed in first-episode bipolar patients at medication-free baseline were not altered following 52 weeks treatment with lithium [86]. Additional evidence suggests that erythrocyte EPA and/or DHA deficits are present in medication-free [85,89] as well as selective serotonin reuptake inhibitor (SSRI)-treated [83] MDD patients. Furthermore, chronic treatment with the SSRI fluoxetine, which resulted in clinically-relevant plasma concentrations, did not significantly alter rat erythrocyte EPA+DHA levels under controlled dietary conditions [146]. In contrast, chronic treatment with the atypical antipsychotics risperidone or quetiapine significantly increased erythrocyte DHA composition in rats under controlled dietary conditions [147,148]. However, 52 week treatment with quetiapine did not significantly alter erythrocyte DHA deficits observed in medication-free bipolar patients at baseline [86]. Taken collectively, extant translational evidence would suggest that the blood EPA+DHA deficits observed in mood disorder patients cannot be wholly attributed to chronic exposure to psychotropic medications.
4.4. Dietary n-3 PUFA insufficiency
Several lines of evidence suggest that the n-3 PUFA deficit observed in patients with MDD or bipolar disorder are attributable to dietary n-3 PUFA insufficiency. This is directly supported by evidence that patients with mood disorders consume less EPA+DHA in their habitual diet [84,149,150]. Perhaps most compelling is the observation that dietary supplementation with fish oil significantly increases erythrocyte EPA+DHA levels in patients with mood disorders [83,151,152]. The latter finding additionally suggests that patients can efficiently absorb EPA+DHA from the gut and incorporate these fatty acids into erythrocyte membranes. In contrast, dietary supplementation with flax oil (a rich source of α-linolenic acid) does not significantly increase erythrocyte DHA levels in youth with bipolar I disorder [153], a result also observed in healthy subjects [42]. While greater intake of linoleic acid would have the potential to decrease n-3 PUFAs in patients with mood disorders [36], extant evidence suggests that patients with mood disorders do not exhibit elevated blood linoleic acid or n-6 PUFA levels compared with healthy subjects [80,81]. Therefore, while the etiology of the EPA+DHA deficits observed in patients with mood disorders may be multifactorial, extant evidence suggests that increasing dietary EPA+DHA intake is sufficient to correct this deficit.
5. n-3 PUFA supplementation studies
There has been considerable interest in evaluating whether n-3 PUFA supplementation has acute psychotherapeutic effects in patients with mood disorders. Over the past 20 years, numerous open-label and placebo-controlled n-3 PUFA supplementation trials have been conducted, and more recently several independent meta-analyses have been performed on placebo-controlled trials. While there have been discrepancies among the results of placebo-controlled trials, independent meta-analyses have reported a modest but statistically significant advantage of n-3 PUFA interventions over placebo for reducing depression symptom severity in patients with MDD [154–157] or bipolar disorder [155,158]. Controlled and open-label trials have also found that n-3 PUFA supplementation, administered either adjunctively or as monotherapy, significantly reduce depression and manic symptom severity in pediatric and adolescent patients [83,151,152,159]. Secondary analyses suggest that a larger EPA/DHA ratio has greater antidepressant efficacy [157], and a recent study found that higher EPA+DHA biostatus at baseline, which was associated with higher endpoint EPA+DHA biostatus following n-3 PUFA supplementation, was associated with increased antidepressant efficacy in depressed coronary heart disease patients [160]. Emerging evidence from controlled and open-label trials further suggests that n-3 PUFAs may augment the therapeutic efficacy of SSRI medications [83,161–164] and may be protective against adverse cardiometabolic [165–168] and hepatic [169,170] side-effects associated with second generation antipsychotic medications. Additional evidence suggests that increasing n-3 PUFA biostatus may be protective against the initial onset of depressive symptoms in hepatitis C patients during treatment with IFN-α [171], and to reduce suicidality in MDD patients [164]. Although extant evidence suggests that n-3 PUFA supplementation may have acute antidepressant and/or mood-stabilizing effects, large-scale controlled trials will be required to confirm and extend this body of evidence.
6. Neuropathophysiological mechanisms
Translational studies have elucidated several plausible biological mechanisms that may mediate the association between low n-3 PUFA status and mood dysregulation (Fig. 2). Consistent with a neurodevelopmental etiology, the initial onset of mood disorders frequently occurs during childhood and adolescence [1,2], a developmental period associated with the rapid cortical accrual of DHA [100] and both regressive (i.e., synaptic pruning) and progressive (i.e., myelination) cortical maturational processes [172–174]. Rodent neurodevelopmental studies have provided critical insight into the role of dietary n-3 fatty acids in normal brain development, and have the advantage of systematic and selective manipulation of n-3 fatty acid intake while controlling myriad potentially confounding variables that can impact clinical research. Additionally, converging evidence from neuroimaging studies is providing clarification regarding brain regions and neuropathological biomarkers associated with mood disorders, and more recently, associations between these biomarkers and n-3 PUFA biostatus.
6.1. Rodent studies
In brief, rodent studies have demonstrated that deficits in brain DHA accrual during perinatal development are associated with perturbations in neurogenesis [175,176], neuroblast migration [177,178], neurotrophic factor expression [179,180], and synaptogenesis [181]. Deficits in cortical DHA accrual during perinatal development are also associated with elevations in peripheral [182] and central [183,184] pro-inflammatory signaling cascades which have been found in separate studies to be down-regulated by mood-stabilizer medications [185]. Moreover, greater cortical DHA levels increase resilience to neurodegenerative processes associated with inflammation [186], lipid peroxidation [187,188] and glutamate excitotoxicity [189–191], and promotes neurovascular coupling [192]. Rodent studies also suggest that perinatal n-3 PUFA deficiency leads to long-standing alterations in neurotransmitter systems, including dopamine [193–195] and serotonin [196,197], and neuroendocrine systems, including corticosterone [198] and melatonin [199], implicated in mood regulation. n-3 PUFA deficiency also leads to elevated behavioral indices of depression, aggression, and anxiety [198,200,201] whereas dietary fish oil fortification decreases depression-like behavior [202,203]. These and other rodent findings suggest that n-3 PUFA deficiency during perinatal development can recapitulate key neuropathological, neurochemical, and behavioral features associated with mood disorders.
6.2. Clinical neuroimaging studies
There is emerging consensus from neuroimaging evidence that mood disorders are associated with abnormalities in connectivity between the prefrontal cortex and limbic emotional structures mediated in part by frontal white matter pathology [204–214]. Frontal lobe connectivity with limbic structures is mediated in part by the uncinate fasciculus and superior longitudinal fasciculus, prominent white matter tracts that develop during gestation and undergo significant maturation during childhood and adolescence. Accumulating evidence suggests that n-3 PUFAs promote cortical white matter microstructural integrity [215], and a recent study found that fish oil supplementation increased white matter microstructural integrity and decreased depressive symptom severity in MDD patients [216]. Moreover, perinatal n-3 PUFA deficiency in monkeys [217], or low erythrocyte DHA biostatus in typically developing children [218], are both associated with reduced functional connectivity within prefrontal cortical networks. These preliminary findings suggest that lower n-3 PUFA intake and biostatus may contribute to reduced functional connectivity within prefrontal cortical networks.
The third trimester of human gestation is a period associated with the initial formation of connections between brain regions including the uncinate fasciculus and superior longitudinal fasciculus [219], and preterm birth, which leads to deficits in third trimester cortical DHA accrual [220–222], is associated with both decreased white matter tract integrity and reduced connectivity within frontal lobe cortical networks [223–232]. In view of the high rate of ADHD in youth with bipolar disorder, it is relevant that preterm birth and/or low birth weight is associated with increased risk for developing ADHD in childhood [233–235] and mood, anxiety, and psychotic disorders during adolescence and young adulthood independent of multiple confounding variables including maternal history of psychiatric illness [236–239]. While these associations suggest that DHA deficits during critical neurodevelopmental periods may contribute to suboptimal frontal lobe cortical network maturation, it is not currently known whether early deficits in cortical DHA accrual directly contribute to frontal lobe cortical network pathology in patients with mood disorders.
Evidence from neuroimaging studies also suggest that n-3 PUFA intake and biostatus is associated with cortical structural integrity over the lifespan [240–245]. For example, greater habitual dietary n-3 PUFA intake [240] and erythrocyte EPA+DHA composition [245] are associated with larger hippocampal volumes among healthy adults. It is relevant, therefore, that hippocampal gray matter volume deficits are among the most consistent and robust neurostructural abnormalities observed in patients with MDD [246]. Furthermore, greater habitual dietary n-3 PUFA intake is associated with larger amygdala volumes among healthy adults [240], and structural imaging studies have consistently observed smaller amygdala volumes in children and adolescents with bipolar I disorder [247]. Near-infrared spectroscopy [248,249], functional magnetic resonance imaging (fMRI)[250,251], and magnetic resonance spectroscopy (1H MRS) studies [252–254], further suggest that higher n-3 PUFA intake or biostatus promote cerebrovascular efficiency and neuronal integrity. As observed in n-3 PUFA deficient rodents [183], recent neuroimaging evidence suggests that depression is also associated with increased microglial activation indicative of neuroinflammation [255]. While this preliminary neuroimaging evidence supports a potential link between low n-3 PUFA status and the abnormalities in cortical structure and function observed in patients with mood disorders, additional research is needed to establish this link and elucidate whether these abnormalities can be prevented with early n-3 PUFA intervention.
7. Clinical applications
In the previous sections we reviewed a body of translational evidence which suggests that n-3 PUFA deficiency, particularly during perinatal development, may represent a modifiable risk factor for neuropathophysiological processes implicated in mood disorders. Together these and other data provide a compelling rationale to begin to translate this body of evidence into clinical practice in an effort to optimize treatment and improve long-term health outcomes for patients. Below we briefly discuss existing screening and treatment resources and general guidelines required for implementation in psychiatric practice.
7.1. Screening for n-3 PUFA deficiency
Blood fatty acid analysis by gas-liquid chromatography can provide a valid measure of a patient’s fatty acid biostatus [256]. As discussed in greater detail previously, erythrocyte EPA+DHA composition, termed the ‘omega-3 index’, has been widely characterized as a risk biomarker in the context of coronary heart disease [121,257]. In this regard, erythrocyte EPA+DHA composition of ≤4% of total fatty acids has been suggested to represent an appropriate criterion for defining a state of ‘n-3 PUFA deficiency’ warranting corrective intervention. Based on cross-sectional evidence that patients with mood disorders commonly exhibit erythrocyte EPA and/or DHA deficits [80,81], and are at increased risk for cardiovascular-related disease [9,10], erythrocyte EPA+DHA composition of ≤4% may also be considered undesirable in the context of psychiatric practice. It is notable that one study found that 90 percent of adolescents with SSRI-resistant MDD exhibited erythrocyte EPA+DHA composition of ≤4% [83]. Importantly, there are currently laboratories that specialize in determining the fatty acid composition of whole blood obtained from a finger prick, a sampling method that is highly amenable to routine clinical practice. As proof-of-concept, beginning in mid-2014 measurement of whole blood fatty acid levels was implemented as part of the routine laboratory assessment at a university-affiliated mental health care center. To date fatty acid levels have been collected from over 100 patients with mood and anxiety disorders. Our initial results suggest that the rate of whole blood EPA+DHA levels of ≤4 percent of total fatty acid composition in patients is significantly greater than the general U.S. population (75% vs. 25%)[258]. Therefore, analogous to routine cholesterol testing routine testing for n-3 PUFA deficiency is currently feasible within the context psychiatric clinical practice.
7.2. Treating n-3 PUFA deficiency
As discussed, dietary supplementation with n-3 PUFA formulations (i.e., fish oil) is efficacious for the treatment of EPA+DHA deficits observed in patients with mood disorders. Prescription ethyl ester EPA+DHA (Lovaza® in the US, Omacor® in Europe, GlaxoSmithKline), purified ethyl ester EPA containing no DHA (Vascepa®, Amarin Corporation), and a free versus ethyl ester EPA+DHA formulation (Epanova®, AstraZeneca) have been approved by the U.S. FDA for the treatment of hypertriacylglycerolemia (≥500 mg/dL). More recently a generic version of Lovaza has become available (Teva Pharmaceuticals USA, Inc.). Over-the-counter fish oil supplements, as well as formulations derived from vegetarian sources, containing similar EPA+DHA concentrations are also widely available. It is important to note, however, that no n-3 PUFA formulation is currently approved by the FDA for the treatment of any psychiatric disorder, and reimbursement for off-label use is ultimately at the discretion of the insurance provider.
Regarding guidelines for dosing, the U.S. Food and Drug Administration (FDA) considers n-3 PUFA doses up to 3 g/d to be ‘generally regarded as safe’, and the European Food Safety Authority (EFSA) considers doses up to 5 g/d to be safe. The American Psychiatric Association has adopted the consensus recommendations of the American Heart Association for an EPA+DHA dose of 1 g/d in patients with MDD [259]. The American Heart Association also recommends 3 g/d EPA+DHA for reducing elevated triacylglycerol levels. Controlled dose-response studies suggest that daily EPA+DHA doses of 1–2 g are sufficient to increase erythrocyte EPA+DHA composition to levels ≥4% [79]. As with other psychotropic medications, upward dose titration may be required as clinically indicated (e.g., [151]) and lower initial starting doses may be appropriate for children.
Potential acute adverse events associated with n-3 PUFA supplementation include gastrointestinal disturbances, including nausea, diarrhea, gastroesophageal reflux, belching, and less commonly vomiting. To minimize these gastrointestinal adverse events patients should be instructed to take their pills with meals. Although taking fish oil at high doses (>3 g/d) has been associated in isolated cases with increased bleeding time in subjects also taking anticoagulant medications [260], controlled clinical trials have found that chronic high dose EPA+DHA alone or in combination with aspirin does not increase risk for clinically-significant increases in bleeding time [261]. Extant evidence further suggests that there is no relationship between n-3 PUFAs and prostate cancer risk [262]. Another safety consideration involves the potential threat of contamination of fish and seafood with methyl mercury and other environmental pollutants. However, most fish oil supplements are highly purified and do not exceed U.S. FDA limits for methyl mercury and other environmental contaminants. As with all medications, patients should be informed of potential risks associated with fish oil-based products.
8.0. Summary and conclusions
Emerging translational evidence over the past three decades suggests that habitual dietary n-3 PUFA insufficiency, particularly during perinatal development, may represent a modifiable risk factor for mood disorders. This is indirectly supported by cross-national as well as cross-sectional evidence for an inverse association between dietary n-3 PUFA intake and prevalence rates of mood disorders in the general population. Meta-analyses of cross-sectional fatty acid composition studies provide strong evidence that pediatric, adolescent, and adult patients with mood disorders exhibit blood n-3 PUFA deficits compared with healthy age-matched controls. While controversial, evidence also suggests that n-3 PUFA deficiency may decrease risk for suicide and cardiovascular disease, two primary causes of excess premature mortality in patients with mood disorders. Although the etiology of the n-3 PUFA deficits observed in patients with mood disorders may be multifactorial, extant evidence suggests that increasing dietary n-3 PUFA intake is sufficient to correct this deficit. Several independent meta-analyses of controlled trials suggest that acute n-3 PUFA supplementation may reduce mood symptom severity, and recent evidence further suggests that increasing n-3 PUFA biostatus is protective against the initial development of mood symptoms in response to inflammation as well as cardiometabolic risk factors.
Neuroimaging and rodent studies are beginning to elucidate plausible biological mechanisms that may link low n-3 PUFA status and mood dysregulation. Evidence from rodent neurodevelopmental studies suggest that n-3 PUFA deficiency during perinatal development can recapitulate key neuropathological, neurochemical, and behavioral features associated with mood disorders including enduring impairments in dopamine and serotonin neurotransmission. Clinical neuroimaging evidence further suggests that low n-3 PUFA biostatus may contribute to abnormalities in cortical structure and function that are consistently observed in patients with mood disorders. Consistent with biochemical evidence that DHA and EPA, and their bioactive metabolites, have anti-inflammatory and inflammation resolving properties, one potential neuropathogenic mechanism linking n-3 PUFA deficiency and relevant neuropathological processes is neuroinflammation. Neuroinflammation may contribute to white matter pathology and associated deficits in regional network connectivity. As discussed, this mechanism is directly supported by the recent observation that fish oil supplementation increased white matter microstructural integrity in MDD patients in conjunction with reductions in depression symptom severity. Nevertheless, additional research is needed to better characterize the link between n-3 PUFA intake and biostatus, neuroinflammation, neurodevelopment, and risk of mood dysregulation.
When taken collectively, this body of evidence provides a strong empirical foundation in support of dietary n-3 PUFA deficiency being relevant to the pathophysiology and pathoetiology of mood disorders. Because n-3 PUFA deficiency can be corrected through dietary n-3 PUFA supplementation, it represents a ‘modifiable’ risk factor that is amenable to treatment and prevention. The reviewed translational evidence provides a compelling rationale to begin translating this knowledge into clinical practice in an effort to optimize treatment response and improve long-term health outcomes for patients and their offspring. As an initial step, screening for and treating n-3 PUFA deficiency in patients with mood disorders is currently feasible in routine psychiatric practice, as evidenced by our recent pilot program conducted in a mental health care center. Furthermore, because n-3 PUFA monotherapy is safe and well-tolerated it is ideally suited as a preemptive intervention for youth at increased risk for developing mood disorders. The latter approach is supported by the observation that fish oil supplementation prevented or delayed the onset of psychosis in ultra-high risk youth [263,264]. Within a ‘clinical staging’ framework, n-3 PUFA monotherapy would also represent a safe first-line intervention for the treatment of early moderate mood symptoms, particularly in those that may be at increased risk for adverse events associated with conventional pharmaceutical medications. While the notion of nutritional medicine has been slow to impact conventional psychiatric training and practice [265], dietary n-3 PUFA deficiency represents a primary therapeutic candidate that warrants incorporation into psychiatric practice.
Acknowledgments
This work was supported in part by National Institute of Health grants DK097599, MH097818, and MH107378 to R.K.M.
Footnotes
DISCLOSURES
R.K.M. has received research support from NARSAD, Martek Biosciences/DSM Inc, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, Kyowa Hakko Bio Co., LTD, and the Inflammation Research Foundation (IRF), was a member of the IRF scientific advisory board, and served as a paid consultant for VAYA Pharma Inc., and Vifor Pharma Inc. The NIH did not have any role in the design, implementation, analysis or interpretation of the research. The other authors do not have any financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, Sachs G. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. doi: 10.1111/j.1399-5618.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conus P, Ward J, Hallam KT, Lucas N, Macneil C, McGorry PD, Berk M. The proximal prodrome to first episode mania--a new target for early intervention. Bipolar Disord. 2008;10:555–565. doi: 10.1111/j.1399-5618.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000;39:1245–1252. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, Sakolsky D, Diler R, Hafeman D, Merranko J, Iyengar S, Brent D, Kupfer D, Birmaher B. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: A longitudinal study. Am J Psychiatry. 2015;172:638–646. doi: 10.1176/appi.ajp.2014.14010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh MK, DelBello MP, Kowatch RA, Strakowski SM. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8:710–720. doi: 10.1111/j.1399-5618.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 7.Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, De Herdt A, Sienaert P, Probst M, De Hert M. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170:265–274. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 8.Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, Probst M, Scheewe TW, De Hert M. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014;44:2017–2028. doi: 10.1017/S0033291713002778. [DOI] [PubMed] [Google Scholar]
- 9.Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. J Affect Disord. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- 10.Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 11.Ehringer MA, Rhee SH, Young S, Corley R, Hewitt JK. Genetic and environmental contributions to common psychopathologies of childhood and adolescence: a study of twins and their siblings. J Abnorm Child Psychol. 2006;34:1–17. doi: 10.1007/s10802-005-9000-0. [DOI] [PubMed] [Google Scholar]
- 12.Merikangas KR, Chakravarti A, Moldin SO, Araj H, Blangero JC, Burmeister M, Crabbe J, Jr, Depaulo JR, Jr, Foulks E, Freimer NB, Koretz DS, Lichtenstein W, Mignot E, Reiss AL, Risch NJ, Takahashi JS. Future of genetics of mood disorders research. Biol Psychiatry. 2002;52:457–477. doi: 10.1016/s0006-3223(02)01471-3. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 14.DelBello MP, Geller B. Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord. 2001;3:325–334. doi: 10.1034/j.1399-5618.2001.30607.x. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60:1209–1215. doi: 10.1001/archpsyc.60.12.1209. [DOI] [PubMed] [Google Scholar]
- 16.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- 17.McNamara RK, Strawn JR. Non-heritable risk factors for bipolar disorder. In: Strakowski SM, DelBello MP, Adler CM, editors. Progression of Bipolar Disorder in Youth: Presentation, Treatment, and Neurobiology. Oxford University Press; NY, U.S.A: 2014. pp. 109–130. [Google Scholar]
- 18.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 19.Reardon HT, Brenna JT. Microsomal biosynthesis of omega-3 fatty acids. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Nova Science Publishers, Inc; U.S.A: 2013. pp. 3–17. [Google Scholar]
- 20.Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr. 2006;96:93–99. doi: 10.1079/bjn20061801. [DOI] [PubMed] [Google Scholar]
- 21.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 22.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. The polyunsaturated fatty acid composition of hepatic and plasma lipids differ by both sex and dietary fat intake in rats. J Nutr. 2010;140:245–250. doi: 10.3945/jn.109.115691. [DOI] [PubMed] [Google Scholar]
- 23.Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- 24.McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: Role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner RR, Rimoldi OJ, Lombardo YB, González MS, Bernasconi AM, Chicco A, Basabe JC. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids. 2003;38:733–742. doi: 10.1007/s11745-003-1121-x. [DOI] [PubMed] [Google Scholar]
- 26.Lattka E, Illig T, Koletzko B, Heinrich J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr Opin Lipidol. 2010;21:64–69. doi: 10.1097/MOL.0b013e3283327ca8. [DOI] [PubMed] [Google Scholar]
- 27.Hoile SP, Clarke-Harris R, Huang RC, Calder PC, Mori TA, Beilin LJ, Lillycrop KA, Burdge GC. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS One. 2014;9:e109896. doi: 10.1371/journal.pone.0109896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoile SP, Irvine NA, Kelsall CJ, Sibbons C, Feunteun A, Collister A, Torrens C, Calder PC, Hanson MA, Lillycrop KA, Burdge GC. Maternal fat intake in rats alters 20:4n-6 and 22:6n-3 status and the epigenetic regulation of Fads2 in offspring liver. J Nutr Biochem. 2013;24:1213–1220. doi: 10.1016/j.jnutbio.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdinandusse S, Denis S, Mooijer PA, Zhang Z, Reddy JK, Spector AA, Wanders RJ. Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J Lipid Res. 2001;42:1987–95. [PubMed] [Google Scholar]
- 30.Steinberg S, Chen L, Wei L, Moser A, Moser H, Cutting G, Braverman N. The PEX Gene Screen: molecular diagnosis of peroxisome biogenesis disorders in the Zellweger syndrome spectrum. Mol Genet Metab. 2004;83:252–263. doi: 10.1016/j.ymgme.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31.Harris WS, Pottala JV, Lacey SM, Vasan RS, Larson MG, Robins SJ. Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis. 2012;225:425–431. doi: 10.1016/j.atherosclerosis.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Adam O, Wolfram G, Zöllner N. Influence of dietary linoleic acid intake with different fat intakes on arachidonic acid concentrations in plasma and platelet lipids and eicosanoid biosynthesis in female volunteers. Ann Nutr Metab. 2003;47:31–36. doi: 10.1159/000068906. [DOI] [PubMed] [Google Scholar]
- 34.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 35.Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond) 2011;8:36. doi: 10.1186/1743-7075-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taha AY, Cheon Y, Faurot KF, Macintosh B, Majchrzak-Hong SF, Mann JD, Hibbeln JR, Ringel A, Ramsden CE. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot Essent Fatty Acids. 2014;90:151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochim Biophys Acta. 2009;1791:132–139. doi: 10.1016/j.bbalip.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 39.Hofacer RD, Rider T, Jandacek RJ, Tso P, Magrisso IJ, Benoit SC, McNamara RK. Omega-3 fatty acid deficiency selectively up-regulates delta6-desaturase expression and activity indices in rat liver: Prevention by normalization of omega-3 fatty acid status. Nutr Res. 2011;31:715–722. doi: 10.1016/j.nutres.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson GJ, Neuringer M, Lin DS, Connor WE. Can prenatal N–3 fatty acid deficiency be completely reversed after birth? Effects on retinal and brain biochemistry and visual function in rhesus monkeys. Pediatr Res. 2005;58:865–872. doi: 10.1203/01.pdr.0000182188.31596.5a. [DOI] [PubMed] [Google Scholar]
- 41.Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 42.Barceló-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88:801–809. doi: 10.1093/ajcn/88.3.801. [DOI] [PubMed] [Google Scholar]
- 43.Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–233. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- 44.Su HM, Bernardo L, Mirmiran M, Ma XH, Nathanielsz PW, Brenna JT. Dietary 18:3n-3 and 22:6n-3 as sources of 22:6n-3 accretion in neonatal baboon brain and associated organs. Lipids. 1999;34(Suppl):S347–350. doi: 10.1007/BF02562339. [DOI] [PubMed] [Google Scholar]
- 45.Portanova JP, Zhang Y, Anderson GD, et al. Selective neutralization of prostaglandin E2 blocks inflammation, hyperalgesia, and interleukin 6 production in vivo. J Exp Med. 1996;184:883–891. doi: 10.1084/jem.184.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groeger AL, Cipollina C, Cole MP, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 53.McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: A new therapeutic target? Expert Rev Neurother. 2012;12:1143–1161. doi: 10.1586/ern.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 55.Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N., Jr Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016;63:132–152. doi: 10.1016/j.plipres.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization, World Health Organization Fish and Fishery Products. World Apparent Consumption Based On Food Balance Sheets (1961–1993) Food and Agriculture Organization; Rome: 1996. FAO Fisheries Circular, No. 821 Rev. 3. [Google Scholar]
- 57.Itomura M, Fujioka S, Hamazaki K, Kobayashi K, Nagasawa T, Sawazaki S, Kirihara Y, Hamazaki T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo. 2008;22:131–135. [PubMed] [Google Scholar]
- 58.Sands SA, Reid KJ, Windsor SL, Harris WS. The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids. 2005;40:343–347. doi: 10.1007/s11745-006-1392-2. [DOI] [PubMed] [Google Scholar]
- 59.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 60.Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. doi: 10.1192/bjp.184.5.404. [DOI] [PubMed] [Google Scholar]
- 61.Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 62.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 63.McGrath-Hanna NK, Greene DM, Tavernier RJ, Bult-Ito A. Diet and mental health in the Arctic: is diet an important risk factor for mental health in circumpolar peoples? A review Int J Circumpolar Health. 2003;62:228–241. doi: 10.3402/ijch.v62i3.17560. [DOI] [PubMed] [Google Scholar]
- 64.Wolfe AR, Ogbonna EM, Lim S, Li Y, Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:972–977. doi: 10.1016/j.pnpbp.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 65.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai JS, Hiles S, Bisquera A, Hure AJ, McEvoy M, Attia J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am J Clin Nutr. 2014;99:181–197. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- 67.Raeder MB, Steen VM, Vollset SE, Bjelland I. Associations between cod liver oil use and symptoms of depression: the Hordaland Health Study. J Affect Disord. 2007;101:245–249. doi: 10.1016/j.jad.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Allen KL, Mori TA, Beilin L, Byrne SM, Hickling S, Oddy WH. Dietary intake in population-based adolescents: support for a relationship between eating disorder symptoms, low fatty acid intake and depressive symptoms. J Hum Nutr Diet. 2013;26:459–469. doi: 10.1111/jhn.12024. [DOI] [PubMed] [Google Scholar]
- 69.Harel Z, Riggs S, Vaz R, White L, Menzies G. Omega-3 polyunsaturated fatty acids in adolescents: knowledge and consumption. J Adolesc Health. 2001;28:10–15. doi: 10.1016/s1054-139x(00)00179-8. [DOI] [PubMed] [Google Scholar]
- 70.Lauritzen L, Harsløf LB, Hellgren LI, Pedersen MH, Mølgaard C, Michaelsen KF. Fish intake, erythrocyte n-3 fatty acid status and metabolic health in Danish adolescent girls and boys. Br J Nutr. 2012;107:697–704. doi: 10.1017/S0007114511002418. [DOI] [PubMed] [Google Scholar]
- 71.Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus Child Health Study. Pediatrics. 2010;126:623–630. doi: 10.1542/peds.2009-3277. [DOI] [PubMed] [Google Scholar]
- 72.Oddy WH, Hickling S, Smith MA, O'Sullivan TA, Robinson M, de Klerk NH, Beilin LJ, Mori TA, Syrette J, Zubrick SR, Silburn SR. Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depress Anxiety. 2011;28:582–588. doi: 10.1002/da.20822. [DOI] [PubMed] [Google Scholar]
- 73.O'Sullivan TA, Ambrosini GL, Mori TA, Beilin LJ, Oddy WH. Omega-3 Index correlates with healthier food consumption in adolescents and with reduced cardiovascular disease risk factors in adolescent boys. Lipids. 2011;46:59–67. doi: 10.1007/s11745-010-3499-8. [DOI] [PubMed] [Google Scholar]
- 74.Swenne I, Rosling A, Tengblad S, Vessby B. Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatr. 2011;100:1610–1615. doi: 10.1111/j.1651-2227.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 75.Sichert-Hellert W, Wicher M, Kersting M. Age and time trends in fish consumption pattern of children and adolescents, and consequences for the intake of long-chain n-3 polyunsaturated fatty acids. Eur J Clin Nutr. 2009;63:1071–1075. doi: 10.1038/ejcn.2009.40. [DOI] [PubMed] [Google Scholar]
- 76.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82:1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 77.Karlsson M, Mårild S, Brandberg J, Lönn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity. 2006;14:1931–1939. doi: 10.1038/oby.2006.225. [DOI] [PubMed] [Google Scholar]
- 78.Appleton KM, Peters TJ, Hayward RC, Heatherley SV, McNaughton SA, Rogers PJ, Gunnell D, Ness AR, Kessler D. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Soc Psychiatry Psychiatr Epidemiol. 2007;42:100–104. doi: 10.1007/s00127-006-0142-3. [DOI] [PubMed] [Google Scholar]
- 79.Flock MR, Skulas-Ray AC, Harris WS, Etherton TD, Fleming JA, Kris-Etherton PM. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2:e000513. doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 81.McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18:300–306. doi: 10.1111/bdi.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pottala JV, Talley JA, Churchill SW, Lynch DA, von Schacky C, Harris WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot Essent Fatty Acids. 2012;86:161–165. doi: 10.1016/j.plefa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 83.McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, Strawn JR, DelBello MP. Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. PharmaNutrition. 2014;2:38–46. doi: 10.1016/j.phanu.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–1038. doi: 10.1007/s11745-008-3224-z. [DOI] [PubMed] [Google Scholar]
- 85.McNamara RK, Jandacek RJ, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, Strakowski SM, DelBello MP. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic and eicosapentaenoic acid deficits: A candidate prodromal risk biomarker. Early Interv Psychiatry. 2016;10:203–211. doi: 10.1111/eip.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNamara RK, Jandacek RJ, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, DelBello MP, Strakowski SM. First-episode bipolar disorder is associated with erythrocyte membrane docosahexaenoic acid deficits: Dissociation from clinical response to lithium or quetiapine. Psychiatry Res. 2015;230:447–453. doi: 10.1016/j.psychres.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin-Jones S, Russ MJ, Rapoport SI. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9:759–765. doi: 10.1111/j.1399-5618.2007.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31:S157–161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 89.McNamara RK, Jandacek RJ, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bigornia SJ, Harris WS, Falcón LM, Ordovás JM, Lai CQ, Tucker KL. The omega-3 index is inversely associated with depressive symptoms among individuals with elevated oxidative stress biomarkers. J Nutr. 2016;146:758–766. doi: 10.3945/jn.115.222562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunayevich E, Keck PE., Jr Prevalence and description of psychotic features in bipolar mania. Curr Psychiatry Rep. 2000;2:286–290. doi: 10.1007/s11920-000-0069-4. [DOI] [PubMed] [Google Scholar]
- 92.Hoen WP, Lijmer JG, Duran M, Wanders RJ, van Beveren NJ, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 93.Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev. 2014;34:496–505. doi: 10.1016/j.cpr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McNamara RK. Mitigation of inflammation-induced mood dysregulation with long-chain omega-3 fatty acids. J Am Coll Nutr. 2015;34:48–55. doi: 10.1080/07315724.2015.1080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2013;31:48–53. doi: 10.1016/j.bbi.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lotrich FE, Sears B, McNamara RK. Polyunsaturated fatty acids moderate the effect of poor sleep on depression risk. Prostaglandins Leukot Essent Fatty Acids. 2016;106:19–25. doi: 10.1016/j.plefa.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013;75:475–483. doi: 10.1016/j.jpsychores.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157–163. doi: 10.1016/j.plefa.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 101.Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82:111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44:688–693. doi: 10.1016/j.jpsychires.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hamazaki K, Hamazaki T, Inadera H. Fatty acid composition in the postmortem amygdala of patients with schizophrenia, bipolar disorder, and major depressive disorder. J Psychiatr Res. 2012;46:1024–1028. doi: 10.1016/j.jpsychires.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 104.Hamazaki K, Hamazaki T, Inadera H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2013;210:346–350. doi: 10.1016/j.psychres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 105.Hamazaki K, Maekawa M, Toyota T, Dean B, Hamazaki T, Yoshikawa T. Fatty acid composition of the postmortem prefrontal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2015;227:353–359. doi: 10.1016/j.psychres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, Rao JS. Brain lipid concentrations in bipolar disorder. J Psychiatr Res. 2010;44:177–182. doi: 10.1016/j.jpsychires.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. J Psychiatry Neurosci. 2007;32:363–370. [PMC free article] [PubMed] [Google Scholar]
- 108.McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 109.McNamara RK, Jandacek R, Rider T, Tso P, Stanford K, Hahn C-G, Richtand NM. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Roberts RC, Conley RR, Pandey GN. Fatty acid composition of the postmortem prefrontal cortex of male and female adolescent suicide victims. Prostaglandins Leukot Essent Fatty Acids. 2009;80:19–26. doi: 10.1016/j.plefa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 111.McNamara RK, Jandacek R, Tso P, Dwivedi Y, Ren X, Pandey GN. Lower docosahexaenoic acid concentrations in the postmortem prefrontal cortex of adult depressed suicide victims compared with controls without cardiovascular disease. J Psychiatr Res. 2013;47:1187–1191. doi: 10.1016/j.jpsychires.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McNamara RK, Rider T, Jandacek R, Tso P. Abnormal fatty acid pattern in the superior temporal gyrus distinguishes bipolar disorder from major depression and schizophrenia and resembles multiple sclerosis. Psychiatry Res. 2014;215:560–567. doi: 10.1016/j.psychres.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tatebayashi Y, Nihonmatsu-Kikuchi N, Hayashi Y, Yu X, Soma M, Ikeda K. Abnormal fatty acid composition in the frontopolar cortex of patients with affective disorders. Transl Psychiatry. 2012;2:e204. doi: 10.1038/tp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McNamara RK, Jandacek RJ. Investigation of postmortem brain polyunsaturated fatty acid composition in psychiatric disorders: Limitations, challenges, and future directions. J Psychiatr Res. 2011;45:44–46. doi: 10.1016/j.jpsychires.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McNamara RK. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins Leukot Essent Fatty Acids. 2013;88:33–42. doi: 10.1016/j.plefa.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Baek D, Park Y. Association between erythrocyte n-3 polyunsaturated fatty acids and biomarkers of inflammation and oxidative stress in patients with and without depression. Prostaglandins Leukot Essent Fatty Acids. 2013;89:291–296. doi: 10.1016/j.plefa.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 117.Baghai TC, Varallo-Bedarida G, Born C, Häfner S, Schüle C, Eser D, Rupprecht R, Bondy B, von Schacky C. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 Index. J Clin Psychiatry. 2011;72:1242–1247. doi: 10.4088/JCP.09m05895blu. [DOI] [PubMed] [Google Scholar]
- 118.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 119.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. ω-3 Polyunsaturated fatty scid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern Med. 2016 doi: 10.1001/jamainternmed.2016.2925. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, et al. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 121.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 122.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 123.Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, Harkin A, Conroy RM. Lipids and essential fatty acids in patients presenting with self-harm. Br J Psychiatry. 2007;190:112–117. doi: 10.1192/bjp.bp.105.019562. [DOI] [PubMed] [Google Scholar]
- 124.Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biol Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 125.Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 126.Harmon SD, Kaduce TL, Manuel TD, Spector AA. Effect of the delta6-desaturase inhibitor SC-26196 on PUFA metabolism in human cells. Lipids. 2003;38:469–476. doi: 10.1007/s11745-003-1086-9. [DOI] [PubMed] [Google Scholar]
- 127.Sublette ME, Vaquero C, Baca-Garcia E, Pachano G, Huang YY, Oquendo MA, Mann JJ. Lack of association of SNPs from the FADS1-FADS2 gene cluster with major depression or suicidal behavior. Psychiatr Genet. 2016;26:81–86. doi: 10.1097/YPG.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haghighi F, Galfalvy H, Chen S, Huang YY, Cooper TB, Burke AK, Oquendo MA, Mann JJ, Sublette ME. DNA methylation perturbations in genes involved in polyunsaturated Fatty Acid biosynthesis associated with depression and suicide risk. Front Neurol. 2015;6:92. doi: 10.3389/fneur.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McNamara RK, Liu Y. Reduced expression of fatty acid biosynthesis genes in the prefrontal cortex of patients with major depressive disorder. J Affect Disord. 2011;129:359–363. doi: 10.1016/j.jad.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lalovic A, Klempan T, Sequeira A, Luheshi G, Turecki G. Altered expression of lipid metabolism and immune response genes in the frontal cortex of suicide completers. J Affect Disord. 2010;120:24–31. doi: 10.1016/j.jad.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, McNamara RK. Elevated Delta-6 desaturase (FADS2) gene expression in the prefrontal cortex of patients with bipolar disorder. J Psychiatr Res. 2011;45:269–272. doi: 10.1016/j.jpsychires.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McNamara RK, Moser AB, Jones RI, Jandacek RJ, Patino LR, Strawn JR, Strakowski SM, DelBello MP. Familial risk for bipolar disorder is not associated with impaired peroxisomal function: Dissociation from docosahexaenoic acid deficits. Psychiatry Res. 2016;246:803–807. doi: 10.1016/j.psychres.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 134.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 135.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 136.Palozza P, Sgarlata E, Luberto C, Piccioni E, Anti M, Marra G, Armelao F, Franceschelli P, Bartoli GM. n-3 fatty acids induce oxidative modifications in human erythrocytes depending on dose and duration of dietary supplementation. Am J Clin Nutr. 1996;64:297–304. doi: 10.1093/ajcn/64.3.297. [DOI] [PubMed] [Google Scholar]
- 137.Thorlaksdottir AY, Skuladottir GV, Petursdottir AL, Tryggvadottir L, Ogmundsdottir HM, Eyfjord JE, Jonsson JJ, Hardardottir I. Positive association between plasma antioxidant capacity and n-3 PUFA in red blood cells from women. Lipids. 2006;41:119–125. doi: 10.1007/s11745-006-5079-5. [DOI] [PubMed] [Google Scholar]
- 138.Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58:241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 139.Ross BM, Maxwell R, Glen I. Increased breath ethane levels in medicated patients with schizophrenia and bipolar disorder are unrelated to erythrocyte omega-3 fatty acid abundance. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:446–453. doi: 10.1016/j.pnpbp.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 140.Scola G, McNamara RK, Croarkin PE, Leffler J, Cullen K, Geske J, Biernacka J, Frye MA, DelBello MP, Andreazza AC. Lipid peroxidation biomarkers in adolescents with or at high-risk for bipolar disorder. J Affect Disord. 2016;192:176–183. doi: 10.1016/j.jad.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kreinin A, Novitski D, Rabinowitz D, Weizman A, Grinshpoon A. Association between tobacco smoking and bipolar affective disorder: clinical, epidemiological, cross-sectional, retrospective study in outpatients. Compr Psychiatry. 2012;53:269–274. doi: 10.1016/j.comppsych.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 142.Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief. 2010;(34):1–8. [PubMed] [Google Scholar]
- 143.Padmavathi P, Reddy VD, Kavitha G, Paramahamsa M, Varadacharyulu N. Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide. 2010;23:181–186. doi: 10.1016/j.niox.2010.05.287. [DOI] [PubMed] [Google Scholar]
- 144.Leng GC, Smith FB, Fowkes FG, Horrobin DF, Ells K, Morse-Fisher N, Lowe GD. Relationship between plasma essential fatty acids and smoking, serum lipids, blood pressure and haemostatic and rheological factors. Prostaglandins Leukot Essent Fatty Acids. 1994;51:101–108. doi: 10.1016/0952-3278(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 145.Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, Shen WW. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103. doi: 10.1016/s0924-977x(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 146.McNamara RK, Able JA, Rider T, Tso P, Jandacek R. Effect of chronic fluoxetine treatment on male and female rat erythrocyte and prefrontal cortex fatty acid composition. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1317–1321. doi: 10.1016/j.pnpbp.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McNamara RK, Able JA, Jandacek RJ, Rider T, Tso P. Chronic risperidone treatment preferentially increases rat erythrocyte and prefrontal cortex omega-3 fatty acid composition: Evidence for augmented biosynthesis. Schizophr Res. 2009;107:150–157. doi: 10.1016/j.schres.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McNamara RK, Rider T, Jandacek RJ, Tso P, Cole-Strauss A, Lipton JW. Differential effects of antipsychotic medications on polyunsaturated fatty acid biosynthesis in rats: Relationship with liver delta6-desaturase expression. Schizophr Res. 2011;129:56–65. doi: 10.1016/j.schres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 150.Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. doi: 10.1016/j.jpsychires.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Cluette-Brown JE, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17:440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 152.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- 153.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12:142–154. doi: 10.1111/j.1399-5618.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015;11:CD004692. doi: 10.1002/14651858.CD004692.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 Fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9:e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. 2016;6:e756. doi: 10.1038/tp.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 159.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163:1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 160.Carney RM, Steinmeyer BC, Freedland KE, Rubin EH, Rich MW, Harris WS. Baseline blood levels of omega-3 and depression remission: a secondary analysis of data from a placebo-controlled trial of omega-3 supplements. J Clin Psychiatry. 2016;77:138–143. doi: 10.4088/JCP.14m09660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016;173:575–587. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- 162.Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jazayeri S, Tehrani-Doost M, Keshavarz SA, Hosseini M, Djazayery A, Amini H, Jalali M, Peet M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42:192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 164.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 165.Pisano S, Gritti A, Catone G, Pascotto A. Antipsychotic-induced dyslipidemia treated with omega 3 fatty acid supplement in an 11-year-old psychotic child: a 1-year follow-up. J Child Adolesc Psychopharmacol. 2013;23:139–141. doi: 10.1089/cap.2012.0060. [DOI] [PubMed] [Google Scholar]
- 166.Peet M, Horrobin DF E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res. 2002;36:7–18. doi: 10.1016/s0022-3956(01)00048-6. [DOI] [PubMed] [Google Scholar]
- 167.Caniato RN, Alvarenga ME, Garcia-Alcaraz MA. Effect of omega-3 fatty acids on the lipid profile of patients taking clozapine. Aust N Z J Psychiatry. 2006;40:691–697. doi: 10.1080/j.1440-1614.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- 168.Freeman MP, McInerney K, Sosinsky AZ, Kwiatkowski MA, Cohen LS. Omega-3 fatty acids for atypical antipsychotic-associated hypertriglyceridemia. Ann Clin Psychiatry. 2015;27:197–202. [PubMed] [Google Scholar]
- 169.Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 170.McNamara RK, Magrisso IJ, Hofacer RD, Jandacek RJ, Rider T, Tso P, Benoit SC. Omega-3 fatty acid deficiency augments risperidone-induced hepatic steatosis in rats: Positive association with stearoyl-CoA desaturase. Pharmacol Res. 2012;66:283–291. doi: 10.1016/j.phrs.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, Chang HC, Pariante CM. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: Results from a randomized, controlled trial. Biol Psychiatry. 2014;76:559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 172.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 173.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 175.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 177.Coti Bertrand P, O'Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–1575. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 178.Yavin E, Himovichi E, Eilam R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience. 2009;162:1011–1122. doi: 10.1016/j.neuroscience.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 179.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 180.Ikemoto A, Nitta A, Furukawa S, Ohishi M, Nakamura A, Fujii Y, Okuyama H. Dietary n-3 fatty acid deficiency decreases nerve growth factor content in rat hippocampus. Neurosci Lett. 2000;285:99–102. doi: 10.1016/s0304-3940(00)01035-1. [DOI] [PubMed] [Google Scholar]
- 181.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McNamara RK, Rider T, Jandacek RJ, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: Relationship with central serotonin turnover. Prostaglandins Leukot Essent Fatty Acids. 2010;83:185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Madore C, Nadjar A, Delpech JC, Sere A, Aubert A, Portal C, Joffre C, Layé S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav Immun. 2014;41:22–31. doi: 10.1016/j.bbi.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 184.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 185.Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target? Mol Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- 186.Orr SK, Palumbo S, Bosetti F, Mount HT, Kang JX, Greenwood CE, Ma DW, Serhan CN, Bazinet RP. Unesterified docosahexaenoic acid is protective in neuroinflammation. J Neurochem. 2013;127:378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Green P, Glozman S, Weiner L, Yavin E. Enhanced free radical scavenging and decreased lipid peroxidation in the rat fetal brain after treatment with ethyl docosahexaenoate. Biochim Biophys Acta. 2001;1532:203–212. doi: 10.1016/s1388-1981(01)00132-9. [DOI] [PubMed] [Google Scholar]
- 188.Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149–157. doi: 10.1080/10284150290003159. [DOI] [PubMed] [Google Scholar]
- 189.Högyes E, Nyakas C, Kiliaan A, Farkas T, Penke B, Luiten PG. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012. doi: 10.1016/s0306-4522(03)00198-2. [DOI] [PubMed] [Google Scholar]
- 190.Ozyurt B, Sarsilmaz M, Akpolat N, Ozyurt H, Akyol O, Herken H, Kus I. The protective effects of omega-3 fatty acids against MK-801-induced neurotoxicity in prefrontal cortex of rat. Neurochem Int. 2007;50:196–202. doi: 10.1016/j.neuint.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 191.Relton JK, Strijbos PJ, Cooper AL, Rothwell NJ. Dietary N-3 fatty acids inhibit ischaemic and excitotoxic brain damage in the rat. Brain Res Bull. 1993;32:223–226. doi: 10.1016/0361-9230(93)90180-j. [DOI] [PubMed] [Google Scholar]
- 192.Ximenes da Silva A, Lavialle F, Gendrot G, Guesnet P, Alessandri JM, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. J Neurochem. 2002;81:1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 193.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res. 2002;43:1209–1219. [PubMed] [Google Scholar]
- 194.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, Durand G, Chalon S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 195.Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, Chalon S. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- 196.Kodas E, Galineau L, Bodard S, Vancassel S, Guilloteau D, Besnard JC, Chalon S. Serotoninergic neurotransmission is affected by n-3 polyunsaturated fatty acids in the rat. J Neurochem. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- 197.McNamara RK, Able JA, Liu Y, Jandacek RJ, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J Psychiatr Res. 2009;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen HF, Su HM. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J Nutr Biochem. 2013;24:70–80. doi: 10.1016/j.jnutbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Lavialle M, Champeil-Potokar G, Alessandri JM, Balasse L, Guesnet P, Papillon C, Pévet P, Vancassel S, Vivien-Roels B, Denis I. An (n-3) polyunsaturated fatty acid-deficient diet disturbs daily locomotor activity, melatonin rhythm, and striatal dopamine in Syrian hamsters. J Nutr. 2008;138:1719–1724. doi: 10.1093/jn/138.9.1719. [DOI] [PubMed] [Google Scholar]
- 200.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 201.Weiser MJ, Wynalda K, Salem N, Jr, Butt CM. Dietary DHA during development affects depression-like behaviors and biomarkers that emerge after puberty in adolescent rats. J Lipid Res. 2015;56:151–166. doi: 10.1194/jlr.M055558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carlezon WA, Jr, Mague SD, Parow AM, Stoll AL, Cohen BM, Renshaw PF. Antidepressant-like effects of uridine and omega-3 fatty acids are potentiated by combined treatment in rats. Biol Psychiatry. 2005;57:343–350. doi: 10.1016/j.biopsych.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 203.Huang SY, Yang HT, Chiu CC, Pariante CM, Su KP. Omega-3 fatty acids on the forced-swimming test. J Psychiatr Res. 2008;42:58–63. doi: 10.1016/j.jpsychires.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 204.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, DelBello MP, Frangou S, McIntosh A, Phillips ML, Sussman JE, Townsend JD. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 206.Bellani M, Brambilla P. Diffusion imaging studies of white matter integrity in bipolar disorder. Epidemiol Psychiatr Sci. 2011;20:137–140. doi: 10.1017/s2045796011000229. [DOI] [PubMed] [Google Scholar]
- 207.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 208.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Kurma S, Lim KO. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–183. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, Hietala J, Lawrie SM, Martinot JL, McIntosh AM, Meisenzahl E, Frangou S. Common and distinct neural correlates of emotional processing in Bipolar Disorder and Major Depressive Disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–113. doi: 10.1016/j.euroneuro.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 211.Dickstein DP, Gorrostieta C, Ombao H, Goldberg LD, Brazel AC, Gable CJ, Kelly C, Gee DG, Zuo XN, Castellanos FX, Milham MP. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68:839–846. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Guo WB, Liu F, Xue ZM, Gao K, Wu RR, Ma CQ, Liu ZN, Xiao CQ, Chen HF, Zhao JP. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci Lett. 2012;522:139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 213.Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. J Affect Disord. 2011;131:299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 214.Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 215.Peters BD, Voineskos AN, Szeszko PR, Lett TA, DeRosse P, Guha S, Karlsgodt KH, Ikuta T, Felsky D, John M, Rotenberg DJ, Kennedy JL, Lencz T, Malhotra AK. Brain white matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. J Neurosci. 2014;34:6367–6376. doi: 10.1523/JNEUROSCI.2818-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chhetry BT, Hezghia A, Miller JM, Lee S, Coopera TB, Oquendoa MA, Mann JJ, Sublette ME. Omega-3 polyunsaturated fatty acid supplementation and white matter changes in major depression. J Psychiatr Res. 2016;75:65–74. doi: 10.1016/j.jpsychires.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065–2074. doi: 10.1523/JNEUROSCI.3038-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Almeida DM, Jandacek RJ, Weber WA, McNamara RK. Docosahexaenoic acid biostatus is associated with event-related functional connectivity in cortical attention networks of typically developing children. Nutr Neurosci. 2016 doi: 10.1179/1476830515Y.0000000046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 219.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 220.Sarkadi-Nagy E, Wijendran V, Diau GY, Chao AC, Hsieh AT, Turpeinen A, Nathanielsz PW, Brenna JT. The influence of prematurity and long chain polyunsaturate supplementation in 4-week adjusted age baboon neonate brain and related tissues. Pediatr Res. 2003;54:244–252. doi: 10.1203/01.PDR.0000072795.38990.F2. [DOI] [PubMed] [Google Scholar]
- 221.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 222.Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- 223.Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, Delancy S, Katz KH, Schneider KC, Schafer RJ, Makuch RW, Reiss AR. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 224.Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lubsen J, Vohr B, Myers E, Hampson M, Lacadie C, Schneider KC, Katz KH, Constable RT, Ment LR. Microstructural and functional connectivity in the developing preterm brain. Semin Perinatol. 2011;35:34–43. doi: 10.1053/j.semperi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, Makuch RW, Reiss AL, Constable RT, Ment LR. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- 228.Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, Ment LR. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 230.Smyser CD, Snyder AZ, Shimony JS, Blazey TM, Inder TE, Neil JJ. Effects of white matter injury on resting state fMRI measures in prematurely born infants. PLoS One. 2013;8:e68098. doi: 10.1371/journal.pone.0068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 232.White TP, Symington I, Castellanos NP, Brittain PJ, Froudist Walsh S, Nam KW, Sato JR, Allin MP, Shergill SS, Murray RM, Williams SC, Nosarti C. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage Clin. 2014;4:352–365. doi: 10.1016/j.nicl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 234.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years. J Child Psychol Psychiatry. 1997;38:931–941. doi: 10.1111/j.1469-7610.1997.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 235.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. [PubMed] [Google Scholar]
- 236.Indredavik MS, Vik T, Evensen KA, Skranes J, Taraldsen G, Brubakk AM. Perinatal risk and psychiatric outcome in adolescents born preterm with very low birth weight or term small for gestational age. J Dev Behav Pediatr. 2010;31:286–294. doi: 10.1097/DBP.0b013e3181d7b1d3. [DOI] [PubMed] [Google Scholar]
- 237.Lindström K, Lindblad F, Hjern A. Psychiatric morbidity in adolescents and young adults born preterm: a Swedish national cohort study. Pediatrics. 2009;123:47–53. doi: 10.1542/peds.2008-1654. [DOI] [PubMed] [Google Scholar]
- 238.Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, MacCabe J, Rifkin L, Hultman CM. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69:1–8. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- 239.Patton GC, Coffey C, Carlin JB, Olsson CA, Morley R. Prematurity at birth and adolescent depressive disorder. Br J Psychiatry. 2004;184:446–447. doi: 10.1192/bjp.184.5.446. [DOI] [PubMed] [Google Scholar]
- 240.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 241.Samieri C, Maillard P, Crivello F, Proust-Lima C, Peuchant E, Helmer C, Amieva H, Allard M, Dartigues JF, Cunnane SC, Mazoyer BM, Barberger-Gateau P. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology. 2012;79:642–650. doi: 10.1212/WNL.0b013e318264e394. [DOI] [PubMed] [Google Scholar]
- 242.Titova OE, Sjögren P, Brooks SJ, Kullberg J, Ax E, Kilander L, Riserus U, Cederholm T, Larsson EM, Johansson L, Ahlström H, Lind L, Schiöth HB, Benedict C. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age (Dordr) 2013;35:1495–1505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Virtanen JK, Siscovick DS, Lemaitre RN, Longstreth WT, Spiegelman D, Rimm EB, King IB, Mozaffarian D. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the Cardiovascular Health Study. J Am Heart Assoc. 2013;2:e000305. doi: 10.1161/JAHA.113.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Walhovd KB, Storsve AB, Westlye LT, Drevon CA, Fjell AM. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 2014;35:1055–1064. doi: 10.1016/j.neurobiolaging.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 245.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology. 2014;82:435–442. doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 247.Pfeifer JC, Welge J, Strakowski SM, Adler CM, DelBello MP. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–1298. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 248.Jackson PA, Reay JL, Scholey AB, Kennedy DO. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. Br J Nutr. 2012;107:1093–1098. doi: 10.1017/S0007114511004041. [DOI] [PubMed] [Google Scholar]
- 249.Pu S, Nakagome K, Yamada T, Matsumura H, Yokoyama K, Kaneko K, Kurosawa Y. Association between fish consumption and prefrontal function during a cognitive task in male Japanese workers: A multi-channel near-infrared spectroscopy study. PLoS One. 2015;10:e0123972. doi: 10.1371/journal.pone.0123972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bauer I, Hughes M, Rowsell R, Cockerell R, Pipingas A, Crewther S, Crewther D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum Psychopharmacol. 2014;29:133–144. doi: 10.1002/hup.2379. [DOI] [PubMed] [Google Scholar]
- 252.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. doi: 10.1192/bjp.188.1.46. [DOI] [PubMed] [Google Scholar]
- 253.McNamara RK, Jandacek RJ, Rider T, Tso P, Weber W, Chu W-J, Strakowski SM, Adler CM, DelBello MP. Low docosahexaenoic acid status is associated with reduced indices of cortical integrity in the anterior cingulate of healthy male children: A 1H MRS study. Nutr Neurosci. 2013;16:183–190. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McNamara RK, Jandacek RJ, Rider T, Tso P, Chu WJ, Weber WA, Welge JA, Strawn JR, Adler CM, DelBello MP. Effects of fish oil supplementation on prefrontal metabolite concentrations in adolescents with major depressive disorder: A preliminary 1H MRS study. Nutr Neurosci. 2016;19:145–155. doi: 10.1179/1476830514Y.0000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Harris WS, von Schacky C, Park Y. Standardizing methods for assessing omega-3 fatty acid biostatus. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Nova Science Publishers, Inc; U.S.A: 2013. pp. 385–398. [Google Scholar]
- 257.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. doi: 10.1093/ajcn/87.6.1997S. [DOI] [PubMed] [Google Scholar]
- 258.Messamore E, McNamara RK. Detection and treatment of omega-3 fatty acid deficiency in psychiatric practice: Rationale and implementation. Lipids Health Dis. 2016;15:1–13. doi: 10.1186/s12944-016-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell LB, Richardson AJ, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 260.Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Ann Pharmacother. 2004;38:50–52. doi: 10.1345/aph.1D007. [DOI] [PubMed] [Google Scholar]
- 261.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99:44C–46C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 262.Aucoin M, Cooley K, Knee C, Fritz H, Balneaves LG, Breau R, Fergusson D, Skidmore B, Wong R, Seely D. Fish-derived omega-3 fatty acids and prostate cancer: A systematic review. Integr Cancer Ther. 2016 doi: 10.1177/1534735416656052. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 264.Amminger GP, Schäfer MR, Schlögelhofer M, Klier CM, McGorry PD. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. doi: 10.1038/ncomms8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sarris J, Logan AC, Akbaraly TN, Amminger GP, Balanzá-Martínez V, Freeman MP, Hibbeln J, Matsuoka Y, Mischoulon D, Mizoue T, Nanri A, Nishi D, Ramsey D, Rucklidge JJ, Sanchez-Villegas A, Scholey A, Su KP, Jacka FN International Society for Nutritional Psychiatry Research. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry. 2015;2:271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]