Abstract
Under physiological conditions, the arterial endothelium exerts a powerful protective influence to maintain vascular homeostasis. However, during the development of vascular disease, these protective activities are lost and dysfunctional endothelial cells actually promote disease pathogenesis. Numerous investigations have analyzed the characteristics of dysfunctional endothelium with a view to understanding the processes responsible for the dysfunction and to determining their role in vascular pathology. The present review adopts an alternate approach: reviewing the mechanisms that contribute to the initial formation of a healthy protective endothelium and on how those mechanisms may be disrupted, precipitating the appearance of dysfunctional endothelial cells and the progression of vascular disease. This approach, which highlights the role of endothelial adherens junctions and VE-cadherin in endothelial maturation and endothelial dysfunction, provides new insight into the remarkable biology of this important cell layer and its role in vascular protection and vascular disease.
Keywords: VE-cadherin, adherens junctions, endothelial dysfunction, postnatal development, aging, developmental originals of vascular disease, atherosclerosis
Normal endothelial function is crucial for maintaining vascular and organismal health. Endothelial cells are key regulators of blood vessel constriction, thrombogenicity, inflammation, permeability, and vascular remodeling. Under normal conditions, the endothelium exerts a protective influence to inhibit these processes and maintain vascular stability and homeostasis. However, during the development of vascular disease, endothelial cells with an altered phenotype, namely ‘dysfunctional’ endothelium, promote these same processes and contribute to pathological changes in vascular structure and reactivity. Numerous investigations have analyzed and described characteristics of dysfunctional endothelium, with a view to understanding the processes responsible for the dysfunction and to determining their role in the development of vascular disease. The present review will focus on an alternate approach: discussing the mechanisms that contribute to the initial formation of a healthy protective endothelium and on how those continuing mechanisms can be disrupted contributing to the emergence of dysfunctional endothelial cells and the progression of vascular disease. The approach emphasizes that the protective activity of the vascular endothelium is an active rather than passive function of the endothelium, and that disruption of those active mechanisms can precipitate pathological changes in endothelial and vascular activity.
The Normal Maturation of Protective Arterial Endothelium
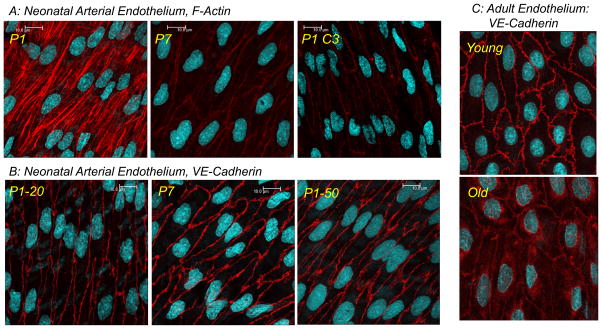
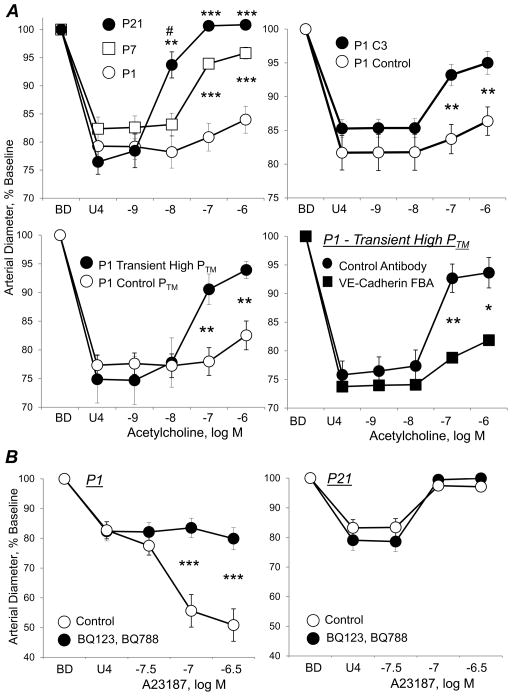
The early postnatal period is associated with remarkable changes in arterial structure and function, including the morphology and activity of endothelial cells. The endothelial cells lining fetal or newborn central arteries have a highly unusual phenotype. They have poorly-organized cellular junctions and prominent transcytoplasmic actin stress fibers (Figure 1) (1–8). Although common in cultured cells, native arterial endothelial cells normally lack stress fibers, and the actin cytoskeleton is restricted to a faint cortical network at the cell periphery (1, 4, 9, 10). Endothelial stress fibers are however a prominent feature in vascular disease and in areas susceptible to atherosclerosis (9, 11). Despite high expression levels of endothelial NO synthase (eNOS), endothelial cells lining fetal or newborn arteries have an impaired ability to initiate NO-mediated endothelial dilatation when compared to mature arteries (Figure 2) (7, 12–19). For example, in newborn mice carotid arteries, endothelial stimulation by acetylcholine, thrombin or A23187 was either poorly able or unable to generate endothelium-dependent dilatation (Figure 2) (7, 8, 20). Indeed, thrombin or A23187, which are powerful endothelial secretagogues, actually caused rapid constrictions of newborn arteries that were capable of causing complete occlusion of the arterial lumen (Figure 2) (20). These constrictions were mediated by endothelin-1 (ET-1) generated by the newborn endothelium, and were abolished by antagonizing ET-1 receptors (ETA or ETA plus ETB receptor antagonists), by inhibiting endothelin converting enzyme (ECE) or by endothelial denudation (Figure 2) (20). Indeed, endothelial cells lining newborn central arteries had high expression of ET-1 peptides and rapidly generated and released ET-1 in response to endothelial activation (20). Therefore, endothelial cells lining newborn arteries have highly unusual morphological and functional features, which are reminiscent of dysfunctional endothelium. Indeed, the extent of the impairment in NO-mediated dilatation and intensity of ET-1 mediated constriction of these immature arteries was far greater than was observed during endothelial dysfunction (20, 21).
Figure 1.
Representative LSM fluorescent images highlighting the morphology of native arterial endothelial cells. In A and B, images present the changes occurring in the actin cytoskeleton (A, phalloidin in red) and adherens junctions (B, VE-cadherin in red) of endothelial cells lining mouse arteries during the early postnatal period. Carotid arteries were isolated from newborn (postnatal day 1 or P1) and P7 mice. In A, the endothelial actin cytoskeleton displayed prominent stress fibers in P1 arteries, but had significantly reduced intensity and was restricted to a cortical actin structure delineating cell borders in P7 arteries. In P1 arteries, Rho inhibition (P1 C3, C3 transferase) reduced the appearance of endothelial stress fibers, and the actin cytoskeleton was were restricted to a cortical network in the cell periphery. In B, there was reduced staining of VE-Cadherin and organization of endothelial adherens junctions in P1 compared to P7 arteries. If P1 arteries were exposed to a transient increase in transmural pressure (PTM) (50 mmHg, 60 mins) (P1-50), the intensity and complexity of VE-cadherin and adherens junctions were increased compared to arteries maintained at a PTM of 20 mmHg (P1-20). Panel C presents VE-cadherin staining in the endothelial cells lining young (4 months) and old (22 months) rat tail arteries. Old age caused marked disruption of endothelial adherens junctions, which was associated with internalization of VE-cadherin. Images in A and B are taken from Flavahan et al, 2013 (7) and Flavahan and Flavahan 2014 (8). Images in C are unpublished observations.
Figure 2.
Regulation of endothelium-dependent responses to acetylcholine (A) or to calcium ionophore A23187 (B) in mice carotid arteries in the immediate postnatal period. Arteries were isolated from postnatal day 1 (P1, newborn), P7 and P21 mice, and analyzed in a microperfusion system at a transmural pressure (PTM) of 20 mmHg, the mean blood pressure of P1 mice. Functional responses are expressed as a percentage of the baseline diameter of the arteries (BD), and presented as means ± SEM. To observe dilatation or constriction, arteries were initially constricted with the thromboxane receptor agonist U46619 (U4). A: In P1 arteries, acetylcholine caused significant endothelium-dependent dilatation only at the highest dose tested, but caused markedly increased dilatation in P7 and P21 arteries (top left panel). In P1 arteries, the minimal dilator responses to acetylcholine were dramatically increased by inhibition of Rho signaling (P1 C3, C3 transferase) (top right panel) or by transiently increasing PTM to 50 mmHg (60 mins), corresponding to the mean blood pressure of P7 mice (middle left panel). This effect of increased pressure to amplify the dilator response to acetylcholine was prevented by a function blocking antibody to VE-cadherin (compared to a control antibody) (middle right panel). B: A23187 caused endothelium-dependent constriction in P1 arteries (bottom left panel), but endothelium-dependent dilatation in P21 arteries (bottom tight panel). Combined antagonism of endothelin ETA and ETB receptors (BQ123 plus BQ788) abolished constriction to A23187 in P1 arteries, but had no effect on the dilatation to A23187 in P21 arteries. Data taken from Flavahan et al, 2013 (7), Flavahan and Flavahan 2014 (8). and Chang et al 2016 (20).
The unusual structural and functional features of newborn arterial endothelial cells change dramatically during the first few weeks of postnatal life as the cells acquire normal protective features. Morphologically, the actin cytoskeleton transforms from transcytoplasmic stress fibers to formation of a cortical actin network, and the endothelial intercellular connections become more highly organized (Figures 1) (1–8). Thrombin or A23187 no longer evoke endothelium-dependent constriction and instead generate endothelium-dependent dilatation (Figure 2), which is paralleled by a diminution in endothelial expression of ET-1 peptides and a loss in the stimulated generation and release of ET-1 (20). Despite a gradual decrease in endothelial eNOS expression in this immediate postnatal period, there is a dramatic increase in endothelium-dependent NO-mediated dilatation (Figure 2) (7, 20). The emerging endothelium-dependent NO-mediated dilatation evoked by acetylcholine was associated with increased phosphorylation of eNOS (Ser1177) and abolished by inhibition of phosphoinositide-3-kinase (PI3K)/Akt signaling (7).
Signaling through the Rho family of GTPases have divergent roles in regulating endothelial morphology and function. RhoA and its downstream effectors, in particular Rho kinase (ROCK), stimulate endothelial stress fiber formation and attachment to the extracellular matrix, via focal adhesions (22–25). ROCK inhibits myosin light chain (MLC) phosphatase, increases levels of phosphorylated MLCs and facilitates actomyosin interaction. The contractile force generated by this ROCK-dependent actomyosin interaction contributes to disruption of intercellular endothelial junctions (22–26). In contrast, activation of Rac1 via PI3K causes disassembly of stress fibers and focal adhesion complexes and stimulates assembly of a cortical actin network and strengthening of intercellular adhesion (24, 27–31). The divergent effects of PI3K and RhoA/ROCK pathways are also apparent in the regulation of eNOS. ROCK inhibits eNOS activity by suppressing PI3K-dependent activation of Akt and eNOS Ser1177 phosphorylation (32, 33). ROCK can also reduce eNOS mRNA stability and promote eNOS protein uncoupling (32, 34, 35). Not surprisingly, endothelial Rho/ROCK signaling is considered to be minimal under normal physiological conditions, but contributes to endothelial dysfunction during the initiation of vascular disease (36, 37).
The unusual morphology and function of newborn endothelium is mediated by heightened activity of endothelial Rho/ROCK signaling (8). Endothelial cells lining newborn carotid arteries have increased levels of phosphorylated MLCs, which is associated with the prominent actin stress fibers, compared to more mature arteries (8). Acute inhibition of Rho or ROCK in isolated newborn arteries reduced the levels of phosphorylated MLCs and reorganized the endothelial actin cytoskeleton: the transcytoplasmic stress fibers were reduced and filamentous actin was restricted to a cortical actin network in the cell periphery (Figure 1) (8). Inhibition of Rho or ROCK also markedly increased endothelial NO-mediated dilatation to acetylcholine in newborn carotid arteries (Figure 2) (8). However, in more mature arteries, inhibition of Rho or ROCK had no effects on endothelial morphology or function (8). Therefore, acutely inhibiting Rho and ROCK signaling in newborn endothelial cells mimics the structural and functional maturation process of neonatal endothelial cells. Heightened activity of Rho/ROCK signaling in newborn endothelium, including in combination with reduced NO activity, may also contribute to increased expression and activity of ET-1 (20, 38).
Increased Rho/ROCK activity in newborn endothelial cells may reflect an unusual sub-endothelial matrix in newborn arteries. Native endothelial cells normally reside on a matrix composed primarily of laminin and collagen IV (39). However, the subendothelial matrix in fetal or newborn arteries is enriched in fibronectin, with endothelial stress fibers connecting via focal adhesion complexes to fibronectin fibrils (3, 5, 6, 40). As endothelial cells mature during the immediate postnatal period, they reorganize their underlying matrix, decreasing the levels of fibronectin and increasing laminin and collagen IV (1, 3, 4, 40). Fibronectin promotes activation of RhoA but not Rac1, whereas laminins enable persistent activation of Rac1 and suppression of RhoA (39, 41). As a result, endothelial adhesion to fibronectin stimulates formation of stress fibers and strong focal adhesions, which is mediated by Rho/ROCK signaling (41). Increased deposition of fibronectin may also contribute to increased Rho/ROCK signaling and the presence of actin stress fibers in dysfunctional endothelium during vascular disease (9, 42–44). Additional mechanisms may contribute to Rho/ROCK signaling under both conditions, including increased generation of reactive oxygen species (ROS) (7, 15, 45–48)
The neonatal maturation of arterial endothelium therefore appears to be associated with a change in endothelial signaling from heightened Rho/ROCK activity in newborn to increased PI3K/Akt/eNOS signaling in maturing endothelium (7, 8). Although Rho/ROCK can inhibit endothelial NO activity through multiple mechanisms, a key inhibitory step appears to be activation of PTEN, which counteracts PI3K activity and reduces Akt activity (33, 49, 50). Therefore, the emerging activity of PI3K/Akt/eNOS signaling in the immediate postnatal period may reflect a diminution in Rho/ROCK and PTEN signaling. This possibility is also evident from the ability of Rho/ROCK inhibition to mimic the process of developmental endothelial maturation (8).
The unusual phenotype of endothelial cells lining fetal and newborn central arteries including prominent expression, production and contractile activity of ET-1, the presence of stress fibers and a provisional fibronectin matrix, minimal NO-mediated dilatation, and heightened Rho/ROCK signaling, is highly reminiscent of dysfunctional endothelial cells that contribute to vascular disease (7, 8, 20). Indeed, eNOS may be uncoupled in neonatal endothelial cells and be associated with increased production of ROS (7, 15, 45, 46). This unusual endothelial phenotype may reflect a developmental requirement for embryonic and fetal growth, including a role for ET-1 in the development of the heart and great vessels (51–53). Presumably, the fetal and newborn cardiovascular system are protected from this unusual endothelial phenotype because of concomitant immaturity in other systems, including inflammatory and thrombotic processes. However, the presence of this immature endothelial phenotype in an adult setting would be expected to promote pathological effects within the cardiovascular system. Therefore, mechanism(s) responsible for initiating and maintaining the protective maturation of arterial endothelium are likely to be of fundamental importance to vascular health.
Endothelial adherens junctions are formed by engagement and clustering of VE-cadherin (CD144) molecules on adjacent endothelial cells (Figure 1). This clustering of VE-cadherin stimulates and supports the assembly of a macromolecular signaling complex that regulates endothelial morphology and function, including inhibition of RhoA/ROCK signaling and amplification of PI3K/Akt signaling (27, 54–59). The width and complexity of endothelial adherens junctions increases during the early postnatal period, consistent with increased clustering of VE-cadherin (Figure 1) (7). To determine whether this might contribute to endothelial maturation, neonatal arteries were incubated with a function-blocking antibody to VE-cadherin, which inhibits VE-cadherin clustering and so disrupts adherens junctions (7). Blocking VE-cadherin clustering inhibited the emergence in neonatal arteries of acetylcholine-induced increases in endothelial NO-mediated dilatation and eNOS phosphorylation, suggesting that increased VE-cadherin and junctional signaling contributes to endothelial maturation (7). This would be consistent with increased clustering of VE-cadherin at adherens junctions acting to reduce Rho/ROCK signaling and amplify PI3K/Akt signaling. The early postnatal maturation of central arterial endothelium occurs in the face of dramatic increases in blood pressure but with relatively stable blood flows in these central arteries (4, 60, 61). Indeed, morphological maturation of the endothelium, including reorganization of the actin cytoskeleton, was proposed to reflect the hemodynamic effects of increasing pressure (4). Remarkably, when isolated newborn mice carotid arteries were exposed to a transient increase in transmural pressure, which was equivalent to mean blood pressure of a 1 week old mouse, the width and complexity of adherens junctions were increased, mimicking the maturation process (Figure 1) (7). Likewise, the transient increase in transmural pressure dramatically amplified endothelial NO-mediated dilatation to acetylcholine in newborn arteries, which was associated with increased phosphorylation of eNOS (Ser1177) and was abolished by inhibition of PI3K/Akt signaling (Figure 2) (7). The function-blocking antibody to VE-cadherin markedly reduced responses evoked by the transient increase in pressure (Figure 2) (7). Therefore, adherens junctions appear to be involved in a novel endothelial mechanotransduction process, with stretch or developmental increases in blood pressure causing increased clustering of VE-cadherin (7). Indeed, in cultured endothelial cells, tugging forces stimulate the growth and stabilization of adherens junctions (62). These results suggest that increasing pressure in developing arteries, through a stretch-mediated increase in clustering of VE-cadherin at endothelial adherens junctions leads to amplification of PI3K-Akt signaling, emergence of eNOS signaling and NO-mediated dilatation in the immediate postnatal period. Increased clustering of VE-cadherin at adherens junctions and VE-cadherin-dependent signaling therefore appears to be of fundamental importance for the protective maturation of arterial endothelium and for actively maintaining vascular homeostasis and stability (7, 63, 64).
Role and Regulation of VE-cadherin and Adherens Junctions in Arterial Endothelial Cells
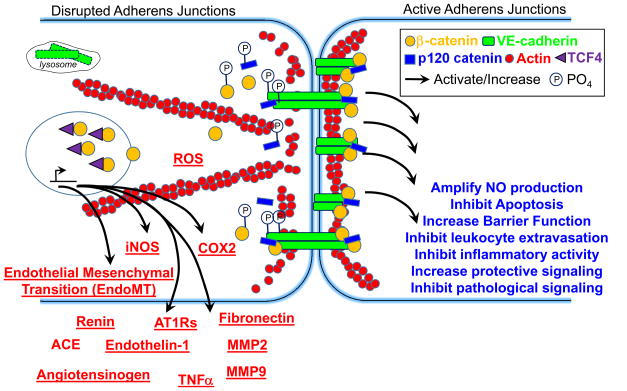
VE-cadherin-dependent signaling at endothelial adherens junctions promotes barrier function, amplifies NO activity, inhibits apoptosis, maintains endothelial differentiation, and inhibits inflammatory activation including inhibiting leukocyte extravasation and the expression of inflammatory mediators (Figure 3) (7, 55, 63, 65, 66). These effects reflect the assembly of a signaling complex in response to junctional clustering of VE-cadherin (27, 54–56, 67). Adherens junctions are stabilized by the interaction of VE-cadherin with catenins including β-catenin, which along with α-catenin connects the adhesion complex to the actin cytoskeleton, and p120 catenin, which inhibits VE-cadherin internalization (27, 65). The clustering of VE-cadherin and β-catenin at adherens junctions recruits additional proteins that stabilize the junctions including Rac1 along with regulators that increase or maintain Rac1 activity (Tiam1, Vav, IQGAP1), and P190RhoGAP, which inhibits RhoA (27, 54–56, 59, 65). Rac1 stimulates assembly of a cortical actin network and maintains junctional stability, whereas RhoA/ROCK promotes actin stress fiber formation and inhibits adherens junctional stability (24, 27, 67). VE-cadherin and β-catenin also interact with and regulate the activity of additional signaling proteins and pathways including PI3K/Akt, growth factor receptors including for VEGF, several non-receptor tyrosine kinases and phosphatases, and additional small G-proteins such as Rap1 (63, 68, 69).
Figure 3.
Schematic representation of endothelial adherens junctions, with protective activity of normal active junctions (right) and the potential pathological effects of disrupted junctions (left). The red underlined mediators and processes reflect potential effects induced by disruption of adherens junctions.
VE-cadherin appears to be a crucial and versatile mediator of endothelial responses to hemodynamic forces. Shear stress stimulates interaction between VE-cadherin, PECAM1 (CD31) and VEGFR2, which contributes to flow-induced activation of eNOS (70, 71). This shear stress mechanotransduction pathway is thought to initially involve PECAM-1 with subsequent transactivation of VEGFR2, leading to VEGFR2-dependent activation of PI3K/Akt, increased eNOS (Ser1177) phosphorylation and NO production (70–75). Although PECAM-1−/− arteries have reduced dilatation to shear stress, responses to acetylcholine are normal, suggesting this proposed pathway may be specific for shear stress (76). Within this model, VE-cadherin:β-catenin acts merely as a scaffolding intermediary to facilitate the transactivation of VEGFR2 (72, 73, 77). Indeed, shear stress-induced signaling does not actually require junctional localization of VE-cadherin or PECAM-1 and is not inhibited by VE-cadherin function blocking antibodies (65, 71, 78). This role is therefore distinct from the activity of VE-cadherin in mediating pressure-induced and developmental maturation of endothelial dilatation in neonatal arteries, which required junctional localization of VE-cadherin (7). In addition to facilitating VEGFR2-dependent activation of PI3K/Akt signaling, VE-cadherin can directly activate PI3K/Akt signaling independently of VEGFR2 (55–57). Moreover, this direct effect is dependent on VE-cadherin clustering at adherens junctions and is inhibited by function-blocking antibodies to the protein (55–57). Interestingly, in cultured endothelial cells, shear stress activates Akt in a VEGFR2-dependent manner, whereas pulsatile stretch activates Akt independently of VEGFR2 (71, 73–75, 79). This latter effect is likely mediated by clustering and activation of VE-cadherin-dependent signaling at adherens junctions. Therefore, stretch and shear stress activate distinct signaling pathways in endothelial cells, both of which are dependent on VE-cadherin.
Endothelial adherens junctions are a primary target during inflammatory responses: inflammatory cells and mediators disrupt endothelial adherens junctions by initiating tyrosine phosphorylation of VE-cadherin and its binding partners, causing dissociation of the signaling complex (Figure 3) (27, 55, 63, 68, 80, 81). Although tyrosine phosphatases, including vascular endothelial protein tyrosine phosphatase (VE-PTP) and SHP2, interact with VE-cadherin and β-catenin to stabilize the junctions, inflammatory stimuli cause dissociation of phosphatases from the complex (55, 82, 83). Src tyrosine kinase is primarily responsible for tyrosine phosphorylation of VE-cadherin (27, 81, 84, 85). Inflammatory stimuli activate and increase the association of Src with VE-cadherin and adherens junctions (81, 85). Once phosphorylated, VE-cadherin is ubiquitinated, then internalized via clathrin-mediated endocytosis for lysosomal degradation (81, 86). Inflammatory mediators can also disrupt adherens junctions by causing proteolysis of VE-cadherin, in particular its extracellular domain (68, 87, 88). Disruption of endothelial adherens junctions by inflammatory mediators reduces their protective influence in endothelial cells and increases endothelial permeability, inflammatory cell extravasation, and the vascular and organismal sensitivity to inflammatory stimuli (Figure 3) (89–91). Reduced activity of endothelium-derived NO and increased activity of Rho/ROCK as a result of diminished VE-cadherin signaling would be expected to amplify this pro-inflammatory activity.
Junctional disruption can also contribute directly to the activity and expression of inflammatory mediators. Once freed from its interaction with VE-cadherin, β-catenin can act as a transcriptional co-regulator by translocating to the nucleus and binding to TCF4 (Figure 3) (92). Although free β-catenin is normally targeted for degradation by a destruction complex, numerous mechanisms enable β-catenin to escape degradation and translocate to the nucleus (25, 55, 92). β-catenin:TCF4 transcriptional activity increases expression of key pathological and inflammatory mediators including the renin angiotensin system (angiotensinogen, renin, angiotensin converting enzyme, AT1 receptors), ET-1, TNFα, IL-6, iNOS, fibronectin, COX-2, MMP2, MMP9 (Figure 3) (93–99). Complementing the actions of these mediators, the disassembly of junctional signaling complexes may directly contribute to endothelial oxidant stress. Rac1 has conflicting roles within the endothelium. VE-cadherin clustering at adherens junctions causes Rac1 to localize at cell junctions, where it stimulates assembly of a cortical actin network, maintaining junctional stability and vascular integrity (27, 54–56). However, Rac1 also contributes to activation of NADPH oxidase (NOX1, NOX2) and increased ROS production (100). Disruption of adherens junctions causes Rac1 to dissociate from the VE-cadherin complex (54, 56), and can increase ROS activity (101), potentially by enabling Rac1 to contribute to NOX activity. Therefore, junctional disruption by inflammatory mediators can amplify inflammatory activity and pathological signaling by contributing to generation of additional inflammatory mediators and oxidant stress (84, 85, 102, 103).
Disruption of endothelial adherens junctions and release of β-catenin is also a key initial step in endothelial mesenchymal transition (EndoMT or EndMT), the trans-differentiation of endothelial cells to a mesenchymal cell phenotype (Figure 3) (104–108). The process is characterized by a progressive loss in endothelial proteins (e.g. VE-cadherin, von Willebrand Factor) and acquisition of mesenchymal markers (e.g. smooth muscle α-actin, SM22, FSP-1) (107, 109, 110). TGFβ and Smad2/3 signaling are considered central mediators of EndoMT and cause increased expression of transcriptional regulators (Snail1, Snail2/Slug, Twist) that suppress VE-cadherin and upregulate mesenchymal genes (92, 104, 107, 109–111). β-catenin is also a dynamic transcriptional mediator of EndoMT, increasing expression of Snail1, Snail2, and Twist, and amplifying the activity of other transcription factors including Smads (92, 104, 111). EndoMT-derived cells have a highly invasive and proliferative phenotype, with high fibrogenic activity and marked production of collagens (I and III) and fibronectin (104–107, 112, 113). Indeed, EndoMT can contribute to intimal lesions, interstitial fibrosis and microvascular rarefaction (104–107, 110, 112–118).
Uncontrolled disruption of endothelial adherens junctions can therefore have severe consequences for vascular health (Figure 3). Such disruption can not only reduce protective signaling and amplify the activity of pathological and inflammatory mediators, it can also threaten the stability and viability of endothelial cells and the blood vessel wall (64, 65).
Adherens Junction Disruption, Endothelial Dysfunction and Vascular Disease
The neonatal period appears to be a crucial transition phase for increased engagement and signaling of VE-cadherin at endothelial adherens junctions, resulting in endothelial maturation and full expression of endothelial protective activity (7). This protective process continues into adulthood with VE-cadherin and adherens junctions promoting vascular stability and homeostasis (Figure 3) (64, 65). Therefore, interruption of this process in developing arteries or its reversal in mature arteries could be equivalent to enabling the retention or re-emergence of the newborn endothelial phenotype, and would be expected to promote endothelial and vascular destabilization and progression of vascular disease. Although this concept is likely valid for numerous vascular diseases, the following examples are selected to highlight the potential role of endothelial junctional disruption on pathological processes initiated in an adult setting (Vascular Aging), in a developmental setting (‘Developmental Origins of Vascular Disease’), and in the complex combined setting of Atherosclerosis, which begins in childhood and progresses throughout our lifetime.
The Aging Arterial System
Old age is associated with a structural and functional deterioration of the arterial system that contributes to organ dysfunction and progression of cardiovascular disease. Endothelial dysfunction is a key contributor to this process of vascular aging (122, 123). Aging arterial endothelial cells are severely compromised: their production of protective NO is reduced, their barrier function is impaired, they are highly susceptible to apoptosis, and they have a prominent pro-inflammatory phenotype, including production of pathological mediators such as ROS, angiotensin II (ANGII), cytokines (e.g. TNFα, IL-6), and ET-1 (21, 123–140). Aging endothelial cells are therefore caught in a cycle of chronic stress, both producing and responding to pathological mediators, and their resulting frailty is considered a important instigator of vascular aging (123, 141).
A key aspect of vascular aging is central arterial stiffening, which results from increased fibrotic and inflammatory activity that transforms the arterial wall into a less compliant structure. Elastic central arteries normally expand during systole to store a portion of the heart’s stroke volume, which is then released during diastole to support continuous organ blood flow (142–145). This process is facilitated by a partial reflection of the pulse wave, which normally returns during diastole to augment central diastolic pressure (142–145). However, in aging, remodeling of central arteries reduces their compliance and increases pulse wave velocity, causing the reflected wave to return during systole and resulting in summation of the incident and reflected waves, which augments central pulse or systolic pressure (142–145). This disruption in central arterial dynamics can cause systemic vascular dysfunction and precipitate or exacerbate cardiovascular diseases including hypertension (142–145).
Aging central arteries have prominent intimal fibrous lesions with deposition of poorly-distensible proteins, collagen (I, III) and fibronectin, and degradation of highly-distensible elastin fibers (127, 134, 137–139, 146–149). Stiffness is further enhanced by increased fragility and reduced compliance of aging elastin fibers and by increased crosslinking and resilience of collagen and fibronectin matrices (127, 150). The expression of key inflammatory and fibrotic mediators is localized predominantly to endothelial cells and intimal smooth muscle cells (SMCs), and appears to be coordinated by local generation of ANGII (135, 137, 139, 140, 148, 149, 151, 152). ANGII-dependent increases in fibrotic (e.g. TGFβ), inflammatory (e.g. MCP-1, TNFα) and proteolytic activity (e.g. MMP2, MMP9) by endothelial and intimal SMCs drive the remodeling process by sustaining inflammatory stress, by promoting the secretory and invasive phenotype of intimal SMCs and by degrading elastin (135, 137, 139, 140, 148, 149, 151–153).
We have observed that endothelial adherens junctions are severely compromised in aging arteries with internalization and degradation of VE-cadherin (22–24 month old compared to 3–4 month old F344 rat aorta and tail arteries) (Figure 1) (154, and unpublished observations). Endothelial NO-mediated dilatation evoked by acetylcholine was significantly decreased in old compared to young arteries, although responses to an NO donor were not affected by aging. A function blocking antibody to VE-cadherin, which blocked clustering of the protein at adherens junctions, inhibited endothelium-dependent dilatation to acetylcholine in young arteries, but did not affect the already reduced response to the agonist in old arteries (154). These results are consistent with the structural disruption of endothelial adherens junctions and suggest that the functional activity of VE-cadherin and adherens junctions are diminished in aging arteries. Interestingly, in the presence of the VE-cadherin function blocking antibody, we no longer observed any significant difference between dilatation to acetylcholine in young and aged arteries (154). Therefore, the disruption in activity of VE-cadherin and adherens junctions in aging arteries can account entirely for the aging-induced impairment in endothelial dilatation to acetylcholine.
In addition to contributing to a diminution in endothelial NO-mediated dilator activity, an aging-induced disruption in endothelial adherens junctions could contribute to other aspects of endothelial and vascular dysfunction present in aging arteries, including increased endothelial permeability and apoptosis, and increased activity of inflammatory mediators. Indeed, β-catenin:TCF4 transcriptional activity increases expression of key pathological mediators, including the renin angiotensin system (angiotensinogen, renin, angiotensin converting enzyme, AT1 receptors), ET-1, TNFα, IL-6, iNOS, fibronectin, COX-2, MMP2, MMP9 (93–99), that are increased in aging endothelium and contribute to vascular aging (Figure 3) (132, 137, 141, 148, 151). Although transcriptional activity of β-catenin is minimal in normal arterial endothelium (25, 95, 155), it may contribute to cellular and organismal aging (156–159). β-catenin and β-catenin/TCF4 can interact with and regulate the activity of other transcriptional factors, including FOXO1 and NFκB (57, 92, 111, 160). NFκB activity is known to be increased in aging endothelial cells and is considered a key mediator of their inflammatory activity (161–164). During oxidant stress, β-catenin can switch from TCF4 to FOXO leading to expression of FOXO-dependent genes, which may also contribute to vascular aging (157, 158).
The intimal lesion of aging central arteries is formed by fibrogenic SMCs (127, 141). When cultured in vitro, ‘SMCs’ from aging arteries have increased proliferative, migratory and secretory capacity compared to ‘SMCs’ from young arteries, including increased ROS production, increased NFκB activity, and increased expression of inflammatory and fibrotic mediators (135, 137–141, 148, 149, 151, 153, 164–167) In the intact artery, the increased expression of inflammatory and fibrotic mediators mostly localize to endothelial and intimal ‘SMC’-like cells (135, 137, 139, 140, 148, 149, 151), and these cells are considered to play a dominant pathological role in aging arteries promoting the remodeling and stiffening of the arterial wall. In contrast to expanding intimal SMCs, the mature contractile SMCs in the media appear to have reduced viability in aging arteries (134, 139, 141). The intima of aging arteries should present a highly permissive environment for EndoMT. The expression and activity of TGFβ1 is increased in aging endothelial and intimal ‘SMCs’ (137, 149). Combined with the disruption of adherens junctions and release of β-catenin, aging endothelial cells are likely exposed to synergistic activation of the EndoMT process, which could contribute to vascular and interstitial fibrosis and to microvascular rarefaction in aging. Indeed, EndoMT-derived cells rather than traditional SMCs may be responsible for the intimal lesions and increased stiffness of aging central arteries.
Therefore, an aging-induced disruption of endothelial adherens junctions and VE-cadherin-dependent signaling could be a major driving force behind the process of vascular aging.
Atherosclerosis
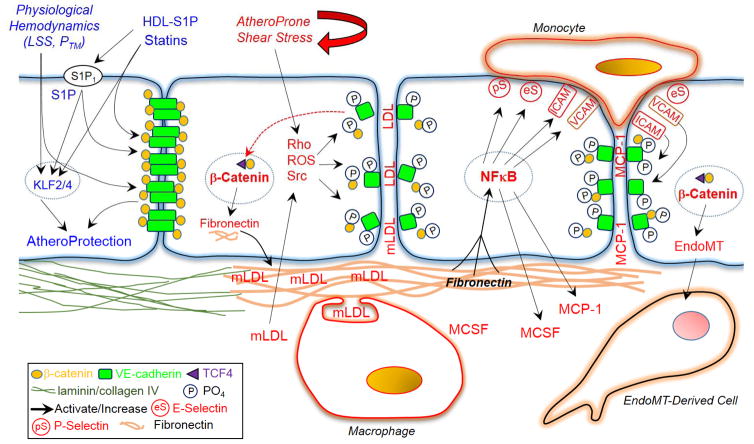
Atherosclerotic lesions reflect focal expansion of the arterial intima by vascular and immune/inflammatory cells, extracellular matrix proteins, native and modified lipids, and cellular debris (206–209). Lesions develop preferentially at sites of disturbed blood flow, such as arterial branches or curvatures, where normal high laminar shear stress is distorted by flow separation and directional changes, resulting in low and reciprocating shear stress (209–213). Although exposure of cultured endothelial cells to prolonged normal shear stress is anti-inflammatory and atheroprotective, disturbed or atheroprone shear stress stimulates expression of inflammatory and thrombotic mediators (44, 209–213). Atheroprone shear stress increases transcriptional activity of NFκB, which is considered a master regulator of the atherosclerotic process, resulting in increased endothelial expression of its target genes including inflammatory mediators ICAM-1, VCAM-1 and MCP-1 (monocyte chemoattractant protein) (42, 44, 214, 215). Likewise, NFκB activity and expression of these inflammatory mediators is increased in atherosclerosis-prone regions of the vasculature, and occurs before lesion formation suggesting they are very early events in the atherosclerotic process (44, 214, 215). Disturbed blood flow patterns can therefore sustain local inflammatory activity that provides the basis for atherosclerotic lesion development (44, 209–213). The atherosclerotic process begins in childhood and progresses through adolescence and young adulthood into middle age (119–121). As described above, the early postnatal maturation of arterial endothelial cells is associated with reorganization of the actin cytoskeleton from transcytoplasmic stress fibers to a cortical actin network and with increased organization and clustering of VE-cadherin at adherens junctions (7, 8). These interdependent processes enable protective maturation of the endothelium (7, 8). However, in arterial regions prone to atherosclerosis, endothelial cells retain transcytoplasmic stress fibers and have poorly organized adherens junctions (1, 4, 95, 213, 216). An interruption in endothelial maturation and disruption of adherens junctions and VE-cadherin-dependent protective signaling in these lesion-prone sites is likely a key driving force for the initiation and progression of the atherosclerotic process (Figure 4).
Figure 4.
Schematic representation highlighting the potential importance of adherens junction disruption in the atherosclerotic process. Abbreviations not defined in manuscript text: LSS, laminar shear stress; PTM, transmural pressure; KLF, Kruppel-Like Factor.
Arterial regions prone to atherosclerosis are sites of increased endothelial proliferation and increased endothelial permeability to macromolecules including low density lipoprotein (LDL) (209, 210, 212, 217), both effects consistent with reduced junctional clustering of VE-cadherin (Figure 4). Indeed, atheroprone shear stress disrupts adherens junctions leading to formation of gaps and increased endothelial permeability (95, 213, 215). The disruption in the endothelial barrier and the subendothelial deposition and modification of apoB-containing lipoproteins, predominantly LDL, is considered an important early amplification step in the atherosclerotic process (206–208). Modified lipids, as with atheroprone shear stress, cause inflammatory activation of the endothelium reducing the activity of protective mediators such as NO and increasing activity of inflammatory mediators including NFκB, ROS, VCAM-1, MCP-1 and MCSF (macrophage colony stimulating factor) (206–209). Indeed, a high cholesterol diet strongly amplifies the inflammatory activity of the endothelium at atherosclerosis-prone regions (44). Inflammatory mediators increase recruitment of monocytes, and stimulate their subsequent survival and differentiation to macrophages (206–209). The transmigration of leukocytes including monocytes across the endothelium is critically dependent on disruption of adherens junctions (80, 218, 219). As with atheroprone shear stress, oxidized lipids derived from modified LDL disrupt endothelial adherens junctions, which is mediated by a ROS-dependent activation of Src and increased tyrosine phosphorylation and internalization of VE-cadherin (Figure 4) (84, 220). Likewise, subendothelial oxidized LDL increases the transendothelial migration of monocytes, which is associated with decreased expression of VE-cadherin and reduced intensity of adherens junctions (219). Monocyte adhesion to endothelial cells also triggers disruption of adherens junctions, which is mediated by a VCAM-1 and ICAM-1-dependent increase in Src activity and tyrosine phosphorylation of VE-cadherin (Figure 4) (80, 218). Therefore, transmigration augments subsequent monocyte transmigration and recruitment to the lesion-prone site (221). Although these subendothelial monocyte/macrophages attempt to clear the modified LDL particles from the subendothelial space, they become engorged with lipids, are unable to leave and are transformed into foam cells (206–209). The evolving atherosclerotic lesion reflects a coordinated interaction between vascular, immune and inflammatory cells, with continual remodeling of the atheroma resulting in formation of a lipid-rich necrotic core comprising extracellular lipid droplets and cellular debris that is capped by a subendothelial fibrous layer of SMCs and extracellular matrix, which provides stability to the plaque (206–209).
Consistent with studies in cultured endothelial cells, a high cholesterol diet increases degradation of VE-cadherin, further disrupting endothelial adherens junctions and increasing endothelial permeability in atherosclerosis-prone regions (88). Several cellular mechanisms have been proposed to explain this increased degradation of VE-cadherin. The expression of ADAM10 and ADAM15 are increased in atherosclerosis and may contribute to extracellular cleavage of VE-cadherin and to Src-mediated phosphorylation and internalization of VE-cadherin, respectively (87, 222, 223). Miyazaki and colleagues demonstrated a clear role for m-calpain in causing cleavage of VE-cadherin and disruption of endothelial adherens junctions in cholesterol-fed mice (88). Expression of m-calpain was selectively increased in the endothelium of human atherosclerotic lesions, in cholesterol-fed mice, and in endothelial cells exposed to modified or oxidized LDL (88). Pharmacological or molecular inhibition of m-calpain prevented VE-cadherin degradation and disruption of adherens junctions in cholesterol-fed LDLR−/− and ApoE−/− mice (88). The proteolysis likely reflected VE-cadherin degradation by m-calpain following its internalization in clathrin-enriched domains (224). Inhibition of m-calpain suppressed atherosclerosis in cholesterol-fed mice, acting to reduce endothelial hyperpermeability, monocyte/macrophage infiltration into the vascular wall, and activity of inflammatory mediators including NFκB, ICAM-1, VCAM-1 and E-selectin (88). This confirms that disruption of endothelial adherens junctions may contribute importantly to endothelial inflammatory activity and to the initiation and progression of atherosclerotic lesions (Figure 4).
In addition to disrupting endothelial adherens junctions, atheroprone shear stress increases the nuclear accumulation and transcriptional activity of β-catenin, resulting in increased expression of β-catenin target genes, including fibronectin, IL-8 and cyclin D1 (Figure 4) (95). Inhibition of β-catenin transcriptional activity blocked expression of these mediators, and also markedly reduced the activation of NFκB by atheroprone shear stress (95). This reflects the key inflammatory activity of fibronectin in endothelial cells (44, 215). Indeed, molecular inhibition of fibronectin expression reduced the ability of atheroprone shear stress to increase NFκB activity and to increase expression of inflammatory mediators including MCP-1 and E-selectin (Figure 4) (42). These in vitro results are consistent with in vivo analyses. Inhibiting fibronectin in vivo reduces inflammatory activity and leukocyte infiltration into the vessel wall (42, 225). Moreover, fibronectin is preferentially deposited beneath the endothelium of human atherosclerotic plaques and in atherosclerosis-prone regions of control and ApoE−/− mice (42, 44, 215). In ApoE−/− mice, the endothelial fibronectin expression is paralleled by increased endothelial nuclear translocation and transcriptional activity of β-catenin (95). These effects appear to be early events in the atherosclerotic process (44, 95). Therefore, activation of a key regulator of the atherosclerotic process, NFκB, and the resulting endothelial inflammatory activity that sustains the atherosclerotic process, is in turn dependent on disruption of endothelial adherens junctions (Figure 4).
Because of the important role of adherens junctions in maintaining a stable endothelial phenotype, the degradation of VE-cadherin, disruption of endothelial adherens junctions and increased transcriptional activity of β-catenin in atherosclerosis-prone regions would be expected to activate EndoMT at these sites (Figures 3 and 4). Indeed, cells expressing endothelial and mesenchymal marker proteins, a characteristic feature of EndoMT, are present in the neointima of human atherosclerotic plaques (226, 227). Furthermore, lineage tracking of endothelium-derived cells demonstrated that a significant number of intimal fibroblast-like cells in ApoE−/− mice are derived from EndoMT (227). EndoMT-derived cells are also present in the porcine vasculature in regions susceptible to atherosclerosis, but not in atherosclerosis-resistant areas with normal flow patterns (226). Indeed, exposure of endothelial cells to atheroprone shear stress stimulates EndoMT and increases expression of mesenchymal marker proteins (228), whereas normal laminar shear stress increases resistance to EndoMT (226). Likewise, oxidized LDL amplifies EndoMT, whereas native LDL does not (229). Production of extracellular matrix by Endo-MT-derived mesenchymal cells might be expected to contribute to expansion of the atherosclerotic cap and stabilization of the plaque. However, EndoMT was increased in unstable compared to stable human atherosclerotic lesions and an increased number of EndoMT-derived cells were present in ruptured versus non-ruptured plaques (227). Indeed, there was an inverse relationship between cap thickness and the amount of EndoMT-derived cells within the plaque (227). The phenotype of EndoMT cells may be determined by the nature and strength of the inciting stimulus, with EndoMT-derived cells in atherosclerotic lesions having increased expression of MMPs resulting in degradation of the cap and plaque destabilization (227). So far, the focus has been on TGFβ as a potential mediator of EndoMT in atherosclerosis (227, 228). However, it is highly likely that disruption of adherens junctions and transcriptional activity of β-catenin plays a key role in the process.
Therefore, the status of endothelial adherens junctions likely plays a key role in the initiation and progression of the atherosclerotic process. Junctional disruption would contribute to a breakdown in the endothelial barrier at atherosclerosis-prone regions, enabling the subendothelial accumulation of atherogenic lipids, increased inflammatory activity and increased monocyte transmigration at these sites and contribute to formation and expansion of the atherosclerotic plaque. Furthermore, junctional disruption and release of transcriptionally active β-catenin will amplify endothelial inflammatory activity and lead to destabilization of the plaque via formation of EndoMT-derived cells, precipitating clinically significant and life threatening events (Figure 4).
Developmental Origins of Vascular Disease
The concept that adult disease can be influenced by events occurring in utero was first popularized by David Barker. Epidemiological studies by Barker and others demonstrated that compared to infants born ‘appropriate for gestational age’ (AGA), those born ‘small for gestational age’ (SGA) are at increased risk for developing cardiovascular and metabolic diseases (168–170). This led to the ‘Barker hypothesis’: that a suboptimal environment or poor nutrition during fetal or neonatal development predisposes an individual to develop cardiovascular and metabolic diseases later in life. The theory has received considerable support from animal models demonstrating that intrauterine growth restriction (IUGR) causes pathological changes in adult offspring (170).
The vascular pathological changes resulting from intrauterine stress have similarities to vascular aging, highlighting the possibility that it may mimic premature aging of the cardiovascular system. Indeed, Barker originally observed that systolic, but not diastolic, blood pressure was inversely related to birth weight (171). Numerous studies have since confirmed that birth weight or size has a greater impact on systolic compared to diastolic blood pressure (172–175). Indeed, compared to AGA youth, those born SGA have increased pulse wave velocity, Augmentation Index (measure of premature wave reflection) and central arterial stiffness (172, 174, 176–179).
The neonatal period is likely to be a crucial stage for understanding pathogenic mechanisms initiated by intrauterine stress. For example, endothelial dilator dysfunction is evident in SGA infants soon after birth (180), earlier than pathological changes in blood pressure or metabolic processes (168, 169, 181–184). Martyn and Greenwald originally proposed that intrauterine stress might disrupt the crucial neonatal synthesis of elastin resulting in a lifelong reduction in the protein and increased arterial stiffness (185). Elastin is long-lived protein and the immediate postnatal period represents a crucial period for its synthesis (1, 185–188). Certainly, in adult offspring from stressed pregnancies, central arteries have markedly reduced elastin and increased collagen content, with a concomitant increase in arterial stiffness and an increase in immature dedifferentiated SMCs (189). Furthermore, transient inhibition of angiotensin activity in the early postnatal period prevents the subsequent development of cardiovascular disease resulting from intrauterine stress (190, 191).
As with endothelial cells, SMC maturation or differentiation also increases in the immediate postnatal period, with a concomitant reduction in immature SMCs (1, 187, 192–198). Interestingly, immature or dedifferentiated SMCs have increased capacity to synthesize collagens and fibronectin, which contribute to arterial stiffness, whereas mature SMCs have increased ability to generate elastin, which increases arterial compliance (199). Endothelial cells promote the maturation of SMCs, and stimulate these mature differentiated SMCs to express elastin (200–203). Therefore, disruption of postnatal endothelial maturation could result in inefficient SMC maturation, with retention of immature SMCs, an imbalance in structural proteins (including reduced elastin) and arterial stiffness. Interestingly, EndoMT may be involved in generating vascular mesenchymal cells during arterial development (105, 204), and failure of endothelial adherens junctions to fully engage in the early postnatal period could amplify EndoMT and increase the activity of immature SMCs.
We have observed that intrauterine stress disrupts the protective maturation of central arterial endothelial cells occurring in the early postnatal period (205, and unpublished observations). The normal emergence of endothelial-dependent NO-mediated dilatation to acetylcholine, and which is dependent on VE-cadherin clustering at adherens junctions, was disrupted in mice models of IUGR (205). This was associated with a failure of endothelial adherens junctions to expand in size and complexity during the immediate postnatal period, suggesting that the normal increased clustering of VE-cadherin at adherens junctions is impaired by intrauterine stress (205).
By delaying or preventing the protective maturation of arterial endothelial cells in the early postnatal period, intrauterine stress could lead to the retention of an inappropriate endothelial phenotype and could disrupt the normal maturation of the arterial wall. The developmental origins of adult vascular disease likely reflects the continued presence of an abnormal endothelial and vascular phenotype, rather than a sudden triggering of pathological activity later in life.
Amplifying Endothelial Adherens Junctions as a Therapeutic Approach in Vascular Disease
Adherens junctions appear to function as an amplification nexus for endothelial signaling, both in terms of protective and pathological signaling. For example, PI3K/Akt signaling increases VE-cadherin clustering and stabilization of adherens junctions, which in turn augments PI3K/Akt signaling and protective endothelial activity. Likewise, pathological and inflammatory stimuli specifically target endothelial adherens junctions, with subsequent junctional disruption leading to additional pathological and inflammatory changes in endothelial and vascular structure and function (Figures 3 and 4). Indeed, targeted strengthening or protection of endothelial adherens junctions, by interrupting such pathological amplification, should be a powerful therapeutic approach to treating vascular disease.
Amplifying the activity of endothelial adherens junctions has already emerged as an innovative approach to treat pathological inflammatory processes (89, 230). Molecular strategies to increase VE-cadherin clustering at adherens junctions reduced inflammation-induced increases in endothelial permeability and inflammatory cell extravasation (83, 231, 232). Likewise, increased VE-cadherin clustering (via Robo4/Slit signaling) decreased vascular inflammation, tissue injury and mortality caused by LPS or infection (89, 233). The role of endothelial adherens junctions was confirmed by demonstrating that the protective effects were inhibited by a function blocking antibody to VE-cadherin (89). Several factors act as protective vascular mediators by increasing the activity of endothelial adherens junctions. For example, angiopoietin-1 (Angpt1) or sphingosine 1-phosphate (S1P) S1P1 receptor agonists (or Robo4/Slit signaling) increase VE-cadherin clustering at endothelial adherens junctions, and prevent phosphorylation and internalization of VE-cadherin and the junctional disruption occurring in response to inflammatory mediators (24, 29, 81, 89, 230, 234–238). Increasing VE-clustering at endothelial adherens junctions can also inhibit the transcriptional activity of β-catenin, presumably by restricting its ability to translocate to the nucleus (57, 239, 240). As a result, this approach should reduce β-catenin-dependent processes such as amplification of inflammatory activity and EndoMT. Protecting and amplifying endothelial adherens junctions inhibit increases in permeability and leukocyte extravasation, reduce endothelial cell apoptosis, and protect tissues from injury and reduce mortality in inflammatory models (234–238, 241–246).
Approaches to increase the activity of endothelial adherens junctions in vascular disease may actually be ‘replacement therapy’. For example, S1P and Angpt1 are local endogenous promoters of vascular stability (242, 247). Indeed, laminar shear stress increases production of S1P from endothelial cells (247), which might contribute to stabilization of adherens junctions (213). Likewise, S1P is a constituent of high density lipoprotein (HDL) and a key mediator of HDL’s anti-inflammatory effects on the endothelium, with loss of S1P contributing to ‘HDL dysfunction’ in atherosclerosis (248–250). Inflammatory stimuli increase endothelial expression of alternate S1P receptors, such as S1P2 that counter the stabilizing influence of S1P1 (50, 244, 251), and the Angpt1 antagonist Angpt2, which contribute to inflammatory responses (242, 252). These disruptive processes can also be upregulated during the development of vascular disease (253, 254). Interestingly, a very early feature of the atherosclerotic process is a selective impairment in endothelial signaling mediated by the pertussis toxin-sensitive Gi-protein (255–258). S1P1 dependent effects to stabilize endothelial adherens junctions are mediated by this Gi-protein (28, 259). The early dysfunction in endothelial Gi-protein signaling may therefore reflect activity of an alternate signaling pathway (e.g. S1P2), which inhibits this protective pathway.
Amplification of endothelial adherens junctions and VE-cadherin-dependent signaling may contribute to the protective effects of existing vascular therapeutic agents. For example, independently of lowering LDL levels, statins have direct, protective effects on endothelial cells that likely contribute to their therapeutic efficacy. These so called ‘pleiotropic’ effects are mediated predominantly by inhibition of Rho/ROCK signaling (108, 260, 261). Statins prevent disruption of endothelial adherens junctions by inflammatory mediators or adherent monocytes by preventing the tyrosine phosphorylation of VE-cadherin and thereby blocking monocyte transmigration (262). This protective effect is mediated by inhibition of Rho/ROCK signaling, which is an upstream regulator of Src and VE-cadherin tyrosine phosphorylation (262). Statins also inhibit the process of EndoMT (108), which is consistent with protection and stabilization of endothelial adherens junctions.
Therapeutic amplification of VE-cadherin signaling and endothelial adherens junctions should therefore be a powerful mechanism to restore protective endothelial signaling and vascular stability, and prevent the initiation and progression of vascular disease (Figures 3 and 4).
References
- 1.Kocher O, Skalli O, Cerutti D, Gabbiani F, Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985 Jun;56(6):829–38. doi: 10.1161/01.res.56.6.829. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H. Electron microscopic study of the prenatal development of the thoracic aorta in the rat. Am J Anat. 1988 Apr;181(4):406–18. doi: 10.1002/aja.1001810409. [DOI] [PubMed] [Google Scholar]
- 3.Davis EC. Endothelial cell connecting filaments anchor endothelial cells to the subjacent elastic lamina in the developing aortic intima of the mouse. Cell Tissue Res. 1993 May;272(2):211–9. doi: 10.1007/BF00302726. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi N, Sakai T. Postnatal reorganization of actin filaments and differentiation of intercellular boundaries in the rat aortic endothelial cells. Cell Tissue Res. 1994 Dec;278(3):471–82. doi: 10.1007/BF00331365. [DOI] [PubMed] [Google Scholar]
- 5.Jinguji Y. Developmental stage dependent expression of the endothelial stress fibers and organization of fibronectin fibrils in the aorta of chick embryos. Zoolog Sci. 2003 Nov;20(11):1359–66. doi: 10.2108/zsj.20.1359. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto K, Fujii S, Takemasa T, Yamashita K. Factors inducing codistribution of marginal actin fibers and fibronectin in rat aortic endothelial cells. Am J Physiol. 1997 May;272(5 Pt 2):H2188–94. doi: 10.1152/ajpheart.1997.272.5.H2188. [DOI] [PubMed] [Google Scholar]
- 7.Flavahan S, Mozayan MM, Lindgren I, Flavahan NA. Pressure-induced maturation of endothelial cells on newborn mouse carotid arteries. Am J Physiol Heart Circ Physiol. 2013 Aug 1;305(3):H321–9. doi: 10.1152/ajpheart.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flavahan S, Flavahan NA. The atypical structure and function of newborn arterial endothelium is mediated by Rho/Rho kinase signaling. Am J Physiol Heart Circ Physiol. 2014 Aug 15;307(4):H628–32. doi: 10.1152/ajpheart.00327.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbiani G, Gabbiani F, Lombardi D, Schwartz SM. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–4. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flavahan NA, Bailey SR, Flavahan WA, Mitra S, Flavahan S. Imaging remodeling of the actin cytoskeleton in vascular smooth muscle cells after mechanosensitive arteriolar constriction. Am J Physiol Heart Circ Physiol. 2005;288(2):H660–9. doi: 10.1152/ajpheart.00608.2004. [DOI] [PubMed] [Google Scholar]
- 11.Guyton JR, Shaffer DR, Henry PD. Stress fibers in endothelial cells overlying atherosclerotic lesions in rabbit aorta. Am J Med Sci. 1989 Aug;298(2):79–82. doi: 10.1097/00000441-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Boegehold MA. Endothelium-dependent control of vascular tone during early postnatal and juvenile growth. Microcirculation. 2010 Jul;17(5):394–406. doi: 10.1111/j.1549-8719.2010.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abman SH, Chatfield BA, Rodman DM, Hall SL, McMurtry IF. Maturational changes in endothelium-derived relaxing factor activity of ovine pulmonary arteries in vitro. Am J Physiol. 1991 Apr;260(4 Pt 1):L280–5. doi: 10.1152/ajplung.1991.260.4.L280. [DOI] [PubMed] [Google Scholar]
- 14.Liu SF, Hislop AA, Haworth SG, Barnes PJ. Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs. Br J Pharmacol. 1992 Jun;106(2):324–30. doi: 10.1111/j.1476-5381.1992.tb14335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol. 2009 Mar;296(3):L555–64. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zellers TM, Vanhoutte PM. Endothelium-dependent relaxations of piglet pulmonary arteries augment with maturation. Pediatr Res. 1991 Aug;30(2):176–80. doi: 10.1203/00006450-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilatation in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2005 Jan;288(1):R149–57. doi: 10.1152/ajpregu.00427.2004. [DOI] [PubMed] [Google Scholar]
- 18.White CR, Hamade MW, Siami K, Chang MM, Mangalwadi A, Frangos JA, Pearce WJ. Maturation enhances fluid shear-induced activation of eNOS in perfused ovine carotid arteries. Am J Physiol Heart Circ Physiol. 2005 Nov;289(5):H2220–7. doi: 10.1152/ajpheart.01013.2004. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LP, Weiner CP. Acetylcholine relaxation of renal artery and nitric oxide synthase activity of renal cortex increase with fetal and postnatal age. Pediatr Res. 1996 Aug;40(2):192–7. doi: 10.1203/00006450-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Chang F, Flavahan S, Flavahan NA. Immature endothelial cells initiate endothelin-mediated constriction of newborn arteries. J Physiol. 2016 Sep 1;594(17):4933–44. doi: 10.1113/JP272176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel A, Su B, Flavahan S, Lowenstein CJ, Berkowitz DE, Flavahan NA. Increased endothelial exocytosis and generation of endothelin-1 contributes to constriction of aged arteries. Circ Res. 2010 Jul 23;107(2):242–51. doi: 10.1161/CIRCRESAHA.109.210229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009 Jan;77(1):53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Nieuw Amerongen GP, Musters RJ, Eringa EC, Sipkema P, van Hinsbergh VW. Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am J Physiol Cell Physiol. 2008 May;294(5):C1234–41. doi: 10.1152/ajpcell.00551.2007. [DOI] [PubMed] [Google Scholar]
- 24.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004 Aug 15;92(6):1075–85. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 25.Beckers CM, Garcia-Vallejo JJ, van Hinsbergh VW, van Nieuw Amerongen GP. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 2008 Sep 1;79(4):679–88. doi: 10.1093/cvr/cvn127. [DOI] [PubMed] [Google Scholar]
- 26.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010 Apr 12;207(4):881–96. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010 Mar 17;72:463–93. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 28.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001 Sep;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999 Oct 29;99(3):301–12. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J. 2002 Jul;16(9):950–62. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- 31.Gunduz D, Thom J, Hussain I, Lopez D, Hartel FV, Erdogan A, Grebe M, Sedding D, Piper HM, Tillmanns H, Noll T, Aslam M. Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3-kinase/Akt-mediated Rac1 activation. Arterioscler Thromb Vasc Biol. 2010 Jun;30(6):1237–45. doi: 10.1161/ATVBAHA.110.203901. [DOI] [PubMed] [Google Scholar]
- 32.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002 Dec;22(24):8467–77. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004 Oct;24(10):1842–7. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002 Jul 2;106(1):57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 35.Ryoo S, Bhunia A, Chang F, Shoukas A, Berkowitz DE, Romer LH. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2010 Nov 4; doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loirand G, Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol. 2010 Nov;7(11):637–47. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Q, Gensch C, Liao JK. Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci. 2011 Mar;32(3):167–73. doi: 10.1016/j.tips.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Perera O, Perez-Sala D, Soria E, Lamas S. Involvement of Rho GTPases in the transcriptional inhibition of preproendothelin-1 gene expression by simvastatin in vascular endothelial cells. Circ Res. 2000 Sep 29;87(7):616–22. doi: 10.1161/01.res.87.7.616. [DOI] [PubMed] [Google Scholar]
- 39.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005 Nov 25;97(11):1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 40.Davis EC. Immunolocalization of microfibril and microfibril-associated proteins in the subendothelial matrix of the developing mouse aorta. J Cell Sci. 1994 Mar;107(Pt 3):727–36. doi: 10.1242/jcs.107.3.727. [DOI] [PubMed] [Google Scholar]
- 41.Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004 Jan 1;292(1):67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Feaver RE, Gelfand BD, Wang C, Schwartz MA, Blackman BR. Atheroprone hemodynamics regulate fibronectin deposition to create positive feedback that sustains endothelial inflammation. Circ Res. 2010 Jun 11;106(11):1703–11. doi: 10.1161/CIRCRESAHA.109.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009 Apr 24;104(8):995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005 Apr 11;169(1):191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, Black SM, Steinhorn RH. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006 Feb;290(2):L232–41. doi: 10.1152/ajplung.00393.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandi M, Leiper J, Arrigoni F, Hislop A, Vallance P, Haworth S. Developmental regulation of GTP-CH1 in the porcine lung and its relationship to pulmonary vascular relaxation. Pediatr Res. 2006 Jun;59(6):767–72. doi: 10.1203/01.pdr.0000219301.19958.a0. [DOI] [PubMed] [Google Scholar]
- 47.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive Oxygen Species from Smooth Muscle Mitochondria Initiate Cold-Induced Constriction of Cutaneous Arteries. Am J Physiol Heart Circ Physiol. 2005 Mar 11; doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
- 48.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004 Oct;287(4):H1495–500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 49.Tamguney T, Stokoe D. New insights into PTEN. J Cell Sci. 2007 Dec 1;120(Pt 23):4071–9. doi: 10.1242/jcs.015230. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 51.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 52.Kurihara Y, Kurihara H, Oda H, Maemura K, Nagai R, Ishikawa T, Yazaki Y. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J Clin Invest. 1995 Jul;96(1):293–300. doi: 10.1172/JCI118033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanagisawa H, Hammer RE, Richardson JA, Williams SC, Clouthier DE, Yanagisawa M. Role of Endothelin-1/Endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest. 1998 Jul 1;102(1):22–33. doi: 10.1172/JCI2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birukova AA, Tian Y, Dubrovskyi O, Zebda N, Sarich N, Tian X, Wang Y, Birukov KG. VE-cadherin trans-interactions modulate Rac activation and enhancement of lung endothelial barrier by iloprost. J Cell Physiol. 2012 Oct;227(10):3405–16. doi: 10.1002/jcp.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb Res. 2007;120(Suppl 2):S1–6. doi: 10.1016/S0049-3848(07)70124-X. [DOI] [PubMed] [Google Scholar]
- 56.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002 Apr;13(4):1175–89. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008 Aug;10(8):923–34. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 58.Su SC, Maxwell SA, Bayless KJ. Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J Biol Chem. 2010 Dec 24;285(52):40624–34. doi: 10.1074/jbc.M110.157271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res. 2011 Apr 1;317(6):859–72. doi: 10.1016/j.yexcr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bendeck MP, Keeley FW, Langille BL. Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol. 1994 Dec;267(6 Pt 2):H2268–79. doi: 10.1152/ajpheart.1994.267.6.H2268. [DOI] [PubMed] [Google Scholar]
- 61.Ishii T, Kuwaki T, Masuda Y, Fukuda Y. Postnatal development of blood pressure and baroreflex in mice. Auton Neurosci. 2001 Dec 10;94(1–2):34–41. doi: 10.1016/S1566-0702(01)00339-3. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):9944–9. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009 Feb;16(2):209–21. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Murakami M, Nguyen LT, Zhuang ZW, Moodie KL, Carmeliet P, Stan RV, Simons M. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008 Oct;118(10):3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013 Sep 16;26(5):441–54. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001 Mar 15;97(6):1679–84. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 67.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010 Jan;103(1):40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 68.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008 Jul 1;121(Pt 13):2115–22. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 69.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010 Oct;22(5):651–8. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009 Jan;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005 Sep 15;437(7057):426–31. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 72.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9462–7. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003 Aug 22;93(4):354–63. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 74.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002 Aug 19;158(4):773–85. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005 Sep 15;118(Pt 18):4103–11. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 76.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005 Aug;25(8):1590–5. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 77.Conway D, Schwartz MA. Lessons from the endothelial junctional mechanosensory complex. F1000 Biol Rep. 2012;4:1. doi: 10.3410/B4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaufman DA, Albelda SM, Sun J, Davies PF. Role of lateral cell-cell border location and extracellular/transmembrane domains in PECAM/CD31 mechanosensation. Biochem Biophys Res Commun. 2004 Aug 6;320(4):1076–81. doi: 10.1016/j.bbrc.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 79.Li M, Chiou KR, Bugayenko A, Irani K, Kass DA. Reduced wall compliance suppresses Akt-dependent apoptosis protection stimulated by pulse perfusion. Circ Res. 2005 Sep 16;97(6):587–95. doi: 10.1161/01.RES.0000181432.73920.b1. [DOI] [PubMed] [Google Scholar]
- 80.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007 Sep 15;179(6):4053–64. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 81.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P, McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wessel F, Winderlich M, Holm M, Frye M, Rivera-Galdos R, Vockel M, Linnepe R, Ipe U, Stadtmann A, Zarbock A, Nottebaum AF, Vestweber D. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014 Mar;15(3):223–30. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 83.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med. 2011 Nov 21;208(12):2393–401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Translational research: the journal of laboratory and clinical medicine. 2013 Jun;161(6):495–504. doi: 10.1016/j.trsl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mittal M, Urao N, Hecquet CM, Zhang M, Sudhahar V, Gao XP, Komarova Y, Ushio-Fukai M, Malik AB. Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):877–87. doi: 10.1161/ATVBAHA.114.304802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003 Nov 10;163(3):535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schulz B, Pruessmeyer J, Maretzky T, Ludwig A, Blobel CP, Saftig P, Reiss K. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ Res. 2008 May 23;102(10):1192–201. doi: 10.1161/CIRCRESAHA.107.169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M, Miyazaki A. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011 Dec 6;124(23):2522–32. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 89.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, Day CW, Barnard DL, Zimmerman GA, Krasnow MA, Li DY. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Science translational medicine. 2010 Mar 17;2(23):23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999 Jun;112(Pt 12):1915–23. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 91.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997 Mar;110(Pt 5):583–8. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 92.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012 Jun 13;31(12):2714–36. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim TH, Xiong H, Zhang Z, Ren B. beta-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005 Jan 20;24(4):597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
- 94.Levy L, Neuveut C, Renard CA, Charneau P, Branchereau S, Gauthier F, Van Nhieu JT, Cherqui D, Petit-Bertron AF, Mathieu D, Buendia MA. Transcriptional activation of interleukin-8 by beta-catenin-Tcf4. J Biol Chem. 2002 Nov 1;277(44):42386–93. doi: 10.1074/jbc.M207418200. [DOI] [PubMed] [Google Scholar]
- 95.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1625–33. doi: 10.1161/ATVBAHA.111.227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun P, Xiong H, Kim TH, Ren B, Zhang Z. Positive inter-regulation between beta-catenin/T cell factor-4 signaling and endothelin-1 signaling potentiates proliferation and survival of prostate cancer cells. Mol Pharmacol. 2006 Feb;69(2):520–31. doi: 10.1124/mol.105.019620. [DOI] [PubMed] [Google Scholar]
- 97.Vikram A, Kim YR, Kumar S, Naqvi A, Hoffman TA, Kumar A, Miller FJ, Jr, Kim CS, Irani K. Canonical Wnt signaling induces vascular endothelial dysfunction via p66Shc-regulated reactive oxygen species. Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2301–9. doi: 10.1161/ATVBAHA.114.304338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2015 Jan;26(1):107–20. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou L, Liu Y. Wnt/beta-catenin signaling and renin-angiotensin system in chronic kidney disease. Current opinion in nephrology and hypertension. 2016 Mar;25(2):100–6. doi: 10.1097/MNH.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012 May 11;110(10):1364–90. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem. 2005 Jun 3;280(22):21129–36. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 102.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009 Sep 18;284(38):25602–11. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004 Nov 15;104(10):3214–20. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]
- 104.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation. 2012 Apr 10;125(14):1795–808. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007 Jul;293(1):L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 106.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, Tournier-Lasserve E, Chapon F, Richichi C, Retta SF, Lampugnani MG, Dejana E. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013 Jun 27;498(7455):492–6. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 107.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002 Jun 14;90(11):1189–96. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 108.Tuuminen R, Syrjala S, Krebs R, Keranen MA, Koli K, Abo-Ramadan U, Neuvonen PJ, Tikkanen JM, Nykanen AI, Lemstrom KB. Donor simvastatin treatment abolishes rat cardiac allograft ischemia/reperfusion injury and chronic rejection through microvascular protection. Circulation. 2011 Sep 6;124(10):1138–50. doi: 10.1161/CIRCULATIONAHA.110.005249. [DOI] [PubMed] [Google Scholar]
- 109.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research. 2007 Nov 1;67(21):10123–8. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 110.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007 Aug;13(8):952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 111.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004 Aug 2;166(3):359–67. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011 Sep;179(3):1074–80. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garcia J, Sandi MJ, Cordelier P, Binetruy B, Pouyssegur J, Iovanna JL, Tournaire R. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO reports. 2012 May;13(5):431–9. doi: 10.1038/embor.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, Gopalan B, Stylianou E, Fiocchi C. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011 Nov;179(5):2660–73. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, Hirata K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010 Jun 8;121(22):2407–18. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 116.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011 Mar;300(3):F721–33. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008 Dec;19(12):2282–7. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell reports. 2012 Dec 27;2(6):1684–96. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGill HC, Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008 Mar 04;117(9):1216–27. doi: 10.1161/CIRCULATIONAHA.107.717033. [DOI] [PubMed] [Google Scholar]
- 120.Berenson GS, Srnivasan SR Bogalusa Heart Study G. Cardiovascular risk factors in youth with implications for aging: the Bogalusa Heart Study. Neurobiol Aging. 2005 Mar;26(3):303–7. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 121.McGill HC, Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000 Nov;72(5 Suppl):1307S–15S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 122.Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012 Jun;30(Suppl):S3–8. doi: 10.1097/HJH.0b013e328353e501. [DOI] [PubMed] [Google Scholar]
- 123.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010 Oct;65(10):1028–41. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002 Jun 14;90(11):1159–66. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 125.Belmin J, Corman B, Merval R, Tedgui A. Age-related changes in endothelial permeability and distribution volume of albumin in rat aorta. Am J Physiol. 1993 Mar;264(3 Pt 2):H679–85. doi: 10.1152/ajpheart.1993.264.3.H679. [DOI] [PubMed] [Google Scholar]
- 126.Ezekowitz MD, Parker KH, Salpidoru N, Oxenham RC. Effect of aging on permeability of cockerel aorta to 125I-albumin. Am J Physiol. 1980 Nov;239(5):H642–50. doi: 10.1152/ajpheart.1980.239.5.H642. [DOI] [PubMed] [Google Scholar]
- 127.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009 May;93(3):583–604. doi: 10.1016/j.mcna.2009.02.008. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000 Jun;20(6):1493–9. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 129.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004 Mar 12;17(1):21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 130.LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2280–8. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004 Jun;286(6):H2249–56. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014 Feb;25(2):72–9. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006 Mar;61(3):232–44. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- 134.Miller SJ, Watson WC, Kerr KA, Labarrere CA, Chen NX, Deeg MA, Unthank JL. Development of progressive aortic vasculopathy in a rat model of aging. Am J Physiol Heart Circ Physiol. 2007 Nov;293(5):H2634–43. doi: 10.1152/ajpheart.00397.2007. [DOI] [PubMed] [Google Scholar]
- 135.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003 Jun;17(9):1183–5. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 136.Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ, Seals DR. Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol. 2011 Sep;301(3):H1025–32. doi: 10.1152/ajpheart.01276.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol. 2005 Nov;167(5):1429–42. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Current opinion in nephrology and hypertension. 2010 Mar;19(2):201–7. doi: 10.1097/MNH.0b013e3283361c0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999 Jan;33(1):116–23. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 140.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1397–402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 141.Wang M, Khazan B, Lakatta EG. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010 Nov 1;6(4):266–81. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Safar ME. Hypertension, systolic blood pressure, and large arteries. Med Clin North Am. 2009 May;93(3):605–19. doi: 10.1016/j.mcna.2009.02.011. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 143.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003 Jun 10;107(22):2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 144.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012 Sep 5;308(9):875–81. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008 Nov;105(5):1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cox RH. Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev. 1983 Sep;23(1):21–36. doi: 10.1016/0047-6374(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 147.Simon G, Danneman KJ. Dilation and reduced distensibility of rat carotid artery with aging. Clin Exp Hypertens. 2005 Aug;27(6):459–66. doi: 10.1081/CEH-200067652. [DOI] [PubMed] [Google Scholar]
- 148.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007 Jul;50(1):219–27. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 149.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006 Jul;26(7):1503–9. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 150.Duca L, Blaise S, Romier B, Laffargue M, Gayral S, El Btaouri H, Kawecki C, Guillot A, Martiny L, Debelle L, Maurice P. Matrix ageing and vascular impacts: focus on elastin fragmentation. Cardiovasc Res. 2016 Mar 22; doi: 10.1093/cvr/cvw061. [DOI] [PubMed] [Google Scholar]
- 151.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003 Jun;41(6):1308–16. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 152.Flavahan S, Chang F, Flavahan NA. Local renin-angiotensin system mediates endothelial dilator dysfunction in aging arteries. Am J Physiol Heart Circ Physiol. 2016 Sep 1;311(3):H849–54. doi: 10.1152/ajpheart.00422.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R, Lakatta EG. A local proinflammatory signalling loop facilitates adverse age-associated arterial remodeling. PLoS One. 2011;6(2):e16653. doi: 10.1371/journal.pone.0016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang F, Flavahan S, Flavahan NA. Endothelial adherens junctions and endothelial dysfunction in aging arteries. FASEB J. 2016;30:1276.5. (abstract) [Google Scholar]
- 155.Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010 Oct 15;107(8):943–52. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- 156.Naito AT, Shiojima I, Komuro I. Wnt signaling and aging-related heart disorders. Circ Res. 2010 Nov 26;107(11):1295–303. doi: 10.1161/CIRCRESAHA.110.223776. [DOI] [PubMed] [Google Scholar]
- 157.DeCarolis NA, Wharton KA, Jr, Eisch AJ. Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. Bioessays. 2008 Feb;30(2):102–6. doi: 10.1002/bies.20709. [DOI] [PubMed] [Google Scholar]
- 158.Jin T, George Fantus I, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal. 2008 Oct;20(10):1697–704. doi: 10.1016/j.cellsig.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 159.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007 Aug 10;317(5839):803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 160.Gonzalez D, Rojas A, Herrera MB, Conlan RS. iNOS activation regulates beta-catenin association with its partners in endothelial cells. PLoS One. 2012;7(12):e52964. doi: 10.1371/journal.pone.0052964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008 Oct;105(4):1333–41. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hasegawa Y, Saito T, Ogihara T, Ishigaki Y, Yamada T, Imai J, Uno K, Gao J, Kaneko K, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Blockade of the nuclear factor-kappaB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation. 2012 Mar 6;125(9):1122–33. doi: 10.1161/CIRCULATIONAHA.111.054346. [DOI] [PubMed] [Google Scholar]
- 163.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 164.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011 Aug;66(8):866–75. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012 Aug;67(8):811–20. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Belmin J, Bernard C, Corman B, Merval R, Esposito B, Tedgui A. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995 Jun;268(6 Pt 2):H2288–93. doi: 10.1152/ajpheart.1995.268.6.H2288. [DOI] [PubMed] [Google Scholar]
- 167.Krug AW, Allenhofer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010 Jun;55(6):1476–83. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005 Apr;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 169.Thompson JA, Regnault TR. In utero origins of adult insulin resistance and vascular dysfunction. Semin Reprod Med. May;29(3):211–24. doi: 10.1055/s-0031-1275522. [DOI] [PubMed] [Google Scholar]
- 170.Morton JS, Cooke CL, Davidge ST. In Utero Origins of Hypertension: Mechanisms and Targets for Therapy. Physiol Rev. 2016 Apr;96(2):549–603. doi: 10.1152/physrev.00015.2015. [DOI] [PubMed] [Google Scholar]
- 171.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989 Mar 4;298(6673):564–7. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lurbe E, Torro MI, Carvajal E, Alvarez V, Redon J. Birth weight impacts on wave reflections in children and adolescents. Hypertension. 2003 Mar;41(3 Pt 2):646–50. doi: 10.1161/01.HYP.0000048341.52293.7C. [DOI] [PubMed] [Google Scholar]
- 173.Lurbe E, Torro I, Rodriguez C, Alvarez V, Redon J. Birth weight influences blood pressure values and variability in children and adolescents. Hypertension. 2001 Sep;38(3):389–93. doi: 10.1161/01.hyp.38.3.389. [DOI] [PubMed] [Google Scholar]
- 174.Lurbe E, Carvajal E, Torro I, Aguilar F, Alvarez J, Redon J. Influence of concurrent obesity and low birth weight on blood pressure phenotype in youth. Hypertension. 2009 Jun;53(6):912–7. doi: 10.1161/HYPERTENSIONAHA.109.129155. [DOI] [PubMed] [Google Scholar]
- 175.Lurbe E, Torro I, Alvarez V, Aguilar F, Redon J. The impact of birth weight on pulse pressure during adolescence. Blood Press Monit. 2004 Aug;9(4):187–92. doi: 10.1097/00126097-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 176.Bradley TJ, Potts JE, Lee SK, Potts MT, De Souza AM, Sandor GG. Early changes in the biophysical properties of the aorta in pre-adolescent children born small for gestational age. J Pediatr. 2010 Mar;156(3):388–92. doi: 10.1016/j.jpeds.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 177.Cheung YF, Wong KY, Lam BC, Tsoi NS. Relation of arterial stiffness with gestational age and birth weight. Arch Dis Child. 2004 Mar;89(3):217–21. doi: 10.1136/adc.2003.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan PY, Morris JM, Leslie GI, Kelly PJ, Gallery ED. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int J Pediatr. 2010;2010:280402. doi: 10.1155/2010/280402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Levent E, Atik T, Darcan S, Ulger Z, Goksen D, Ozyurek AR. The relation of arterial stiffness with intrauterine growth retardation. Pediatr Int. 2009 Dec;51(6):807–11. doi: 10.1111/j.1442-200X.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- 180.Martin H, Lindblad B, Norman M. Endothelial function in newborn infants is related to folate levels and birth weight. Pediatrics. 2007 Jun;119(6):1152–8. doi: 10.1542/peds.2006-2706. [DOI] [PubMed] [Google Scholar]
- 181.Lurbe E, Garcia-Vicent C, Torro I, Fayos JL, Aguilar F, de Llano JM, Fuertes G, Redon J. First-year blood pressure increase steepest in low birthweight newborns. J Hypertens. 2007 Jan;25(1):81–6. doi: 10.1097/HJH.0b013e32801040ec. [DOI] [PubMed] [Google Scholar]
- 182.Koudsi A, Oldroyd J, McElduff P, Banerjee M, Vyas A, Cruickshank JK. Maternal and neonatal influences on, and reproducibility of, neonatal aortic pulse wave velocity. Hypertension. 2007 Jan;49(1):225–31. doi: 10.1161/01.HYP.0000250434.73119.7a. [DOI] [PubMed] [Google Scholar]
- 183.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, Fisher S, Joszi A, Hirshman M, Oge A, Walrond S, Przybyla R, Boozer C, Goodyear LJ, Patti ME. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes. 2005 Mar;54(3):702–11. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 184.Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010 Apr;25(4):669–77. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- 185.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997 Sep 27;350(9082):953–5. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 186.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–88. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 187.Olivetti G, Anversa P, Melissari M, Loud AV. Morphometric study of early postnatal development of the thoracic aorta in the rat. Circ Res. 1980 Sep;47(3):417–24. doi: 10.1161/01.res.47.3.417. [DOI] [PubMed] [Google Scholar]
- 188.Le VP, Knutsen RH, Mecham RP, Wagenseil JE. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011 Jul;301(1):H221–9. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thompson JA, Gros R, Richardson BS, Piorkowska K, Regnault TR. Central stiffening in adulthood linked to aberrant aortic remodeling under suboptimal intrauterine conditions. Am J Physiol Regul Integr Comp Physiol. 2011 Dec;301(6):R1731–7. doi: 10.1152/ajpregu.00274.2011. [DOI] [PubMed] [Google Scholar]
- 190.Sherman RC, Langley-Evans SC. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin Sci (Lond) 1998 Apr;94(4):373–81. doi: 10.1042/cs0940373. [DOI] [PubMed] [Google Scholar]
- 191.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000 Mar;98(3):269–75. [PubMed] [Google Scholar]
- 192.Owens GK, Loeb A, Gordon D, Thompson MM. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986;102(2):343–52. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Eddinger TJ, Murphy RA. Developmental changes in actin and myosin heavy chain isoform expression in smooth muscle. Arch Biochem Biophys. 1991 Feb 1;284(2):232–7. doi: 10.1016/0003-9861(91)90290-y. [DOI] [PubMed] [Google Scholar]
- 194.Frid MG, Printesva OY, Chiavegato A, Faggin E, Scatena M, Koteliansky VE, Pauletto P, Glukhova MA, Sartore S. Myosin heavy-chain isoform composition and distribution in developing and adult human aortic smooth muscle. J Vasc Res. 1993 Sep-Oct;30(5):279–92. doi: 10.1159/000159007. [DOI] [PubMed] [Google Scholar]
- 195.Kocher O, Gabbiani G. Expression of actin mRNAs in rat aortic smooth muscle cells during development, experimental intimal thickening, and culture. Differentiation. 1986;32(3):245–51. doi: 10.1111/j.1432-0436.1986.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 196.Kuro-o M, Nagai R, Tsuchimochi H, Katoh H, Yazaki Y, Ohkubo A, Takaku F. Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem. 1989 Nov 5;264(31):18272–5. [PubMed] [Google Scholar]
- 197.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995 Jul;75(3):487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 198.Belknap JK, Grieshaber NA, Schwartz PE, Orton EC, Reidy MA, Majack RA. Tropoelastin gene expression in individual vascular smooth muscle cells. Relationship to DNA synthesis during vascular development and after arterial injury. Circ Res. 1996 Mar;78(3):388–94. doi: 10.1161/01.res.78.3.388. [DOI] [PubMed] [Google Scholar]
- 199.Wanjare M, Kuo F, Gerecht S. Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc Res. 2013 Feb 1;97(2):321–30. doi: 10.1093/cvr/cvs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rzucidlo EM, Martin KA, Powell RJ. Regulation of vascular smooth muscle cell differentiation. J Vasc Surg. 2007 Jun;45(Suppl A):A25–32. doi: 10.1016/j.jvs.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 201.Rayatpisheh S, Poon YF, Cao Y, Feng J, Chan V, Chan-Park MB. Aligned 3D human aortic smooth muscle tissue via layer by layer technique inside microchannels with novel combination of collagen and oxidized alginate hydrogel. J Biomed Mater Res A. 2012 Aug;98(2):235–44. doi: 10.1002/jbm.a.33085. [DOI] [PubMed] [Google Scholar]
- 202.Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999 Jan-Feb;36(1):2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- 203.Sugitani H, Wachi H, Tajima S, Seyama Y. Nitric oxide stimulates elastin expression in chick aortic smooth muscle cells. Biol Pharm Bull. 2001 May;24(5):461–4. doi: 10.1248/bpb.24.461. [DOI] [PubMed] [Google Scholar]
- 204.Arciniegas E, Neves CY, Carrillo LM, Zambrano EA, Ramirez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium: journal of endothelial cell research. 2005 Jul-Aug;12(4):193–200. doi: 10.1080/10623320500227283. [DOI] [PubMed] [Google Scholar]
- 205.Flavahan S, Chang F, Flavahan NA. Intrauterine stress disrupts the early postnatal maturation of arterial endothelium. FASEB J. 2016;30:1276.6. (abstract) [Google Scholar]
- 206.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004 May-Jun;13(3):125–38. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 207.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England journal of medicine. 2005 Apr 21;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 208.Tabas I. Russell Ross Memorial Lecture in Vascular Biology: Molecular-Cellular Mechanisms in the Progression of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017:37. doi: 10.1161/ATVBAHA.116.308036. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gimbrone MA, Jr, Garcia-Cardena G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res. 2016 Feb 19;118(4):620–36. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995 Jul;75(3):519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999 Dec 01;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 212.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011 Jan;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, Wang Y, Jin G, Usami S, Chien S. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005 Jan-Feb;42(1):77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 214.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):2003–8. doi: 10.1161/ATVBAHA.108.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007 Feb 26;176(5):719–27. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim DW, Langille BL, Wong MK, Gotlieb AI. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ Res. 1989 Jan;64(1):21–31. doi: 10.1161/01.res.64.1.21. [DOI] [PubMed] [Google Scholar]
- 217.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973 May-Jun;17(3):401–17. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 218.Haidari M, Zhang W, Chen Z, Ganjehei L, Warier N, Vanderslice P, Dixon R. Myosin light chain phosphorylation facilitates monocyte transendothelial migration by dissociating endothelial adherens junctions. Cardiovasc Res. 2011 Dec 01;92(3):456–65. doi: 10.1093/cvr/cvr240. [DOI] [PubMed] [Google Scholar]
- 219.Hashimoto K, Kataoka N, Nakamura E, Tsujioka K, Kajiya F. Oxidized LDL specifically promotes the initiation of monocyte invasion during transendothelial migration with upregulated PECAM-1 and downregulated VE-cadherin on endothelial junctions. Atherosclerosis. 2007 Oct;194(2):e9–17. doi: 10.1016/j.atherosclerosis.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 220.Starosta V, Wu T, Zimman A, Pham D, Tian X, Oskolkova O, Bochkov V, Berliner JA, Birukova AA, Birukov KG. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. Am J Respir Cell Mol Biol. 2012 Mar;46(3):331–41. doi: 10.1165/rcmb.2011-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gerhardt T, Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015 Aug 01;107(3):321–30. doi: 10.1093/cvr/cvv147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sun C, Wu MH, Lee ES, Yuan SY. A disintegrin and metalloproteinase 15 contributes to atherosclerosis by mediating endothelial barrier dysfunction via Src family kinase activity. Arterioscler Thromb Vasc Biol. 2012 Oct;32(10):2444–51. doi: 10.1161/ATVBAHA.112.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Donners MM, Wolfs IM, Olieslagers S, Mohammadi-Motahhari Z, Tchaikavski V, Heeneman S, van Buul JD, Caolo V, Molin DG, Post MJ, Waltenberger J. A disintegrin and metalloprotease 10 is a novel mediator of vascular endothelial growth factor-induced endothelial cell function in angiogenesis and is associated with atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(11):2188–95. doi: 10.1161/ATVBAHA.110.213124. [DOI] [PubMed] [Google Scholar]
- 224.Su W, Kowalczyk AP. The VE-cadherin cytoplasmic domain undergoes proteolytic processing during endocytosis. Mol Biol Cell. 2016 Oct 26; doi: 10.1091/mbc.E16-09-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009 Jul;29(7):1074–9. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Moonen JR, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJ, Zeebregts CJ, Krenning G, Harmsen MC. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res. 2015 Dec 01;108(3):377–86. doi: 10.1093/cvr/cvv175. [DOI] [PubMed] [Google Scholar]
- 227.Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun. 2016 Jun 24;7:11853. doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015 Oct 26;125(12):4514–28. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim M, Choi SH, Jin YB, Lee HJ, Ji YH, Kim J, Lee YS, Lee YJ. The effect of oxidized low-density lipoprotein (ox-LDL) on radiation-induced endothelial-to-mesenchymal transition. Int J Radiat Biol. 2013 May;89(5):356–63. doi: 10.3109/09553002.2013.763193. [DOI] [PubMed] [Google Scholar]
- 230.Darwish I, Liles WC. Emerging therapeutic stratgies to prevent infection-related microvascular endothelial activation and dysfunction. Virulence. 2013;4(6):572–82. doi: 10.4161/viru.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Heupel WM, Efthymiadis A, Schlegel N, Muller T, Baumer Y, Baumgartner W, Drenckhahn D, Waschke J. Endothelial barrier stabilization by a cyclic tandem peptide targeting VE-cadherin transinteraction in vitro and in vivo. J Cell Sci. 2009 May 15;122(Pt 10):1616–25. doi: 10.1242/jcs.040212. [DOI] [PubMed] [Google Scholar]
- 232.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011 Oct 19;30(20):4157–70. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee WL, Slutsky AS. Sepsis and endothelial permeability. The New England journal of medicine. 2010 Aug 12;363(7):689–91. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 234.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res. 2010 Nov 1;88(2):344–51. doi: 10.1093/cvr/cvq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.David S, Ghosh CC, Kumpers P, Shushakova N, Van Slyke P, Khankin EV, Karumanchi SA, Dumont D, Parikh SM. Effects of a synthetic PEG-ylated Tie-2 agonist peptide on endotoxemic lung injury and mortality. Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L851–62. doi: 10.1152/ajplung.00459.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000 Sep 29;87(7):603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 237.Wang Y, Pampou S, Fujikawa K, Varticovski L. Opposing effect of angiopoietin-1 on VEGF-mediated disruption of endothelial cell-cell interactions requires activation of PKC beta. J Cell Physiol. 2004 Jan;198(1):53–61. doi: 10.1002/jcp.10386. [DOI] [PubMed] [Google Scholar]
- 238.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, Zhao YY, Malik AB. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res. 2009 Sep 25;105(7):696–704. doi: 10.1161/CIRCRESAHA.109.199778. 8 p following. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, Redell JB, Grill R, Matsuo Y, Guha S, Cox CS, Reitz MS, Holcomb JB, Dash PK. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem cells and development. 2011 Jan;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Glading AJ, Ginsberg MH. Rap1 and its effector KRIT1/CCM1 regulate beta-catenin signaling. Dis Model Mech. 2010 Jan-Feb;3(1–2):73–83. doi: 10.1242/dmm.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003 May;139(2):329–36. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Moss A. The angiopoietin:Tie 2 interaction: A potential target for future therapies in human vascular disease. Cytokine & growth factor reviews. 2013 Jul 6; doi: 10.1016/j.cytogfr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 243.Alfieri A, Watson JJ, Kammerer RA, Tasab M, Progias P, Reeves K, Brown NJ, Brookes ZL. Angiopoietin-1 variant reduces LPS-induced microvascular dysfunction in a murine model of sepsis. Critical care. 2012 Oct 4;16(5):R182. doi: 10.1186/cc11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H33–42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem. 2001 Apr 6;276(14):10627–33. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 246.Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Seminars in immunopathology. 2012 Jan;34(1):73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008 Mar 28;102(6):669–76. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlback B, Kono M, Proia RL, Smith JD, Hla T. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015 Aug 11;8(389):ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ruiz M, Frej C, Holmer A, Guo LJ, Tran S, Dahlback B. High-Density Lipoprotein-Associated Apolipoprotein M Limits Endothelial Inflammation by Delivering Sphingosine-1-Phosphate to the Sphingosine-1-Phosphate Receptor 1. Arterioscler Thromb Vasc Biol. 2017 Jan;37(1):118–29. doi: 10.1161/ATVBAHA.116.308435. [DOI] [PubMed] [Google Scholar]
- 250.Sattler K, Graler M, Keul P, Weske S, Reimann CM, Jindrova H, Kleinbongard P, Sabbadini R, Brocker-Preuss M, Erbel R, Heusch G, Levkau B. Defects of High-Density Lipoproteins in Coronary Artery Disease Caused by Low Sphingosine-1-Phosphate Content: Correction by Sphingosine-1-Phosphate-Loading. J Am Coll Cardiol. 2015 Sep 29;66(13):1470–85. doi: 10.1016/j.jacc.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 251.Zhang G, Yang L, Kim GS, Ryan K, Lu S, O’Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013 Jul 18;122(3):443–55. doi: 10.1182/blood-2012-11-467191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006 Feb;12(2):235–9. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 253.Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, Sanchez T, Wang E, Kontos CD, Lin CY, Hla T, Haribabu B, Lee MJ. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008 Oct 31;283(44):30363–75. doi: 10.1074/jbc.M804392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Feng Y, Pfister F, Schreiter K, Wang Y, Stock O, Vom Hagen F, Wolburg H, Hoffmann S, Deutsch U, Hammes HP. Angiopoietin-2 deficiency decelerates age-dependent vascular changes in the mouse retina. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2008;21(1–3):129–36. doi: 10.1159/000113755. [DOI] [PubMed] [Google Scholar]
- 255.Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85(5):1927–38. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- 256.Shimokawa H, Flavahan NA, Vanhoutte PM. Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries. Possible dysfunction of a pertussis toxin-sensitive G protein. Circ Res. 1989;65(3):740–53. doi: 10.1161/01.res.65.3.740. [DOI] [PubMed] [Google Scholar]
- 257.Shimokawa H, Flavahan NA, Vanhoutte PM. Loss of endothelial pertussis toxin-sensitive G protein function in atherosclerotic porcine coronary arteries. Circulation. 1991;83(2):652–60. doi: 10.1161/01.cir.83.2.652. [DOI] [PubMed] [Google Scholar]
- 258.Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010 May;459(6):807–16. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2011 Jun;300(6):L840–50. doi: 10.1152/ajplung.00404.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhou Q, Liao JK. Rho kinase: an important mediator of atherosclerosis and vascular disease. Current pharmaceutical design. 2009;15(27):3108–15. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhou Q, Liao JK. Pleiotropic effects of statins. - Basic research and clinical perspectives. Circulation journal: official journal of the Japanese Circulation Society. 2010 May;74(5):818–26. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Haidari M, Zhang W, Chen Z, Ganjehei L, Mortazavi A, Warier N, Vanderslice P, Dixon RA. Atorvastatin preserves the integrity of endothelial adherens junctions by inhibiting vascular endothelial cadherin tyrosine phosphorylation. Exp Cell Res. 2012 Aug 15;318(14):1673–84. doi: 10.1016/j.yexcr.2012.05.009. [DOI] [PubMed] [Google Scholar]