Abstract
Introduction
Burns lead to persistent and detrimental muscle breakdown and weakness. Standard treatment at our institution includes a voluntary 12-week rehabilitative exercise program to limit and reverse the effects of increased muscle catabolism. In the present work, we investigated if different durations of exercise, 6 or 12 weeks, produce comparable improvements in muscle strength, body composition, and cardiopulmonary fitness.
Methods
We prospectively enrolled and randomized patients with ≥30% total body surface area (TBSA) burned to receive 6- or 12-weeks of exercise rehabilitation. Patients were evaluated for muscle strength, oxygen consumption capacity, and lean body mass at discharge (n = 42) and post exercise. After 6 weeks (n = 18) or 12 weeks (n = 24) of exercise training, leg muscle strength was assessed as peak torque per body weight using a Biodex Isokinetic Dynamometer. Oxygen consumption capacity, measured as peak VO2, was studied using a standard treadmill-based test, and lean body mass was determined using dual-energy X-ray absorptiometry.
Results
Significant improvements in muscle strength, peak VO2, and lean body mass were seen after 6 weeks of exercise training (p<0.001), with only significant improvements in peak VO2 being seen after 6 weeks more of training.
Conclusion
These data suggest that a 6-week rehabilitative exercise program is sufficient for improving muscle strength, body composition, and cardiopulmonary fitness in pediatric burn patients. However, continuation of at- or near-home cardiopulmonary training following the 6 weeks of at-hospital rehabilitation may be useful.
Keywords: resistance exercise, burns, children, rehabilitation
INTRODUCTION
Burns exceeding 30% of the total body surface area (TBSA) result in a profound hypermetabolic response that includes many organ systems and persists for an extended time [1– 4]. The hypermetabolic response augments proteolysis to cause loss of lean body mass, which is exacerbated by prolonged physical inactivity [5]. The resulting morbidity inhibits patients‘ return to normal societal activities and reduces quality of life [6]. One strategy that has proven effective in combating decreased lean body mass, reduced muscle strength, and reduced cardiopulmonary fitness is physical exercise [7–9]. At our institution, current standard of care comprises a voluntary rehabilitation program entailing occupational and physical therapy with an added component of respiratory therapy. This program is usually implemented for 12 weeks following discharge from the hospital, and involves the use of standard gym equipment and various strength and cardiopulmonary exercises [9]. Patients were discharged from the hospital when 95% of wounds were healed. However, the 12-week exercise program typically requires patients to remain in an unfamiliar environment, away from family and normal daily activities. The effects of the injury and subsequent rehabilitation cause a significant socioeconomic burden on the families. While the facilities at Shriners Hospitals for Children®—Galveston are uniquely adapted to accommodate all patients and families that are affected by severe burns, the location relative to the majority of our patients‘ homes is typically quite far and unfortunately burdensome. In an effort to decrease the duration of time away from family and home, we explored options to reduce the length rehabilitative exercise program without compromising patient care or safety. To this end, we designed a study to determine if a 6-week exercise program would provide similar benefits as the current 12-week exercise program.
METHODS
Subjects
Children 6 to 18 years of age were prospectively enrolled in this study between 2003 and 2014. Written informed consent was obtained, and all procedures were approved by the Institutional Review Board at the University of Texas Medical Branch. Patients were included if the TBSA burned was equal to or greater than 30% and if they were admitted to Shriners Hospitals for Children - Galveston for acute burn care. Burn size was measured using modified Lund and Browder charts [10]. All patients received identical standard-of-care treatment. Exclusion criteria included leg amputation, anoxic brain injury, psychological disorders, quadriplegia, or severe behavior and/or cognitive disorders. Additionally, subjects were only included in this exercise study if they were previously randomized to the ‘control’ drug group of a core randomization scheme, indicating that they would not receive any type of research study drug. Furthermore, as patient volume at our institution decreased or a higher number of randomized protocols increased, there was a smaller population of subjects to approach for the current study. This accounts for an extended study period for our 42 subjects.
Muscle strength, reflected by peak torque, peak oxygen consumption (Peak VO2), and lean body mass, was measured at hospital discharge and at 6 weeks or 12 weeks after starting the rehabilitative exercise program.
Patient Groups
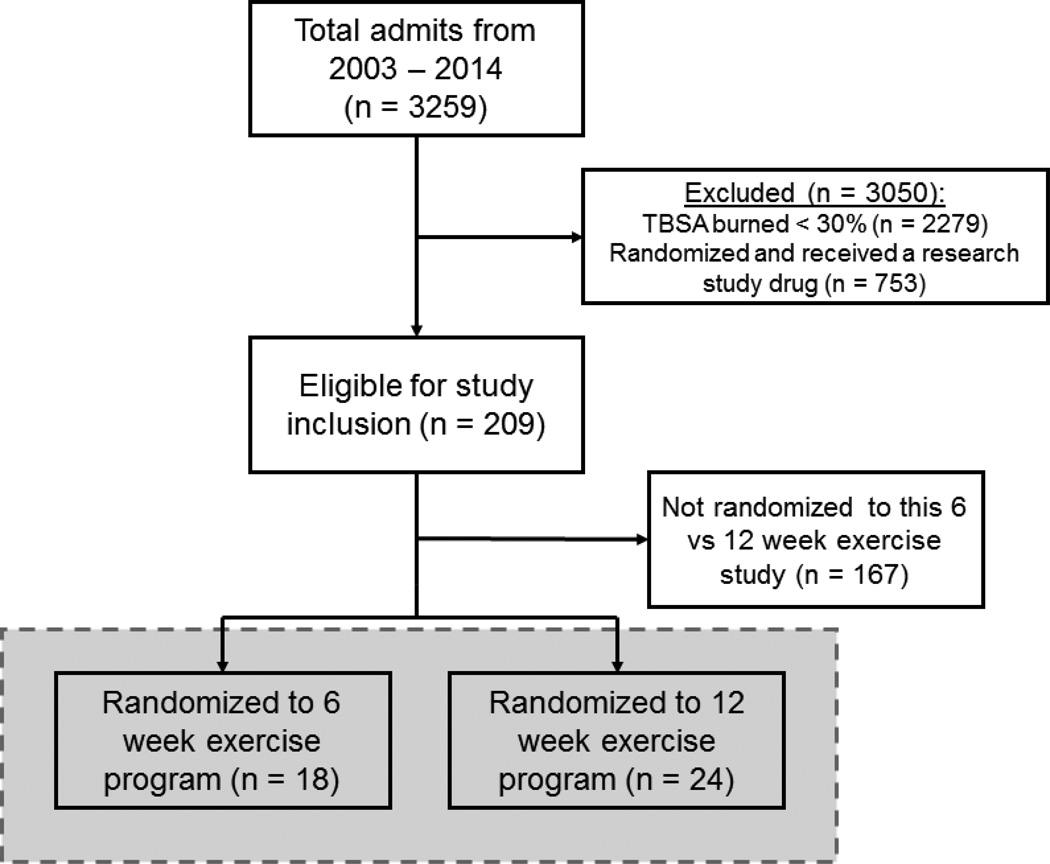
Patients consenting/assenting to the rehabilitative exercise program were randomly placed into a 6- or 12-week exercise-training group (Figure 1) using a randomization scheme that was generated prior to the start of the study. The scheme was generated using the randomization function of Microsoft Excel (Redmond, WA). Values were obtained at discharge, after 6 weeks of exercise, and after 12 weeks of exercise (if in the 12-week exercise program). Of the 42 patients consented, 18 participated in 6 weeks of training, while 24 received 12 weeks of training. Patients enrolled in the 12-week rehabilitative exercise program also had measurements collected at the 6-week time point. Analysis of demographics and measured variables were minimally different between the 6-week exercise group and 12-week exercise group at the 6-week time point and were subsequently grouped together for final analysis (Figures 2–5).
Figure 1. CONSORT diagram showing the randomization process. Gray shaded area shows finalized patient groups.
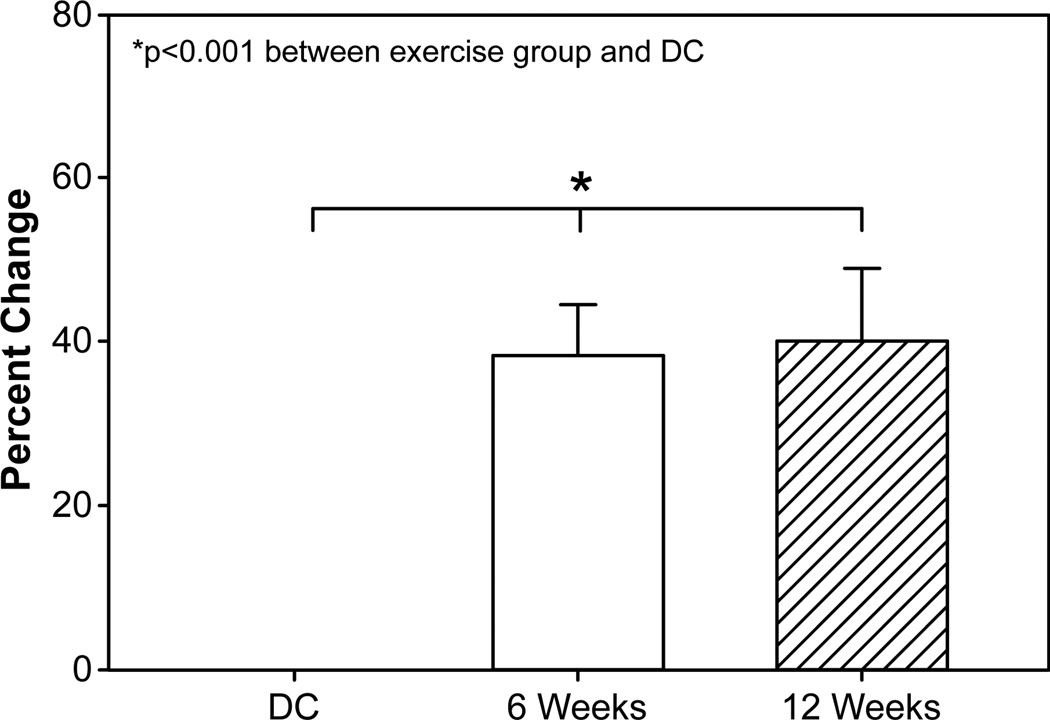
Figure 2. Muscle strength changes following rehabilitative exercise.
Change in relative peak torque from discharge (DC = 42) to 6 weeks (n = 42) and 12 weeks (n = 24). 6- and 12-week subject groups were combined for final analysis. Abbreviations: DC, discharge. 6 wk, 6-week rehabilitative exercise. 12 wk, 12-week rehabilitative exercise.
Figure 5. Lean body mass measured after rehabilitative exercise.
Change in lean body mass from discharge (DC = 42) to 6 weeks (n = 42) and 12 weeks (n = 24). 6- and 12-week subject groups were combined for final analysis. Abbreviations: DC, discharge. 6 wk, 6-week rehabilitative exercise. 12 wk, 12-week rehabilitative exercise. LBM, lean body mass.
Discharge values of patients were obtained upon discharge from the hospital, typically occurring when a patient‘s wounds are 95% healed. The discharge values from the 6- and 12- week rehabilitative exercise programs were grouped together during the final analysis (Figures 2–5). While there was a single variable we found to be significantly different between the two discharge groups (Table 1, % TBSA burned), our statistical model accounted for this difference in the final analysis. All other demographic and experimental outcomes were not statistically different between the 6- and 12-week exercise groups. Demographics data are presented in Table 1.
Table 1.
Patient characteristics.
| 6-week exercise program |
12-week exercise program |
p value | |
|---|---|---|---|
| n = 18 | n = 24 | ||
| Age (years) | 11.3 ± 3.8 | 12.3 ± 3.6 | 0.43 |
| Burn Size (% TBSA) | 46.4 ± 14.5 | 56.1 ± 13.2 | 0.01 |
| % 3rd Degree Burn | 30.0 ± 23.6 | 42.8 ± 21.5 | 0.07 |
| Height (cm) | 140.9 ± 20.4 | 149.3 ± 19.2 | 0.19 |
| Length of Stay (days) | 23.8 ± 20.4 | 33.4 ± 20.4 | 0.22 |
| Weight (kg) | 37.3 ± 12.7 | 43.7 ± 15.6 | 0.17 |
Data are presented as mean ± SD. Table shows the patient characteristics between the two groups (6-week program vs. 12-week program) when discharged from the hospital. TBSA = total body surface. Non-burned, control patient values measured at our institution (reference only, mean ± SD): n = 47; Age, 12.4 ± 2.5 years; Height, 1.53 ± 0.2 m; Weight, 58.7 ± 21.9 kg.
Standard of Care
All patients received identical treatment from admission until discharge (95% healed) and the end of the rehabilitative exercise program [11]. Briefly, fluid resuscitation was performed using the Galveston formula (5000 mL/m2 TBSA burned + 2000 mL/m2 TBSA lactated Ringer‘s solution given during the first 24 hours). Burn wounds were excised and covered with autograft/allograft within 48 hours of admission. Excision and grafting were performed every 6 to 7 days until all wound sites were 95% healed. Patients began ambulation 4 days after each surgery and continued this daily until the following surgical procedure [12].
Patients were fed Vivonex® TENenteral nutrition (Nestlé, Vevey, Switzerland) (6% fat, 15% protein, 82% carbohydrate) through a nasoduodenal or nasogastric tube. Early intake was determined with the formula 1500 kcal/m2 TBSA + 1500 kcal/m2 TBSA burned [13, 14]. After one week of hospitalization, this was changed to 1.4 times the resting energy expenditure, which was determined weekly.
In addition to receiving the aforementioned acute care, all patients received conventional physiotherapy sessions with either an occupational (OT) or physical therapist (PT) twice daily for 1 hour. A typical treatment program included positioning and splinting, range of motion and strengthening activities for specific areas such as hand, feet, and scar management activities. Although patients spent time with PT/OT, this was standardized across all patients and only involved minimal strengthening exercises that would be negligible in regards to the rehabilitative exercise program. Patients were discharged from hospital when 95% of their wounds were considered closed, but may continue to spend time with PT/OT for improvements in mobility and/or range of motion.
Rehabilitative Exercise Program
Rehabilitative exercise was performed as previously described [7, 9, 15] and began after discharge. Training sessions were conducted under the supervision of an American College of Sports Medicine (ACSM)-certified exercise physiologist and included both resistance and aerobic exercises. Exercise training took place in the gymnasium located at our institution. Exercise testing took place in our Exercise Physiology Laboratory. Eight resistance-training exercises were used: bench press, leg press, shoulder press, biceps curl, leg curl, triceps curl, toe raises, and abdominal curls. A combination of free weights or resistance machinery was used for each exercise, but exercises could be altered to accommodate a wide range of equipment. Modifications were allowed on an individual basis, if necessary, due to injury-related limitations. Exercise occurred 5 days each week (Monday through Friday). Patients exercised aerobically daily, when possible, and resistance exercise 3 days each week. Resistance exercise typically occurred on Monday, Wednesday, and Friday with Tuesday and Thursday reserved for rest and recovery. During the first week of exercise rehabilitation, patients were instructed on proper weight lifting techniques and allowed to become familiar with the equipment. Initial weight loads were set at 50 to 60% of each individual‘s 3-repetition maximum (3RM). Weight loads were then increased to allow 3 sets of 70–75% 3RM, with a goal of 8 to 12 repetitions, for weeks 2 to 6 of training. Weeks 6 to 12 set a goal of 8–12 repetitions, but a higher load if needed to account for potential improvement.
In addition to the weight training exercises, rehabilitation sessions included aerobic exercise on a cycle ergometer or treadmill. This was carried out 3 to 5 days a week and typically lasted from 20 to 40 minutes at 70 to 85% of peak VO2. Each session was preceded by a 5-minute warm-up with less than 50% peak VO2 intensity. All exercise sessions were supervised by an exercise specialist. No strength training-related activities were allowed outside of the supervised training sessions; however, normal daily routines and activities were encouraged.
Strength Measurements (Peak Torque)
Muscle strength was measured using the Biodex System – 3 Dynamometer (Shirley, NY, USA). The isokinetic test was performed using the dominant leg extensors at an angular velocity of 150°/s. Patients were instructed to sit upright in the machine, and the anatomic axis of the knee was aligned with the mechanical axis of the dynamometer. Straps were then placed over the mid-thigh, pelvis, and trunk to stabilize the dominant leg. Initially, patients became familiar with the machine through the assistance of the exercise specialist. Following familiarization, patients were given 3 practice attempts without any resistance. Next, 10 maximal voluntary muscle contractions with complete flexion and extension were performed in succession without rest. Peak torque was calculated using Biodex software. The highest peak torque observed in the 10 repetitions was used for analysis. Peak torque was corrected for gravitational movements of the lower leg and lever arm. Relative peak torque is presented to correct for variations in patient body weight.
Peak Oxygen Consumption (peak VO2)
Standardized cardiopulmonary testing was performed after the muscular strength assessment and after allowing patients to rest for 30 minutes to 1 hour. A modified Bruce protocol was used for treadmill exercise testing as outlined previously [16, 17]. Heart rate and peak VO2 were determined throughout testing, as described elsewhere [9, 16]. We used the Medgraphics CardiO2 system (St. Paul, MN, USA) to perform continuous breath-by-breath analyses, which relied on inspired and expired gases, flow, and volume. Initial treadmill speed was set at 1.7 miles/hour, and incline was set at 0%. Patients were strongly encouraged to finish 3-minute stages. Once patients reached their peak volitional effort, the testing was stopped. Both heart rate and peak VO2 were used to determine the intensity target for rehabilitation exercises. Further analysis of peak VO2 was performed to adjust for lean body mass. Analysis of peak VO2 as a function of lean body mass has been shown to give a better representation of actual cardiopulmonary fitness [18, 19].
Lean Body Mass
At the designated time point, lean body mass measurements were performed by dual-energy X-ray absorptiometry (DEXA) using QDR45000A software (Hologic Inc., Waltham, MA, USA). The method of measuring lean body mass in pediatric burned patient has previously been described by our group [18, 19]. Scans were taken according to manufacturer‘s recommendations [20] with the patient lying supine on the scanning table. Analytical software was then used to calculate the patient‘s lean body mass.
Statistical Analysis
Patient demographics (Table 1) and measurable outcomes for the 6-week and 12-week exercise groups (Table 2, Table 3, respectively) were analyzed using GraphPad Prism 7 (La Jolla, CA). Statistically significant differences were determined with the appropriate parametric or nonparametric test.
Table 2.
6–week exercise program outcomes.
| DC | 6 weeks | p value | |
|---|---|---|---|
| n = 18 | n = 18 | ||
| Strength (N*m*kg−1, %) | 102.2 ± 41.0 | 122.6 ± 27.7 | < 0.01 |
| Peak VO2 (ml*kg−1*min−1) | 25.1 ± 5.8 | 28.5 ± 6.0 | < 0.001 |
| Peak VO2-LBM (ml*kg−1*min−1) | 35.0 ± 5.0 | 40.9 ± 7.8 | < 0.001 |
| LBM (kg*m2−1) | 12.86 ± 1.6 | 13.5 ± 1.7 | 0.01 |
Data are presented as mean ± SD. Table shows exercise values measured in the 6-week exercise group at discharge from hospital and after 6 weeks of exercise. Non-burned values as measured at our institution (reference only, mean ± SD): n = 47; Strength, 155.0 ± 39.8 Nm/kg; Peak VO2, 37.0 ± 8.3 ml/kg/min; LBM, 16.1 ± 3.3 kg/m.
Abbreviations: DC, discharge; VO2, oxygen consumption; LBM, lean body mass.
Table 3.
12-week exercise program outcomes.
| DC | 6 weeks | 12 weeks | |
|---|---|---|---|
| n = 24 | n = 24 | n = 24 | |
| Strength (N*m*kg−1, %) | 84.4 ± 32.2 | 132.3 ± 41.1** | 129.5 ± 39.7** |
| Peak VO2 (ml*kg−1*min−1) | 24.9 ± 4.7 | 27.7 ± 7.0**‡ | 31.0 ± 6.4***‡ |
| Peak VO2-LBM (ml*kg−1*min−1) | 34.4 ± 6.5 | 37.9 ± 9.2‡ | 42.1 ± 7.7***‡ |
| LBM (kg*m2−1) | 13.8 ± 1.5 | 14.7 ± 2.1* | 14.8 ± 2.2* |
Data are presented as mean ± SD. Table shows exercise values measured in the 12-week exercise group at discharge from hospital and after 6 and 12 weeks of exercise.
p < 0.05 vs. DC,
p < 0.01 vs. DC,
p < 0.001 vs. DC,
p < 0.01 between 6 and 12 weeks of exercise.
Non-burned values as measured at our institution (reference only, mean ± SD): n = 47; Strength, 155.0 ± 39.8 Nm/kg; Peak VO2, 37.0 ± 8.3 ml/kg/min; LBM, 16.1 ± 3.3 kg/m.
Abbreviations: DC, discharge; VO2, oxygen consumption; LBM, lean body mass.
Analysis of benefit for subjects receiving 6 or 12 weeks of exercise was determined using R statistical software (R Core Team 2015, version 3.2.1, Vienna, Austria). Mixed linear models related each outcome (Peak Torque, peak VO2, lean body mass) to the time point (discharge, 6 weeks, and 12 weeks) and the prognostic covariates age and TBSA burn, clustering on subject to compensate for repeated measures. Differences among time points were assessed by Tukey-adjusted contrasts. Likelihood-ratio tests were used to rule-out the inclusion of interactions between time point and TBSA or age [21], as inclusion of these interactions showed no evidence of improving the model. Normality of model residuals was verified by normal quantile plots. Values for the changes are shown in tables 2 and 3, while figures show the percent change among the time points. Values are given as mean ± SD.
RESULTS
Patient Characteristics
Patient demographics at time of discharge are presented in Table 1. Age was 11.3 ± 3.8 for the 6-week group, and 12.3 ± 3.6 years for the 12-week group. Average burn size was significantly greater in the 12-week group when compared to the 6-week group. (56.1 ± 13.2 % and 46.4 ± 14.5, respectively). Height, weight, length of stay, and % 3rd degree TBSA burn was not significantly different between the groups. Statistical analyses did not show evidence of interaction between time point and burn size or age for any of the outcomes measured.
Peak Torque
Measured strength (N · m · kg −1, %) had a significant relationship with age (p = 0.004). Increases in patient age were associated with an increase in overall strength, while increases in TBSA burned were associated with decreases in overall strength. With age and TBSA burned accounted for, muscle strength was significantly increased (p < 0.001) after 6 weeks and 12 weeks of rehabilitation exercise (Figure 2). Measured strength increased from 102.2 Nm/kg to 122.6 Nm/kg in the 6-week group and from 84.4 Nm/kg to 132.3 Nm/kg in the 12-week group (Table 2 and Table 3, respectively). The 12-week group showed no improvement in muscle strength between 6 and 12 weeks of exercise. Overall, muscle strength increases were 38.3% greater after 6 weeks of exercise training, with only a 2% additional increase being seen after participation in 6 weeks more of rehabilitation exercise (Figure 2). Despite significant increases when compared to discharge, no significant difference was detected between 6 and 12 weeks of rehabilitation training.
Cardiopulmonary Function
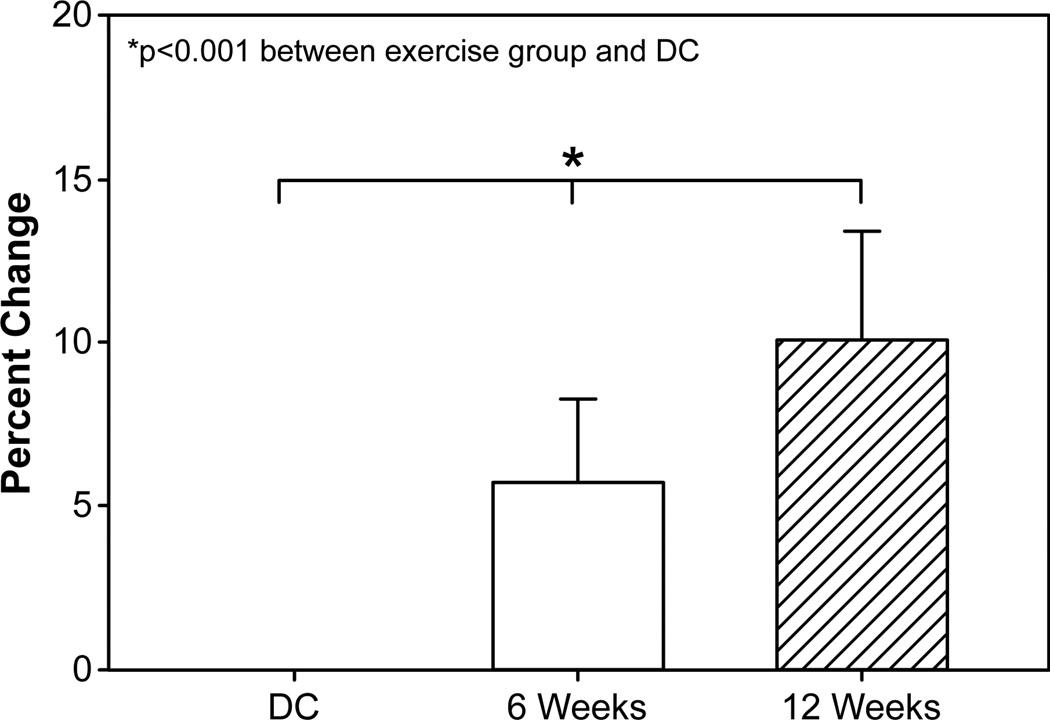
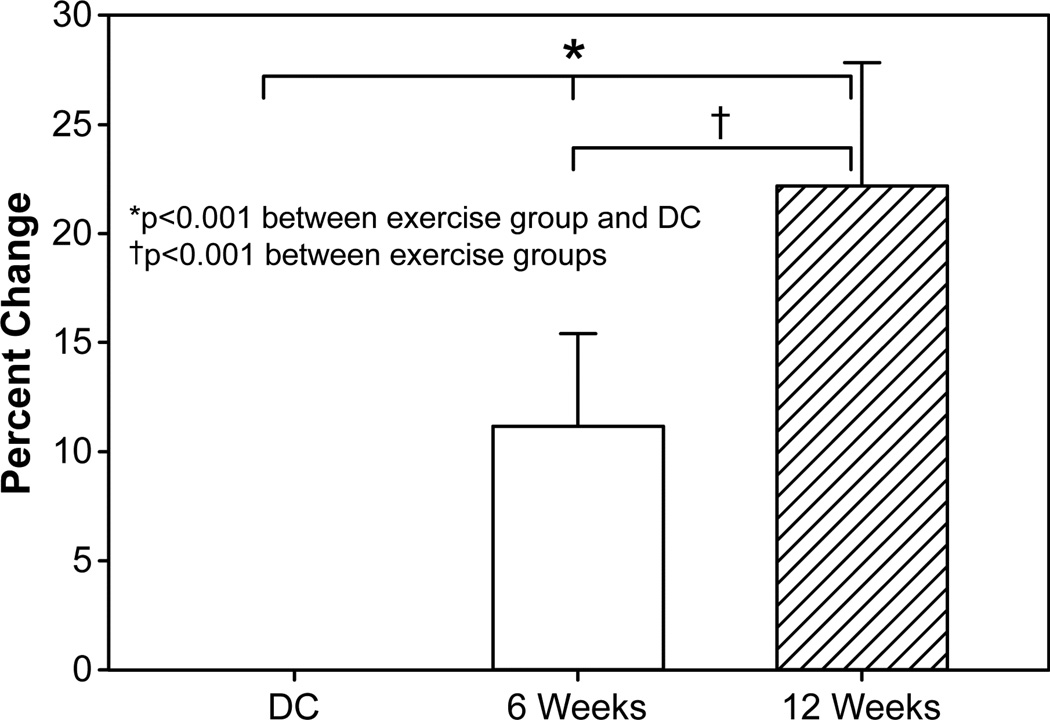
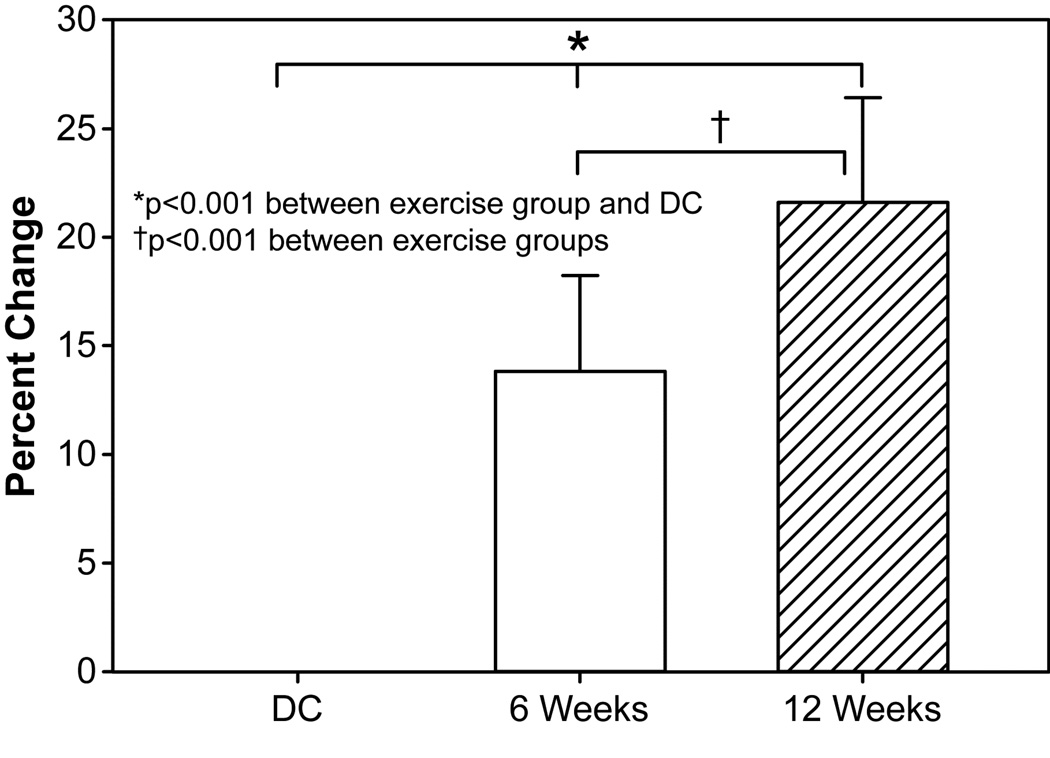
Peak VO2 (ml · kg−1 · min−1) showed no significant relationship to age. Peak VO2 values at discharge were 25.1 ± 5.8 ml/kg/min and 24.9 ± 4.7 ml/kg/min for the 6- and 12-week exercise groups, respectively (Table 2, Table 3). Significant improvements in Peak VO2 were seen after 6 weeks of exercise in both groups (6wk, 28.5 ± 6.0 ml/kg/min; 12wk, 27.7 ± 7.0 ml/kg/min). There was additional benefit noted in patients of the 12-week group receiving an additional 6 weeks of exercise training (Table 3). Percent changes of Peak VO2 from discharge to 6 and 12 weeks were 11.2% and 22.2%, respectively (Figure 3). Cardiopulmonary function, as it relates to lean body mass (as opposed to overall body weight), also showed significant changes in both groups after 6 and 12 weeks of exercise when compared to discharge values (Peak VO2 – LBM; Table 2, Table 3) and differed between the 6- and 12-week time points (Figure 4). An analysis of peak VO2 in relation to lean body mass, as opposed to body weight, revealed that the percent differences at 6 and 12 weeks were 13.7% and 21.5%, respectively, reducing the 11% gap between exercise groups to 7.8%.
Figure 3. Peak Oxygen consumption following rehabilitative exercise.
Change in peak oxygen consumption, measured with body weight, from discharge (DC = 42) to 6 weeks (n = 42) and 12 weeks (n = 24). 6- and 12-week subject groups were combined for final analysis. Abbreviations: DC, discharge. 6 wk, 6-week rehabilitative exercise. 12 wk, 12-week rehabilitative exercise. Peak VO2, peak oxygen consumption.
Figure 4. Cardiopulmonary fitness and lean body mass.
Change in peak oxygen consumption, measured with lean body mass, from discharge (DC = 42) to 6 weeks (n = 42) and 12 weeks (n = 24). 6- and 12-week subject groups were combined for final analysis. Abbreviations: DC, discharge; LBM, lean body mass. 6 wk, 6-week rehabilitative exercise. 12 wk, 12-week rehabilitative exercise. Peak VO2, peak oxygen consumption.
Lean Body Mass
Lean body mass (kg · m2 −1) was measured at discharge and after 6 or 12 weeks of rehabilitation exercise. A significant relationship between lean body mass and age was observed (p < 0.001). Increased age was associated with increase in lean body mass. There were also significant increases in lean body mass between discharge and the 6-week time points for both 6-week (12.86 ± 1.6, 13.5 ± 1.7, respectively) and 12-week groups. (13.8 ±1.5, 14.7 ±2.1, respectively) (Table 2, Table 3). No improvements were seen with an additional 6 weeks of exercise in the 12-week exercise group. Lean body mass showed improvements of 5 and 10% at the 6- and 12-week time points, respectively (Figure 5). As observed with muscle strength, no significant difference was detected between 6 and 12 weeks of exercise rehabilitation.
DISCUSSION
Overall, our results reflect minimal differences between 6 and 12 weeks of exercise training. Each measured variable showed significant improvements at 6 weeks (and 12 weeks) when compared to discharge values, confirming the favorable effects of exercise training in burned children. These results suggest that, in general, a rehabilitation program of 6 weeks is sufficient to increase muscle strength and cardiopulmonary fitness of pediatric burn survivors. A shorter rehabilitation time would allow families to return home more quickly and children to return to their normal environment more quickly as well. As an added benefit, a shorter duration exercise program could lead to greater implementation among burn centers that currently do not advocate post-burn rehabilitation [22].
This study confirms previous findings by our group that an in-hospital rehabilitative exercise program of 12 weeks greatly benefits burn patients in terms of muscle strength and cardiopulmonary fitness [7–9, 15, 23–25]. However, previous training protocols included a 3- to 6-month home physical therapy/occupational therapy rehabilitation period before the return to Shriners Hospitals for Children®—Galveston for a 12-week exercise program [9, 24, 25]. In this study, we show that an exercise rehabilitation program beginning immediately following discharge from the hospital significantly improves patient outcomes. A lack of delay likely reduces the time needed for patients and families to return to normal daily activities and routines, though this needs to be studied in a systemic, prospective study. Furthermore, home environments often have poor rehabilitative support that likely hinders patient improvement during the 6-month hiatus from burn care specialists. Releasing patients home for an extended period of time also increases the chance of patients losing contact with our burn center and not returning for proper rehabilitation training. Altogether, our results suggest that implementation of an early rehabilitation exercise training that immediately follows discharge and lasts for 6 weeks provides beneficial outcomes for burned children, outcomes similar to those observed with a 12-week exercise program.
Cardiopulmonary fitness was measured as peak VO2 and significantly differed between 6 and 12 weeks of rehabilitation. These results indicate that, while muscle strength and lean body mass did not significantly differ, peak VO2 can still be significantly improved with an additional 6 weeks of exercise. The difference in peak VO2 between 6 and 12 weeks of training is less when factoring in lean body mass rather than body weight (Figure 3 vs Figure 4). The percent difference decreases when accounting for lean body mass, which has been shown to be more accurate for evaluating actual peak VO2 since lean body mass uses more oxygen than body fat [19]. While we did see changes at the final measurement for the 6-week exercise program and the 12-week exercise program, we did not see any significant change between the groups at the 6-week time point. This lack of change, we believe, indicates that there was no overall difference between the groups‘ effort or training intensity but indicates that the cardiopulmonary function takes longer to recover from the sequelae that occur after severe burn injury. Overall, these results suggest that continued cardiopulmonary training could benefit patients beyond 6 weeks of exercise training and after cessation of strength training. However, despite the added cardiopulmonary benefit of an additional 6 weeks of training, other factors should be considered. These factors include the emotional stress of being away from home and family, increased economic stress with additional costs to the patient and legal guardian, and more time spent away from school and/or work. These circumstances are highly variable based on the patient and family support. The optimal scenario is yet to be determined, but a combination of hospital-based exercise training and at-home/community training may be the best approach.
Continuing cardiopulmonary training in a home rehabilitation program in the patient‘s common environment beyond 6 weeks is a future direction of this research [26]. Preliminary studies into this “community-based” exercise program (COMBEX) have briefly been explored by our group [26]. In this preliminary study, patients took part in either an in-hospital 12-week rehabilitative exercise program (current standard-of-care) or an at-home/native environment-prescribed exercise protocol, which included a local gym membership and personal trainer. Similar outcomes were measured as in this current study. The outcomes of the COMBEX study showed improvements that were statistically similar to those seen in patients randomized to the in-hospital exercise program [26]. Adaptations to the currently recommended treatment will likely include a combination of in-hospital and at-home training protocols. Fortunately, most acute care institutes with access to physical therapy and/or occupational therapy will be able to accommodate the exercises that we used in our rehabilitative exercise program. All of the exercises use basic and traditional exercise equipment, with the exception of the Biodex system that was only used to standardize our training and testing. Biodex measurements and VO2 measurements could be substituted with a 3RM measurement and a 6 minute walk test, respectively [9, 27]. Using basic equipment for our study should allow for generalization and translation to other institutions.
We are aware of several concerns with the current study. In the current study, randomization occurred over an extended period of time. Prior to the study, our standard therapy was to encourage participation in a voluntary 12-week program. Because of this strategy, most of our patients take part in an extended-length (12-week) exercise program, which we have shown to be beneficial for patients [9]. Due to the uncertainty that 6-weeks of exercise would benefit patients as much as 12-weeks of exercise, we made a conscious decision to limit our patient enrollment each year. We enrolled 20% of eligible patients into our study over the 11-year study period. Patient demographics and measured outcomes were not significantly different between eligible and enrolled patients at hospital discharge. Furthermore, as our study dictates, both groups differ in terms of training length. While the volume load is similar during the first 6 weeks for each group, overall there is less training (total days) in patients randomized to the 6-week exercise program. A longer training length translates to greater total number of repetitions, sets, and total work load. However, these differences did not translate into a difference in peak torque or lean body mass. In contrast, peak VO2 was greater in the children that were part of the 12-week exercise program. An increase in peak VO2 of about 3.3 ml/kg/min was observed from 6 to 12 weeks of exercise. We believe this deficit may be filled and overcome following patients‘ return home by implementation of COMBEX. An additional concern related to this study is that individual training intensity or effort could differ between groups. Our study was designed to regulate training intensity based on previously determined maximum effort analyses (i.e., VO2 max, 3RM, 8–12 RM). Training intensity is adjusted to maintain a certain percentage of the maximum effort and increases the similarity in training regimen between patients. Based on the results obtained at discharge and the 6-week time point, we believe that differences we see are solely based on the rehabilitative exercise program rather than any discrepancies of intensity between the groups. Due to the fact that patients were enrolled over an 11-year period, advances in burn care could have biased outcomes including exercise capacity and other studied endpoints. Nevertheless, we do not believe that those improvements affected our findings because the protocol for the rehabilitative exercise program has not changed over this period of time and we compared results from discharge up to 6- and 12-weeks.
In summary, our results show that children with ≥30% TBSA burns also benefit considerably from rehabilitative exercise training regimens that last at least 6 weeks, relative to the typically recommended 12-week training period. Additionally, although 6 weeks of training is successful in improving strength, oxygen consumption capacity, and lean body mass, extended specific cardiorespiratory training may be required. Thus, we strongly recommend an exercise training program of at least 6 weeks in pediatric burn patients.
HIGHLIGHTS.
6 weeks of exercise provides beneficial outcomes after severe burns
6 weeks of exercise is comparable to 12 weeks for strength and lean body mass
12 weeks of exercise is best for cardiopulmonary fitness recovery after burns
At least 6-weeks of exercise is recommended for children with severe burn injury
Acknowledgments
The authors would like to thank Dr. Kasie Cole for editing and proofreading the manuscript. This study was supported by the National Institutes of Health (P50 GM060338, UL1TR001439, T32 GM008256, R01 GM056687, and R01 HD049471), NIDILRR (90DP00430100), and Shriners Hospitals for Children (84080, 71006, 71008, and 71009).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION
RPC, PW, RPM, DNH, and OES are responsible for the conception and design of the study; RPC and CRA analyzed the data; RPC, PW, RPM, DNH, and OES interpreted the data; RPC, PW, CRA, DNH, and OES were involved in drafting the manuscript and preparing the figures; RPC, PW, CRA, RPM, DNH, and OES revised the manuscript. All authors approve the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest
REFERENCES
- 1.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 2.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Annals of surgery. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft R, Herndon DN, Finnerty CC, Shahrokhi S, Jeschke MG. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Annals of surgery. 2014;259:381–387. doi: 10.1097/SLA.0b013e31828c4d04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol (1985) 1997;82:807–810. doi: 10.1152/jappl.1997.82.3.807. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg M, Celis MM, Meyer W, 3rd, Tropez-Arceneaux L, McEntire SJ, Fuchs H, et al. Effects of a hospital based Wellness and Exercise program on quality of life of children with severe burns. Burns : journal of the International Society for Burn Injuries. 2013;39:599–609. doi: 10.1016/j.burns.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardee JP, Porter C, Sidossis LS, Borsheim E, Carson JA, Herndon DN, et al. Early rehabilitative exercise training in the recovery from pediatric burn. Medicine and science in sports and exercise. 2014;46:1710–1716. doi: 10.1249/MSS.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter C, Hardee JP, Herndon DN, Suman OE. The role of exercise in the rehabilitation of patients with severe burns. Exercise and sport sciences reviews. 2015;43:34–40. doi: 10.1249/JES.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol (1985) 2001;91:1168–1175. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 10.Hettiaratchy S, Papini R. Initial management of a major burn: II--assessment and resuscitation. BMJ. 2004;329:101–103. doi: 10.1136/bmj.329.7457.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Annals of surgery. 2012;256:402–411. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller MJ, Herndon DN. The challenge of burns. Lancet. 1994;343:216–220. doi: 10.1016/s0140-6736(94)90995-4. [DOI] [PubMed] [Google Scholar]
- 13.Hildreth MA, Herndon DN, Desai MH, Duke MA. Caloric needs of adolescent patients with burns. The Journal of burn care & rehabilitation. 1989;10:523–526. doi: 10.1097/00004630-198911000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TT, Gilpin DA, Meyer NA, Herndon DN. Current treatment of severely burned patients. Annals of surgery. 1996;223:14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porro LJ, Al-Mousawi AM, Williams F, Herndon DN, Mlcak RP, Suman OE. Effects of propranolol and exercise training in children with severe burns. The Journal of pediatrics. 2013;162:799–803. e1. doi: 10.1016/j.jpeds.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones NL. Clinical Exercise Testing. Philadelphia, PA: W.B. Saunders; 1997. [Google Scholar]
- 17.McInnis KJ, Balady GJ. Comparison of submaximal exercise responses using the Bruce vs modified Bruce protocols. Medicine and science in sports and exercise. 1994;26:103–107. [PubMed] [Google Scholar]
- 18.Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- 19.Krachler B, Savonen K, Komulainen P, Hassinen M, Lakka TA, Rauramaa R. Cardiopulmonary fitness is a function of lean mass, not total body weight: The DR's EXTRA study. European journal of preventive cardiology. 2015;22:1171–1179. doi: 10.1177/2047487314557962. [DOI] [PubMed] [Google Scholar]
- 20.Hologic. QDR 4500 Fan Beam X-Ray Bone Densitometer: Users Guide. Waltman, MA: Hologic; 1995. [Google Scholar]
- 21.Neyman J, Pearson ES. On the problem of the most efficient tests of statistical hypotheses. Philos Trans R Soc Lond Ser Contain Pap Math Phys Character. 1933;231:289–337. [Google Scholar]
- 22.Diego AM, Serghiou M, Padmanabha A, Porro LJ, Herndon DN, Suman OE. Exercise training after burn injury: a survey of practice. Journal of burn care & research : official publication of the American Burn Association. 2013;34:e311–e317. doi: 10.1097/BCR.0b013e3182839ae9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2007;119:e109–e116. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Archives of physical medicine and rehabilitation. 2007;88:S24–S29. doi: 10.1016/j.apmr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Suman OE, Thomas SJ, Wilkins JP, Mlcak RP, Herndon DN. Effect of exogenous growth hormone and exercise on lean mass and muscle function in children with burns. J Appl Physiol (1985) 2003;94:2273–2281. doi: 10.1152/japplphysiol.00849.2002. [DOI] [PubMed] [Google Scholar]
- 26.Pena R, Ramirez LL, Crandall CG, Wolf SE, Herndon DN, Suman OE. Effects of community-based exercise in children with severe burns: A randomized trial. Burns : journal of the International Society for Burn Injuries. 2016;42:41–47. doi: 10.1016/j.burns.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]