Abstract
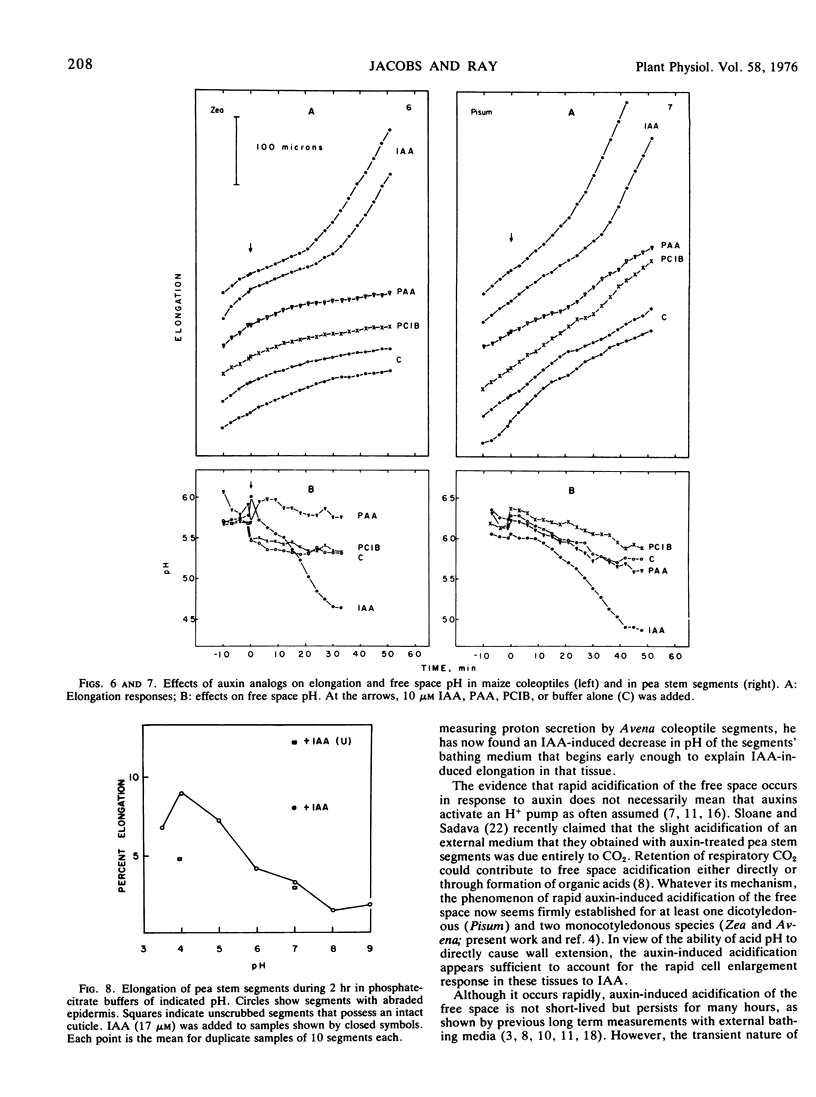
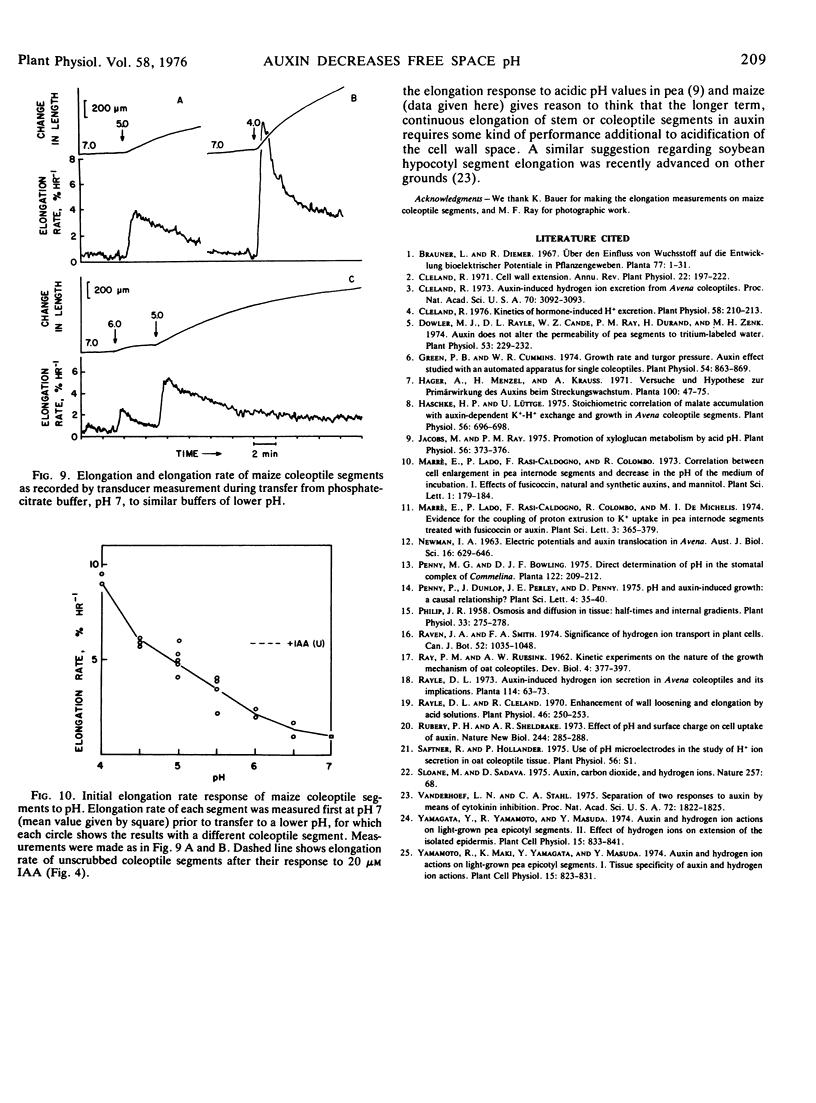
A pH microelectrode has been used to investigate the auxin effect on free space pH and its correlation with auxin-stimulated elongation in segments of pea (Pisum sativum) stem and maize (Zea mays var. Bear Hybrid) coleoptile tissue. Auxin induces a decrease in free space pH in both tissues. In maize coleoptiles, free space pH begins to fall within about 12 minutes of exposure to auxin and decreases by about 1 pH unit by approximately 30 minutes. In pea, pH begins to decrease within an average of 15 to 18 minutes of exposure to auxin and falls by about 0.9 pH unit by approximately 40 minutes. Auxin-stimulated elongation, measured in the same two tissues similarly prepared, appears in maize at the earliest 18 minutes after auxin application, while in pea it appears at the earliest 21 to 24 minutes after auxin application. The auxin analogs p-chlorophenoxyisobutyric acid and phenylacetic acid do not stimulate elongation above control levels in maize or pea tissue segments and do not cause a decrease in free space pH in either tissue. These findings are consistent with the acid secretion theory of auxin action.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland R. E. Kinetics of Hormone-induced H Excretion. Plant Physiol. 1976 Aug;58(2):210–213. doi: 10.1104/pp.58.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Auxin-induced hydrogen ion excretion from Avena coleoptiles. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3092–3093. doi: 10.1073/pnas.70.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler M. J., Rayle D. L. Auxin Does Not Alter the Permeability of Pea Segments to Tritium-labeled Water. Plant Physiol. 1974 Feb;53(2):229–232. doi: 10.1104/pp.53.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg C. Dark CO(2) Fixation in Gladiolus Cormels and Its Regulation during the Break of Dormancy. Plant Physiol. 1975 Jul;56(1):51–55. doi: 10.1104/pp.56.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. B., Cummins W. R. Growth rate and turgor pressure: auxin effect studies with an automated apparatus for single coleoptiles. Plant Physiol. 1974 Dec;54(6):863–869. doi: 10.1104/pp.54.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Ray P. M. Promotion of Xyloglucan Metabolism by Acid pH. Plant Physiol. 1975 Sep;56(3):373–376. doi: 10.1104/pp.56.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery P. H., Sheldrake A. R. Effect of pH and surface charge on cell uptake of auxin. Nat New Biol. 1973 Aug 29;244(139):285–288. doi: 10.1038/newbio244285a0. [DOI] [PubMed] [Google Scholar]
- Vanderhoef L. N., Stahl C. A. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci U S A. 1975 May;72(5):1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]


