Abstract
Purpose
To develop J-difference editing with Parallel Reconstruction in Accelerated Multivoxel (PRIAM) for simultaneous measurement of γ-aminobutyric acid (GABA) and glutathione in two separate brain regions.
Methods
PRIAM separates signals from two simultaneously excited voxels using receiver-coil sensitivity profiles. PRIAM was implemented into Mescher-Garwood- (MEGA)-edited experiments at 3T, and validated by acquiring dual-voxel MEGA-PRIAM (and compared to conventional single-voxel MEGA-PRESS) spectra from a GABA/glutathione phantom, and 11 healthy participants.
Results
MEGA-PRIAM effectively separated phantom spectra with ~3–4% between-voxel contamination. GABA and glutathione measurements agreed well with those obtained using single-voxel MEGA-PRESS (mean difference was below 2% in GABA levels, and below 7% in glutathione levels). In vivo, GABA- and glutathione-edited spectra were successfully reconstructed with a mean in vivo g-factor of 1.025 (typical voxel-center separation: 7–8 cm). MEGA-PRIAM experiments showed higher signal-to-noise than sequential single-voxel experiments of the same total duration (mean improvement 1.38±0.24).
Conclusions
Simultaneous acquisition of J-difference-edited GABA or glutathione spectra from two voxels is feasible at 3T. MEGA-PRIAM increases data acquisition rates compared to MEGA-PRESS by a factor of 2.
Keywords: brain, edited MRS, dual volume, GABA, GSH, multi-voxel
Introduction
Spectral editing is widely used to detect and quantify proton magnetic resonance spectroscopy (1H-MRS) signals from low-concentration brain metabolites with coupled resonances. Detection of such metabolites with conventional MRS at 3T is impeded by overlying signals from other metabolites, such as creatine (Cr), N-acetyl aspartate (NAA), and choline (Cho). J-difference editing involves selective manipulation of coupled spin systems using frequency-selective RF pulses designed to retain the compound of interest, while at the same time eliminating unwanted resonances. The MEGA-PRESS (1) J-difference sequence has been widely used to measure levels of γ-aminobutyric acid (GABA) (2), N-acetyl aspartyl glutamate (NAAG) (3), glutathione (4,5), lactate (6), and 2-hydroxyglutarate (7).
In recent years, MEGA-PRESS has become an important tool to study brain function, development and pathology (8). A major drawback, however, is its limitation to one target metabolite and a single volume of interest per measurement. Typically, edited measurements of low-concentration metabolites have low signal-to-noise ratios (SNR), requiring acquisition times on the order of 10 minutes for volumes on the order of 30 ml (8). Sequential edited measurements of multiple metabolites and/or different brain regions therefore rapidly exceed reasonable research protocol lengths, restricting study designs. This is particularly the case with patient groups that show reduced tolerance for long scanning sessions, and when additional modalities, such as functional or diffusion-based imaging, need to be included in the protocol.
Direct acceleration of MRS experiments is only achievable by reducing the number of averages (or repetition time (TR)), sacrificing SNR. Time efficiency of edited MRS experiments can also be increased by simultaneous acquisition of multiple spatial locations. Spectral editing has been incorporated into MR spectroscopic imaging (MRSI) (9), but suffers from sensitivity to head motion and long scan duration (although substantial progress in this area has been made (10)).
It has recently been shown that dual-band excitation allows the acquisition of MR spectra from two spectroscopic voxels at the same time, which can subsequently be separated using the spatial sensitivity profiles of the multi-channel phased array receiver coil (11). This Parallel Reconstruction In Accelerated Multi-voxel (PRIAM) approach is based on sensitivity encoding (SENSE), a well-established technique that has provided significant acceleration of data acquisition in MRI and MRSI (12–15). In this article, the PRIAM methodology is extended to MEGA-edited MRS, demonstrating MEGA-PRIAM spectroscopy at 3T. This combination enables simultaneous acquisition of MEGA-PRESS data from two separate locations. Using phantom and in vivo measurements, it is shown that MEGA-PRIAM is feasible for the editing of GABA and glutathione (GSH), and that consistency with typical single-excitation experiments is maintained.
Methods
Background
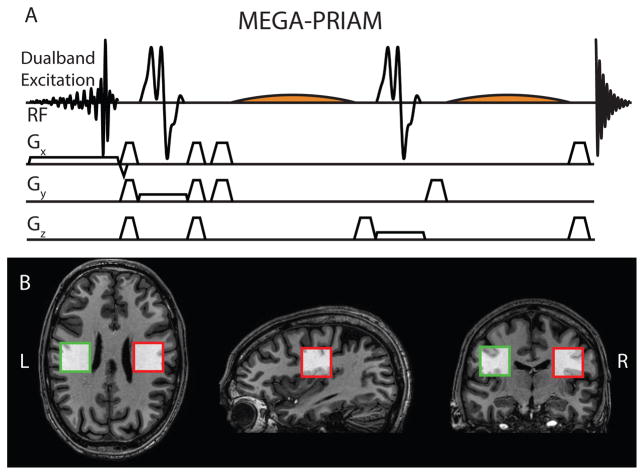
The conventional single-volume MEGA-PRESS sequence was modified by the incorporation of a dual-band excitation pulse, as recently described for conventional PRESS by Boer et al. (11), and as shown in Figure 1A. The dual-band pulse is calculated from a point-by-point summation of the two single minimum-phase excitation pulses (16,17), that would be required for separate excitation of the two voxels. A receiver-coil sensitivity map is determined from a gradient-echo imaging sequence by normalizing the signal from each receiving channel to the body coil signal. Averaging these maps over N voxels (here N = 2) for each of the R receiving channels (here R = 32) yields an N × R complex sensitivity matrix S. The (i,j)-th element of the R × R receiver noise matrix Ψ is calculated according to , where ηi denotes the noise in the i-th receiver channel, which is determined from the first 30 complex data points at the edge of the reference image. Ψ therefore describes the noise levels and their mutual correlation for each receiver channel. With Ψ, an unfolding matrix U can be calculated following the SENSE reconstruction formalism (12):
| Eq. (1) |
where H denotes the transpose complex conjugate operation. Multiplying this matrix with the 1 × R vector containing the time-domain signals from the R receiver channels, the according sensitivity and phase weighting provides distinct optimal coil combinations for the N excited voxels, allowing separate reconstruction of their MR spectroscopic signals with optimal SNR.
Figure 1.
MEGA-PRIAM acquisition. A) Pulse sequence including dual-band excitation and editing pulses for J-difference editing. B) In vivo voxel locations.
SNR in the reconstructed signals can be compromised as a consequence of mutual noise amplification, i.e. when the receiver profiles are very similar for both voxels. The degree of similarity is expressed by the geometry factor
| Eq. (2) |
by which SNR is reduced in the dual-voxel acquisition.
Mapping of the g-factor allows for the estimation of SNR reduction that is associated with the particular geometry of voxel positioning and coil location.
Experimental design
All experimental data were collected on a Philips ‘Achieva’ MRI scanner (Philips Healthcare LLC, Best, Netherlands) at 3T field strength. The body coil was used for transmit, and a 32-channel phased array volume head coil (Invivo, Gainesville, FL) for receive.
After a survey scan, a low flip-angle gradient-echo sequence was performed to establish the complex spatial receiver coil sensitivity profile for each coil. Both voxels were reconstructed within the sensitivity profile maps with an in-house MATLAB (The Mathworks Inc., Natick, MA) routine. For each coil, the complex sensitivity was averaged across each voxel to construct the unfolding matrix.
Six MRS scans were subsequently performed (parameters in common: TR = 2 s, 2048 data points, 2 kHz spectral width, 16-step phase cycling, standard Philips refocusing pulses (bandwidth 1.3 kHz) for single-voxel MRS (16)). Three experiments were set to edit GABA (TE = 68 ms, 30×30×30 mm3 voxel size, 12-ms editing pulses, ON pulse centered at 1.90 ppm, OFF pulse centered at 7.46 ppm), and a further three to edit GSH (TE = 120 ms, 33×33×33 mm3 voxel size, 20-ms editing pulses, ON pulse centered at 4.56 ppm, OFF pulse centered at 8.00 ppm). The first acquisition of each set was performed with dual-band excitation of both voxels (320 averages for GABA; 400 averages for GSH), followed by two acquisitions each exciting a single voxel separately. These single-voxel measurements were performed with half the number of averages (i.e. 160 for GABA; 200 for GSH) of the dual-voxel measurement to maintain the same total acquisition time for both voxels, and demonstrate the temporal SNR gains available. Second-order shimming was performed on a cuboidal volume enclosing both voxels.
Eight water-unsuppressed reference averages were dispersed evenly throughout each acquisition to enable prospective frequency correction and water-scaled metabolite concentration estimation (18).
Signal ‘cross-talk’ between the two voxels (due to imperfect excitation profiles and/or reconstruction) was calculated from the unsuppressed water reference scans of the single-voxel excitation experiments – the unwanted signal (e.g. from Voxel 2 when only Voxel 1 was excited) was quantified as a percentage of the water signal reconstructed from the desired location.
Phantom measurements
In order to demonstrate the MEGA-PRIAM technique, a two-compartment agarose phantom was constructed. One half of the phantom contained 16 mM of GABA and 10 mM of GSH, the second half contained 8 mM of GABA and 14 mM of GSH. For the MRS experiments, one voxel was localized in each compartment of the phantom. 8 water-suppressed averages and 1 water-unsuppressed reference scan were collected for each scan. The concentration ratio between the two excited voxels was calculated for each metabolite, assuming that relaxation behavior is the same in both locations.
In vivo measurements
MEGA-PRIAM data were acquired from 11 healthy participants (7M/4F, mean age 32.6±8.8 y) after obtaining their written informed consent prior to examination, as approved by the local institutional review board.
Two voxels were excited in the left and right hemispheres of the posterior frontal lobe, placed symmetrically about the midline, as shown in Figure 1B. The voxel centers were separated by 76.4±2.2 mm (GABA) and 75.5±2.1 mm (GSH). Water suppression was achieved using VAPOR (19). Automatic first- and second-order projection-based shim optimization was performed on a cuboidal shim volume including both voxels. This shim volume was not altered for the subsequent single-voxel acquisitions.
Gannet 2.0 (20) was used to process each in vivo dataset, perform post-hoc frequency and phase correction, average the spectra, and fit the GABA and GSH resonances. GABA spectra were aligned using Spectral Registration (21). For alignment of the GSH spectra, the NAA peak was instead used as frequency and phase reference, as the water resonance is saturated in the GSH-ON experiment by the editing pulses applied at 4.56 ppm. Post-processing water suppression using singular value decomposition (22) was also performed on GSH-edited spectra to address this differential saturation. The GABA signal at 3 ppm was modeled with a single Gaussian. The GSH-edited spectrum between 2.2 and 3.5 ppm was fit using a model with five Gaussian peaks and a baseline term. Four of the Gaussians modeled the adjacent co-edited NAA and NAAG signals between 2.3 and 2.8 ppm, and the remaining Gaussian modeled the GSH signal at 2.95 ppm. Water-scaled metabolite concentration estimates were provided in ‘institutional units’ (i.u.). Tissue correction was not applied. SNR was calculated by dividing the edited GABA peak height (determined by Gannet fitting) by 2 × (standard deviation of the noise in the frequency range between −3.19 ppm and 0 ppm).
Results
Phantom
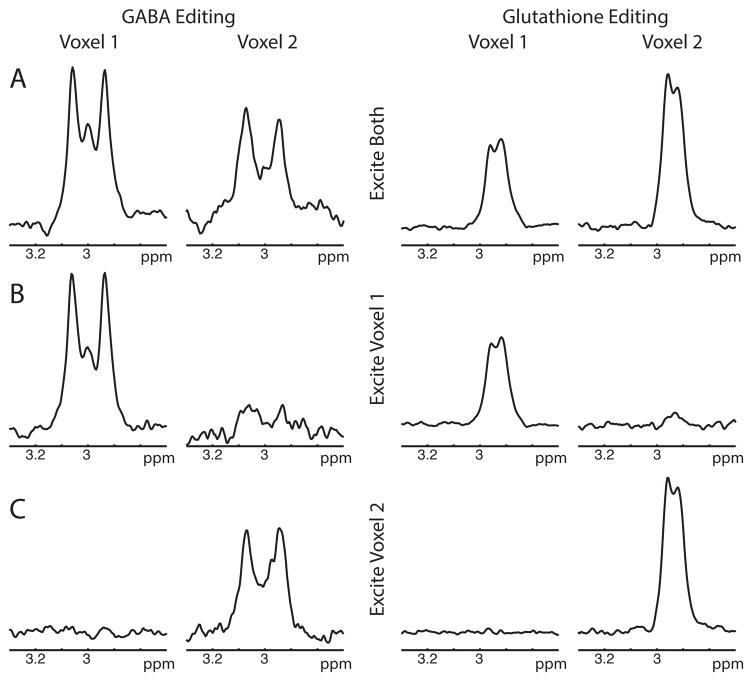
GABA- and GSH-edited spectra from the dual-voxel excitation (shown in Figure 2A) show good agreement of the reconstructed spectra with the single-voxel acquisitions (shown in Figure 2B–C). Average water signal crosstalk was 4.0% in the GABA experiments and 3.6% in the GSH experiments.
Figure 2.
GABA/Glutathione phantom results. A) PRIAM reconstruction of GABA (left) and GSH (right) editing with dual-band (two-voxel) excitation. B) PRIAM reconstruction of GABA (left) and GSH (right) editing with single-voxel (Voxel 1) excitation i.e. PRIAM reconstruction of conventional MEGA-PRESS acquisition. C) PRIAM reconstruction of GABA (left) and GSH (right) editing with single-voxel (Voxel 2) excitation.
The GABA concentration ratio between the two voxel compartments was measured as 2.04 for dual-voxel excitation and 2.03 for single-voxel excitations, in good agreement with the actual concentration ratio of 2.00 (16 mM: 8 mM). The GSH concentration ratio was 0.7576 for dual-voxel excitation and 0.85 for single-voxel excitation, slightly higher than the expected 0.71 ratio (10 mM: 14 mM).
Mean water linewidths were 11.0 Hz for Voxel 1 and 9.9 Hz for Voxel 2, indicating that residual inhomogeneities after shimming were not equal in the two voxels of interest.
In vivo
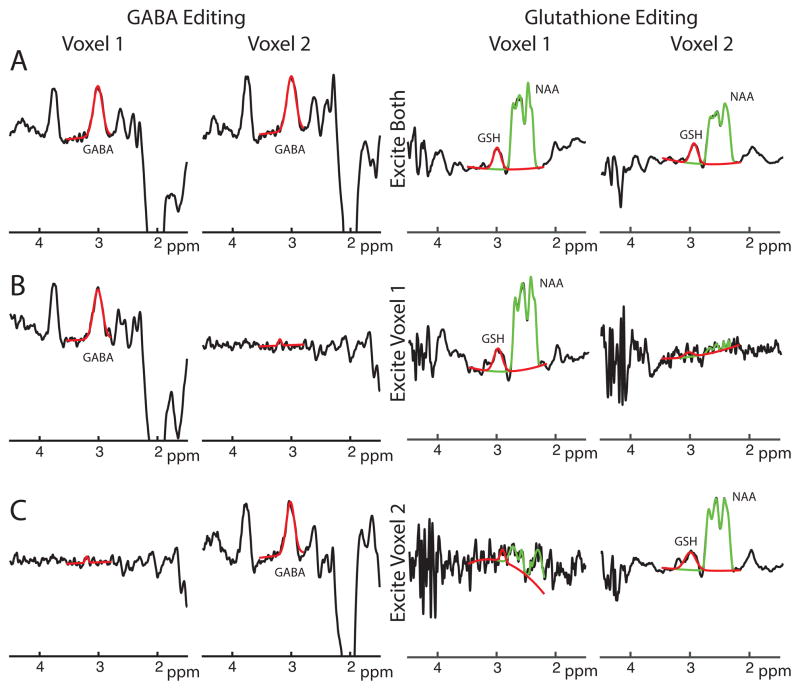
PRIAM successfully reconstructed MEGA-edited spectra from two simultaneously excited voxels in vivo, whether editing for GABA or GSH, as shown in Figure 3A. MEGA-PRIAM spectra show good agreement with sequentially acquired single-voxel spectra (Figure 3B–C). For these particular spectra shown in Figure 3, the water-scaled concentration estimates between the dual-voxel-excited spectra and the respective single-voxel-excited spectra differ by 0.2% (GABA, V1), 8.9% (GABA, V2), −0.5% (GSH, V1) and 4.4% (GSH, V2). As expected, the dual-voxel experiment (which acquired data from both locations for the full 10-minute duration) showed significantly increased SNR compared to the single-voxel experiments (which only acquired data from each voxel for half the total duration). For the spectra shown, the SNR improvement was 1.36 (SNR of 15.0 for dual-voxel and 11.1 for single-voxel) and 1.41 (SNR of 17.0 for dual-voxel and 12.1 for single-voxel) in Voxel 1 and Voxel 2, respectively, close to the theoretical prediction of √2 improvement. The g-factor for this reconstruction was 1.02, suggesting excellent signal separation, low signal crosstalk, and minimal mutual noise amplification between the two voxels.
Figure 3.
In vivo GABA/Glutathione editing with MEGA-PRIAM. A) PRIAM reconstruction of GABA- (left) and GSH-edited data (right) acquired with dual-band (two-voxel) excitation. B) PRIAM reconstruction of data acquired with single-voxel (Voxel 1 only) excitation i.e. PRIAM reconstruction of conventional MEGA-PRESS acquisition. C) PRIAM reconstruction of single-voxel (Voxel 2 only) excitation. Fits for the modeling of GABA and GSH (both red) and NAA (green), using the Gannet program, are overlaid.
MEGA-PRIAM data of GABA could be reconstructed from both voxels for all 11 subjects (Voxel 1: 1.26±0.27 i.u.; Voxel 2: 1.41±0.15 i.u.). Single-voxel MEGA-PRESS spectra of GABA could be reconstructed from both voxels for all 11 subjects (Voxel 1: 1.28±0.23 i.u.; Voxel 2: 1.39±0.20 i.u.). There was no significant difference between MEGA-PRIAM and single-voxel estimates of GABA (paired t-test, Voxel 1: P = 0.49, Voxel 2: P = 0.79). The relative difference between MEGA-PRIAM and single-voxel GABA estimates was −2.5±8.0% (Voxel 1) and 0.7±13.8% (Voxel 2).
MEGA-PRIAM data of GSH could be reconstructed from both voxels for 10 subjects (Voxel 1: 2.44±0.60 i.u.; Voxel 2: 2.81±0.67 i.u.). Single-voxel MEGA-PRESS spectra of GSH could be reconstructed from Voxel 1 for 9 subjects (mean GSH: 2.61±0.50 i.u.) and from Voxel 2 for 10 subjects (mean GSH: 2.95±0.65 i.u.). There was no significant difference between MEGA-PRIAM and single-voxel estimates of GSH (paired t-test, Voxel 1: P = 0.52; Voxel 2: P = 0.32). The relative difference between MEGA-PRIAM and single-voxel GSH estimates was −5.3±15.3% (Voxel 1) and −7.3±18.6% (Voxel 2).
Mean SNR was 14.2±2.8 for the dual-voxel and 10.34±2.3 for the single-voxel GABA acquisitions, yielding an average SNR improvement of 1.38±0.24. The mean g-factor for the GABA voxel was 1.025±0.017 (1.026±0.011 for the GSH voxel).
Discussion
In this study, it is demonstrated that parallel reconstruction in accelerated multi-voxel spectroscopy (PRIAM, (11)) is compatible with J-difference editing (MEGA). The combined MEGA-PRIAM experiment substantially increases the rate at which edited MRS data can be acquired, addressing a major limitation in the application of edited MRS. Provided that the coil geometry is favorable and optimized high order shimming performed, this acceleration should be possible with negligible loss of SNR, and with comparable data quality.
Phantom data and in vivo data from 11 subjects show good agreement between simultaneously acquired MEGA-PRIAM and sequential single-voxel MEGA-PRESS acquisitions, and low signal crosstalk between voxels. On average, the in vivo difference in measured GABA levels between dual-voxel and single-voxel measurements was below 2% and below 7% for GSH, respectively, demonstrating that the dual-voxel approach does not bias concentration estimates.
Improvement in temporal SNR may be carried even further, as the MEGA-PRIAM approach is, in principle, not limited to simultaneous excitation of only two voxels. Implementation of suitable multi-band slice-selective pulses may facilitate parallel reconstruction of three or more voxels; advances in phased array coil design driven by multi-band imaging should ensure that g-factors remain low for efficient signal separation.
Multi-volume localized spectroscopy based on spatial Hadamard excitation (23) is an alternative route to simultaneous acquisition of multiple locations. Multi-voxel spectroscopy could also be achieved by interleaving separate acquisitions within one single TR (24,25). To our knowledge, these approaches, however, have not been implemented for spectral-editing, and lead to increased RF power deposition, and further lack exact simultaneity, which may be important for functional MR spectroscopy experiments, where data acquisition is timed relative to external stimuli (26–29).
The PRIAM approach for spatial acceleration of J-edited MRS can further be combined with simultaneous editing of multiple metabolites with the Hadamard Encoding and Reconstruction of MEGA-edited Spectroscopy (HERMES) editing scheme (30), which has recently been implemented for simultaneous editing of GABA and GSH (31). As PRIAM is acquiring multiple spatial data within TR, while HERMES acquires multiple metabolite data between TR, HERMES and PRIAM are orthogonal. Their combination can therefore accelerate GABA and GSH acquisition from two separate voxels by a factor of 4. Since PRIAM is in principle not limited to two voxels, and HERMES editing is also expandable to simultaneously edit three-or-more metabolites, the combination of HERMES and PRIAM has transformative potential in terms of the kind of edited MRS studies that can be undertaken.
Several limitations of the PRIAM approach arise from the dual-band excitation. Addition of the single-slice excitation RF pulses may exceed the maximum available B1 transmit capacity of the system, and necessitate longer excitation pulses which have lower bandwidth. The loss in bandwidth causes increased chemical shift displacement effects, which may manifest as increased lipid artifacts in superficial voxel locations. Furthermore, the echo time in a J-difference editing experiment is usually constrained by coupling evolution (to ~1/J for doublets or ~1/2J for triplets). Increasing the duration of dual-band excitation pulses may therefore necessitate either shortened editing pulses or longer (compromised) echo times, resulting in either reduced selectivity or efficiency of editing.
Dual-band excitation also restricts the geometry of acquisition volumes. While location and orientation of the individual voxels are completely independent in sequential measurements, they are inherently linked for dual-band slice-selective excitation to the direction of the slice selection gradient. Furthermore, the individual voxels require a certain minimum distance relative to each other, in order to maintain low g-factors for effective signal separability with low mutual noise amplification. A 32-channel coil can be expected to perform superiorly to a coil with fewer channels, as the sensitivity profiles of the individual channels will show less overlap, and SENSE reconstruction is better defined the more channels that are available. Parallel reconstruction has improved g-factors at higher field strengths, i.e. 7T (11).
For edited MRS, voxel sizes are usually larger than for conventional un-edited MRS (typically 3×3×3 cm3 vs. 2×2×2 cm3). Larger voxels will have less homogeneous coil sensitivity values, which leads to inaccuracy in the reconstruction. For the PRIAM approach, the signals from each coil are weighted by the average coil sensitivity within the voxel. More detailed approaches of signal modeling within large voxels have been proposed for sensitivity-encoded spectroscopic imaging (14), and may further improve the performance of PRIAM for large voxels.
Despite these constraints, numerous two-voxel protocols remain feasible with MEGA-PRIAM including: prefrontal and occipital; homologous left and right regions, such as medial temporal lobe; anterior and posterior cingulate, to name a few. Recent advances in the development of multiband refocusing pulses (e.g. (32)) may allow further possibilities. Using dualband-excitation and dualband-refocusing, signals can be acquired simultaneously from four co-planar voxels. Alternatively, such an acquisition could be used to uncouple the orientation and location for a 2-voxel configuration (discarding the other two).
In this study, shimming was, for practical reasons, performed on one single shim volume enveloping both voxels. This is likely not to be optimal, especially in cases where this shim volume spans across the ventricles. More sophisticated shimming methods that only consider the voxels themselves can be expected to substantially improve the outcome of dual-or-more-voxel MRS experiments. This will be especially true for protocols with less favorable voxel geometry and symmetry, such as prefrontal/occipital protocols. The single-voxel comparison scans were acquired with the same shim parameters. Re-shimming of each single voxel would have improved their linewidths and SNR – the reliance of MEGA-PRIAM on a single shim setting for both voxels likely compromises data quality to some degree, but reduces scan preparation times.
The challenging shimming may also be responsible for the greater average difference between MEGA-PRIAM and single-voxel measures of GSH, as the voxel size of (33 mm)3 was slightly larger compared to the GABA-edited experiments at (30 mm)3. Further variability may have arisen from the modeling used to quantify GSH. Fitting of GABA within Gannet is well-established, having been optimized over a period of time (e.g. (21)), while fitting of GSH is a less mature feature.
CONCLUSION
Dual-band excitation and parallel reconstruction allows the simultaneous acquisition of edited MRS measurements in half the scan time compared to sequential acquisition. High quality of shimming provided, the loss in SNR or spectral quality can be expected to be negligible. These advances may further facilitate the investigation of spatial distribution and/or potential interactions of metabolite and neurotransmitter systems in the human brain.
Acknowledgments
This work was supported by NIH grants R01 EB016089, and P41 EB015909.
References
- 1.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edden RAE, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- 4.Terpstra M, Henry P-G, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn Reson Med. 2003;50:19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]
- 5.Chan KL, Puts NAJ, Snoussi K, Harris AD, Barker PB, Edden RAE. Echo time optimization for J-difference editing of glutathione at 3T. Magn Reson Med. 2016:n/a–n/a. doi: 10.1002/mrm.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edden RAE, Schär M, Hillis AE, Barker PB. Optimized detection of lactate at high fields using inner volume saturation. Magn Reson Med. 2006;56:912–917. doi: 10.1002/mrm.21030. [DOI] [PubMed] [Google Scholar]
- 7.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Heiden MGV, Sorensen AG. Detection of 2-Hydroxyglutarate in IDH-Mutated Glioma Patients by In Vivo Spectral-Editing and 2D Correlation Magnetic Resonance Spectroscopy. Sci Transl Med. 2012;4:116ra4–116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puts NAJ, Edden RAE. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Edden RAE, Ouwerkerk R, Barker PB. High resolution spectroscopic imaging of GABA at 3 Tesla. Magn Reson Med. 2011;65:603–609. doi: 10.1002/mrm.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogner W, Gagoski B, Hess AT, et al. 3D GABA imaging with real-time motion correction, shim update and reacquisition of adiabatic spiral MRSI. NeuroImage. 2014;103:290–302. doi: 10.1016/j.neuroimage.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boer VO, Klomp DWJ, Laterra J, Barker PB. Parallel reconstruction in accelerated multivoxel MR spectroscopy. Magn Reson Med. 2015;74:599–606. doi: 10.1002/mrm.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. doi: 10.1002/(SICI)1522-2594(199911)42:5<952::AID-MRM16>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 14.Dydak U, Weiger M, Pruessmann KP, Meier D, Boesiger P. Sensitivity-encoded spectroscopic imaging. Magn Reson Med. 2001;46:713–722. doi: 10.1002/mrm.1250. [DOI] [PubMed] [Google Scholar]
- 15.Bonekamp D, Smith MA, Zhu H, Barker PB. Quantitative SENSE-MRSI of the human brain. Magn Reson Imaging. 2010;28:305–313. doi: 10.1016/j.mri.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murdoch JB, Lent AH, Kritzer MR. Computer-optimized narrowband pulses for multislice imaging. J Magn Reson 1969. 1987;74:226–263. doi: 10.1016/0022-2364(87)90336-2. [DOI] [Google Scholar]
- 17.Pauly J, Macovski A. Direct Design of Self-Refocusing RF Pulses. Proc Soc Magn Reson Med; San Francisco. 1991. [Google Scholar]
- 18.Edden RAE, Oeltzschner G, Harris AD, Puts NAJ, Chan KL, Boer VO, Schär M, Barker PB. Prospective Frequency Correction for Macromolecule-Suppressed GABA Editing Experiments at 3T. J Magn Reson Imaging. 2016 doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tkáč I, Starčuk Z, Choi I-Y, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(SICI)1522-2594(199904)41:4<649::AID-MRM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73:44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabanes E, Confort-Gouny S, Le Fur Y, Simond G, Cozzone PJ. Optimization of Residual Water Signal Removal by HLSVD on Simulated Short Echo Time Proton MR Spectra of the Human Brain. J Magn Reson. 2001;150:116–125. doi: 10.1006/jmre.2001.2318. [DOI] [PubMed] [Google Scholar]
- 23.Bolinger L, Leigh JS. Hadamard spectroscopic imaging (HSI) for multivolume localization. J Magn Reson 1969. 1988;80:162–167. doi: 10.1016/0022-2364(88)90070-4. [DOI] [Google Scholar]
- 24.Ernst T, Hennig J. Double-volume 1H spectroscopy with interleaved acquisitions using tilted gradients. Magn Reson Med. 1991;20:27–35. doi: 10.1002/mrm.1910200104. [DOI] [PubMed] [Google Scholar]
- 25.Lemke C, Hess A, Clare S, Bachtiar V, Stagg C, Jezzard P, Emir U. Two-voxel spectroscopy with dynamic B0 shimming and flip angle adjustment at 7 T in the human motor cortex. NMR Biomed. 2015;28:852–860. doi: 10.1002/nbm.3328. [DOI] [PubMed] [Google Scholar]
- 26.Cleve M, Gussew A, Reichenbach JR. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage. 2015;105:67–75. doi: 10.1016/j.neuroimage.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Landim RCG, Edden RAE, Foerster B, Li LM, Covolan RJM, Castellano G. Investigation of NAA and NAAG dynamics underlying visual stimulation using MEGA-PRESS in a functional MRS experiment. Magn Reson Imaging. 2016;34:239–245. doi: 10.1016/j.mri.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Davis IvH (Hap), Yue Q, Wiebking C, Duncan NW, Zhang J, Wagner N-F, Wolff A, Northoff G. Increase in glutamate/glutamine concentration in the medial prefrontal cortex during mental imagery: A combined functional mrs and fMRI study. Hum Brain Mapp. 2015;36:3204–3212. doi: 10.1002/hbm.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apšvalka D, Gadie A, Clemence M, Mullins PG. Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3 T in a repetition suppression paradigm. NeuroImage. 2015;118:292–300. doi: 10.1016/j.neuroimage.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Chan KL, Puts NAJ, Schär M, Barker PB, Edden RAE. HERMES: Hadamard encoding and reconstruction of MEGA-edited spectroscopy: HERMES. Magn Reson Med. 2016;76:11–19. doi: 10.1002/mrm.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saleh MG, Oeltzschner G, Chan KL, Puts NAJ, Mikkelsen M, Schär M, Harris AD, Edden RAE. Simultaneous Edited MRS of GABA and Glutathione. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma A, Lustig M, Grissom WA. Root-flipped multiband refocusing pulses. Magn Reson Med. 2016;75:227–237. doi: 10.1002/mrm.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]