Abstract
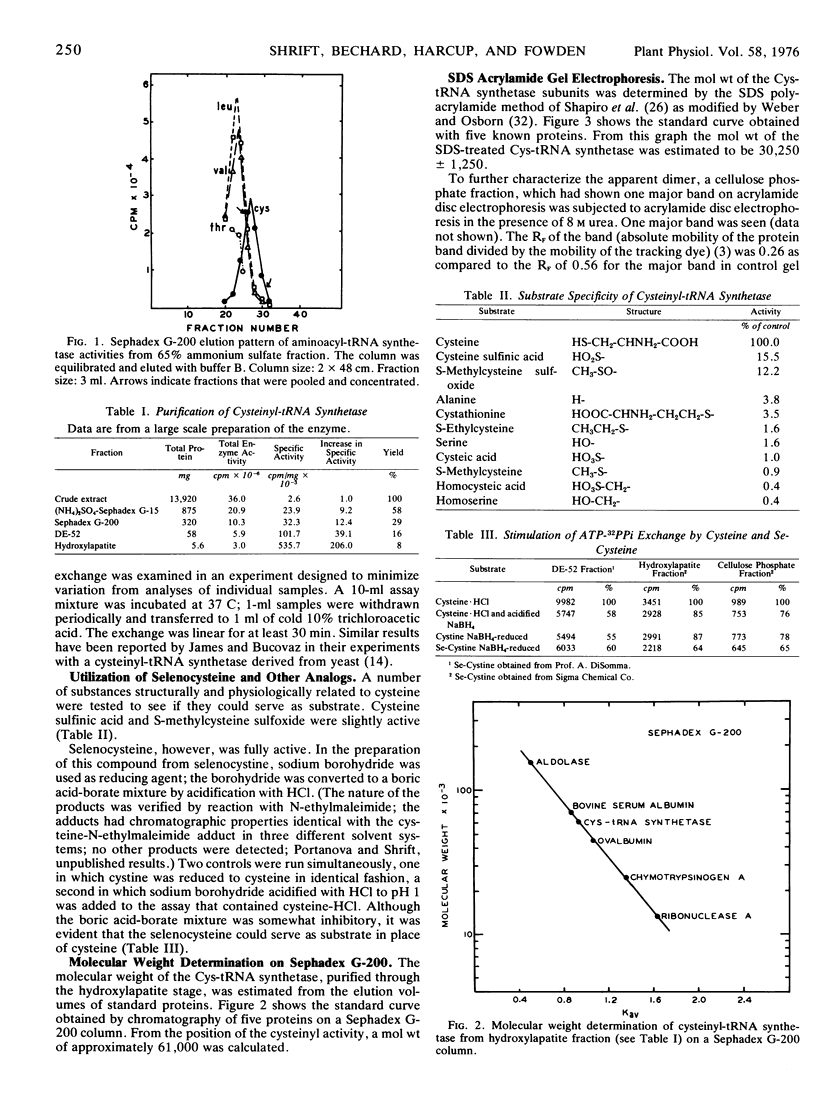
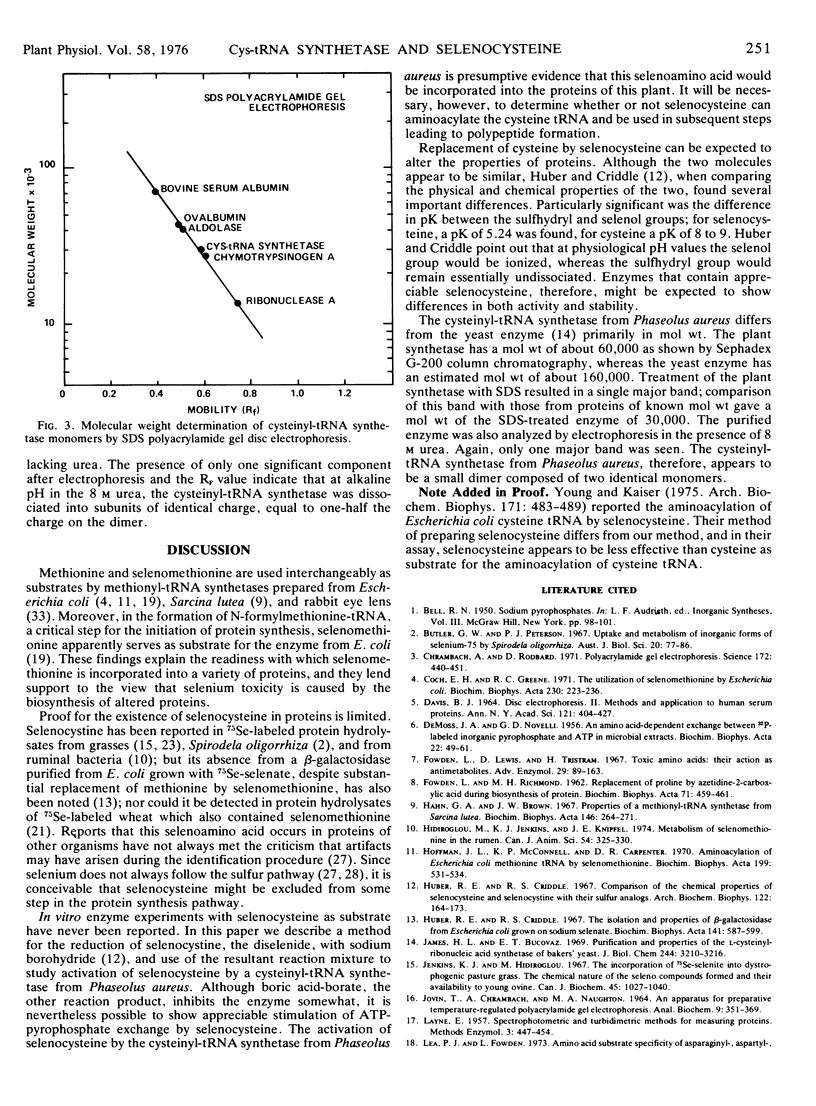
An l-cysteinyl-tRNA synthetase (EC 6.1.1.16) from Phaseolus aureus has been purified approximately 200-fold. The enzyme uses selenocysteine as substrate in the ATP-PPi exchange assay; other cysteine analogs were inactive. The molecular weight as determined by Sephadex G-200 column chromatography is about 61,000; sodium dodecyl sulfate and 8 m urea acrylamide gel electrophoresis indicate that the enzyme is a dimer consisting of two identical monomers of molecular weight 30,000. A method for the preparation of selenocysteine from selenocystine is described.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrambach A., Rodbard D. Polyacrylamide gel electrophoresis. Science. 1971 Apr 30;172(3982):440–451. doi: 10.1126/science.172.3982.440. [DOI] [PubMed] [Google Scholar]
- Coch E. H., Greene R. C. The utilization of selenomethionine by Escherichia coli. Biochim Biophys Acta. 1971 Feb 23;230(2):223–236. doi: 10.1016/0304-4165(71)90207-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A., NOVELLI G. D. An amino acid dependent exchange between 32P labeled inorganic pyrophosphate and ATP in microbial extracts. Biochim Biophys Acta. 1956 Oct;22(1):49–61. doi: 10.1016/0006-3002(56)90222-0. [DOI] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Hahn G. A., Brown J. W. Properties of a methionyl-tRNA synthetase from Sarcina lutea. Biochim Biophys Acta. 1967 Sep 12;146(1):264–271. doi: 10.1016/0005-2744(67)90093-9. [DOI] [PubMed] [Google Scholar]
- Hoffman J. L., McConnell K. P., Carpenter D. R. Aminoacylation of Escherichia coli methionine tRNA by selenomethionine. Biochim Biophys Acta. 1970 Feb 18;199(2):531–534. doi: 10.1016/0005-2787(70)90098-5. [DOI] [PubMed] [Google Scholar]
- Huber R. E., Criddle R. S. Comparison of the chemical properties of selenocysteine and selenocystine with their sulfur analogs. Arch Biochem Biophys. 1967 Oct;122(1):164–173. doi: 10.1016/0003-9861(67)90136-1. [DOI] [PubMed] [Google Scholar]
- Huber R. E., Criddle R. S. The isolation and properties of beta-galactosidase from Escherichia coli grown on sodium selenate. Biochim Biophys Acta. 1967 Aug 29;141(3):587–599. doi: 10.1016/0304-4165(67)90187-0. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- James H. L., Bucovaz E. T. Purification and properties of the L-cysteinyl ribonucleic acid synthetase of bakers' yeast. J Biol Chem. 1969 Jun 25;244(12):3210–3216. [PubMed] [Google Scholar]
- Jenkins K. J., Hidiroglou M. The incorporation of 75-Se-selenite into dystrophogenic pasture grass. The chemical nature of the seleno compounds formed and their availability to young ovine. Can J Biochem. 1967 Jul;45(7):1027–1039. doi: 10.1139/o67-119. [DOI] [PubMed] [Google Scholar]
- McConnell K. P., Hoffman J. L. Methionine-selenomethionine parallels in E. coli polypeptide chain initiation and synthesis. Proc Soc Exp Biol Med. 1972 Jun;140(2):638–641. doi: 10.3181/00379727-140-36520. [DOI] [PubMed] [Google Scholar]
- Peterson P. J. Amino acid selection in protein biosynthesis. Biol Rev Camb Philos Soc. 1967 Nov;42(4):552–613. doi: 10.1111/j.1469-185x.1967.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Virupaksha T. K., Shrift A. Biochemical differences between selenium accumulator and non-accumulator Astragalus species. Biochim Biophys Acta. 1965 Aug 24;107(1):69–80. doi: 10.1016/0304-4165(65)90389-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weller C. A., Green M. Methionyl-tRNA synthetase detected by [75Se]selenomethionine in lenses from normal and galactose-fed rats. Exp Eye Res. 1969 Jan;8(1):84–90. doi: 10.1016/s0014-4835(69)80084-9. [DOI] [PubMed] [Google Scholar]
- Young P. A., Kaiser I. I. Aminoacylation of Escherichia coli cysteine tRNA by selenocysteine. Arch Biochem Biophys. 1975 Dec;171(2):483–489. doi: 10.1016/0003-9861(75)90057-0. [DOI] [PubMed] [Google Scholar]


