Abstract
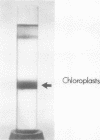
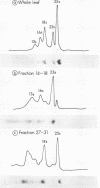
Freshly prepared spinach leaf protoplasts were gently ruptured by mechanical shearing followed by sucrose density gradient centrifugation to separate constituent cell organelles. The isolation of intact Class I chloroplasts (d = 1.21) in high yield, well separated from peroxisomes and mitochondria, was evidenced by the specific localization of ribulose-1,5-bisphosphate carboxylase (EC 4.1.1.39), NADP triose-P dehydrogenase (EC 1.2.1.9), and carbonic anhydrase (EC 4.2.1.1) in the fractions. A clear separation of chloroplastic ribosomes from the soluble cytoplasmic ribosomes was also demonstrated by the band patterns of constituent RNA species in the polyacrylamide gel electrophoresis. Localization of several enzyme activities specific to leaf peroxisomes, e.g. catalase (EC 1.11.1.6), glycolate oxidase (EC 1.1.3.1), glyoxylate reductase (EC 1.1.1.26), glutamate glyoxylate aminotransferase (EC 2.6.1.4), serine glyoxylate aminotransferase, and alanine glyoxylate aminotransferase (EC 2.6.1.12) in the peroxisomal fractions (d = 1.25), was demonstrated. Overall results show the feasibility of the method for the isolation of pure organelle components in leaf tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Schweiger H. G. Sites of synthesis of chloroplast-membrane proteins. Evidence for three types of ribosomes engaged in chloroplast-protein synthesis. Eur J Biochem. 1973 Oct 5;38(2):373–383. doi: 10.1111/j.1432-1033.1973.tb03070.x. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi T., Nishimura M. Regulatory function of malate dehydrogenase isoenzymes in the cotyledons of mung bean. J Biochem. 1973 Feb;73(2):217–225. [PubMed] [Google Scholar]
- Atkins C. A., Patterson B. D., Graham D. Plant Carbonic Anhydrases: I. Distribution of Types among Species. Plant Physiol. 1972 Aug;50(2):214–217. doi: 10.1104/pp.50.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Isolation of microbodies from plant tissues. Plant Physiol. 1971 Nov;48(5):637–641. doi: 10.1104/pp.48.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems R. E., Allison V. F., Butow R. A. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. II. Evidence for the association of cytoplasmic ribosomes with the outer mitochondrial membrane in situ. J Biol Chem. 1974 May 25;249(10):3297–3303. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson C., Albertsson P. A. Photosynthetic 14CO2 fixation by chloroplast populations isolated by a polymer two-phase technique. Biochim Biophys Acta. 1974 Sep 20;357(3):412–419. doi: 10.1016/0005-2728(74)90031-0. [DOI] [PubMed] [Google Scholar]
- Lips S. H. Enzyme content of plant microbodies as affected by experimental procedures. Plant Physiol. 1975 Apr;55(4):598–601. doi: 10.1104/pp.55.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E., Ingle J. Diversity of RNA components in green plant tissues. Nature. 1967 Jul 22;215(5099):363–367. doi: 10.1038/215363a0. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Akazawa T. Photosynthetic activities of spinach leaf protoplasts. Plant Physiol. 1975 Apr;55(4):712–716. doi: 10.1104/pp.55.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Effect of oxygen on photosynthesis by spinach leaf protoplasts. Plant Physiol. 1975 Nov;56(5):718–722. doi: 10.1104/pp.56.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Takabe T., Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. XIX. Dissociation of spinach leaf ribulose-1,5-diphosphate carboxylase by p-mercuribenzoate. J Biochem. 1973 Nov;74(5):945–954. [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Preparation of cellular plant organelles from spinach leaves. Arch Biochem Biophys. 1970 Oct;140(2):398–407. doi: 10.1016/0003-9861(70)90081-0. [DOI] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]