Abstract
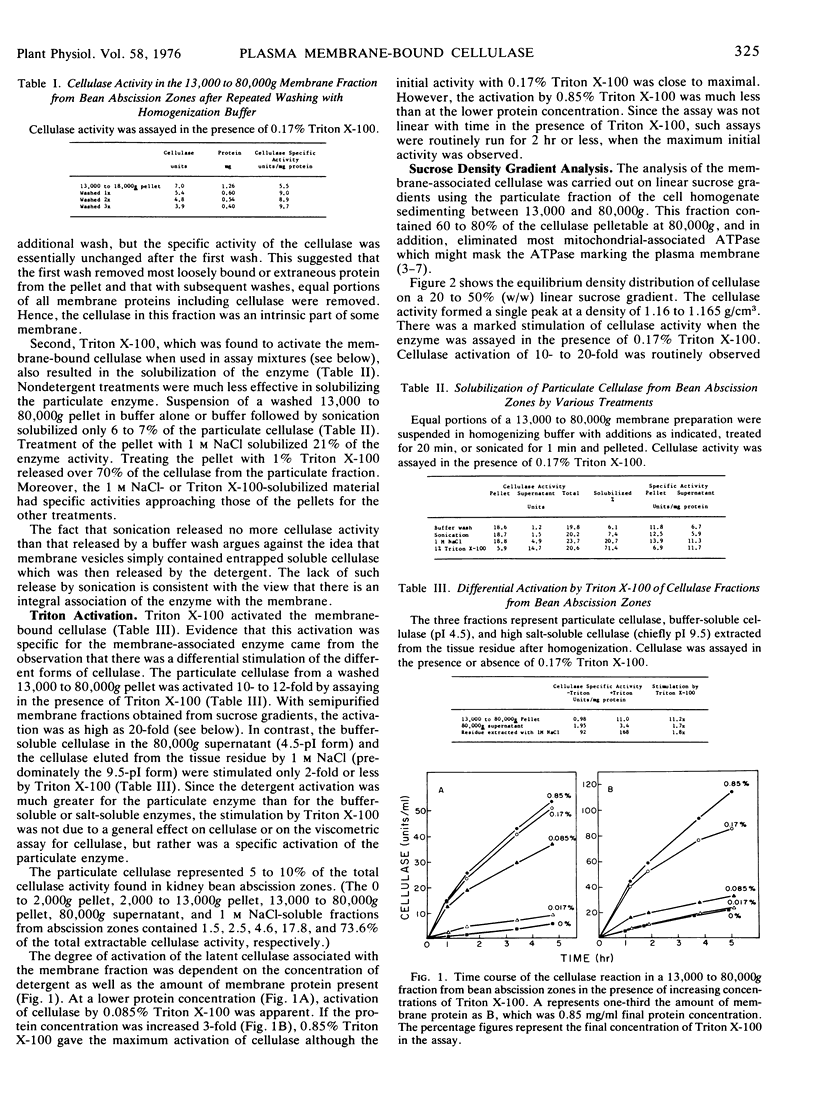
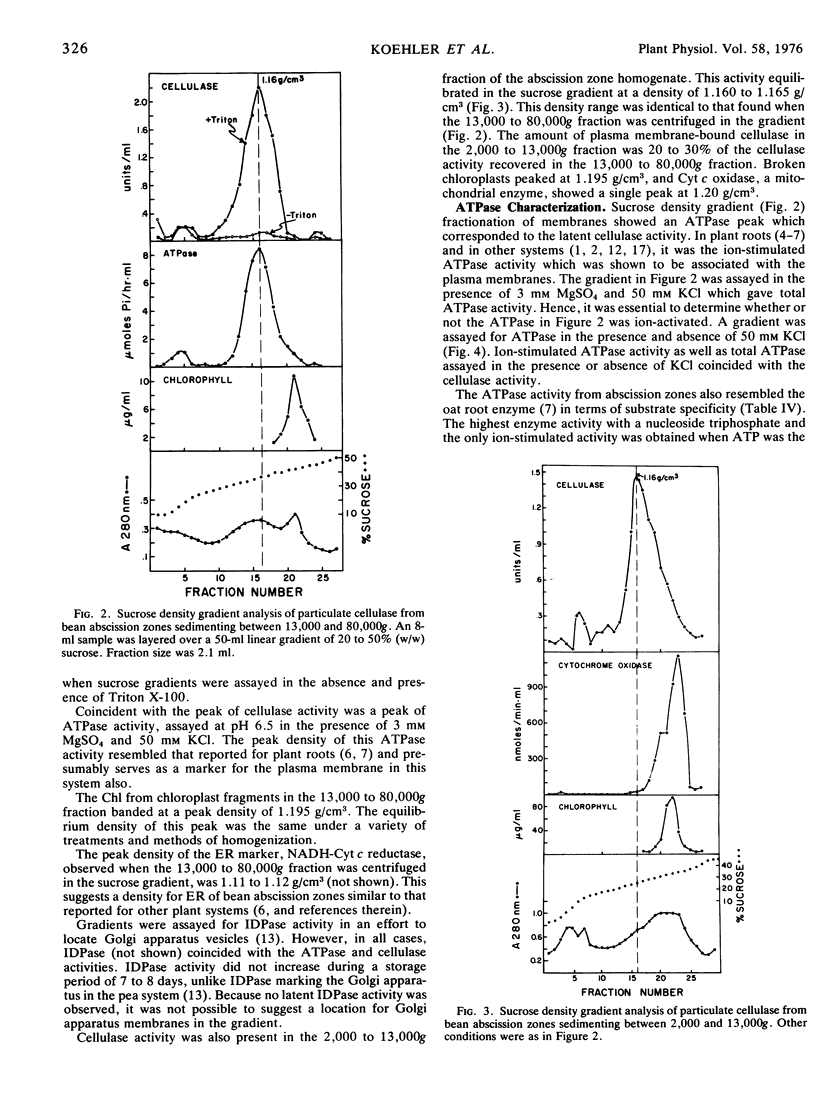
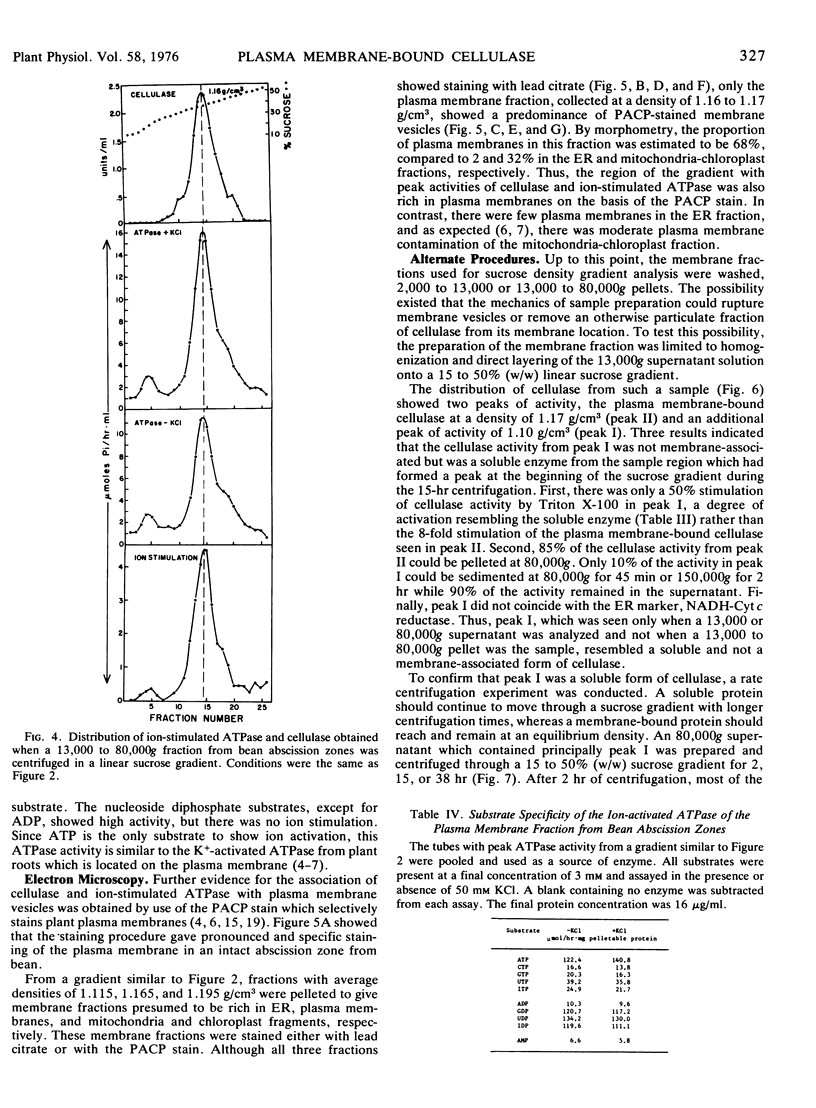
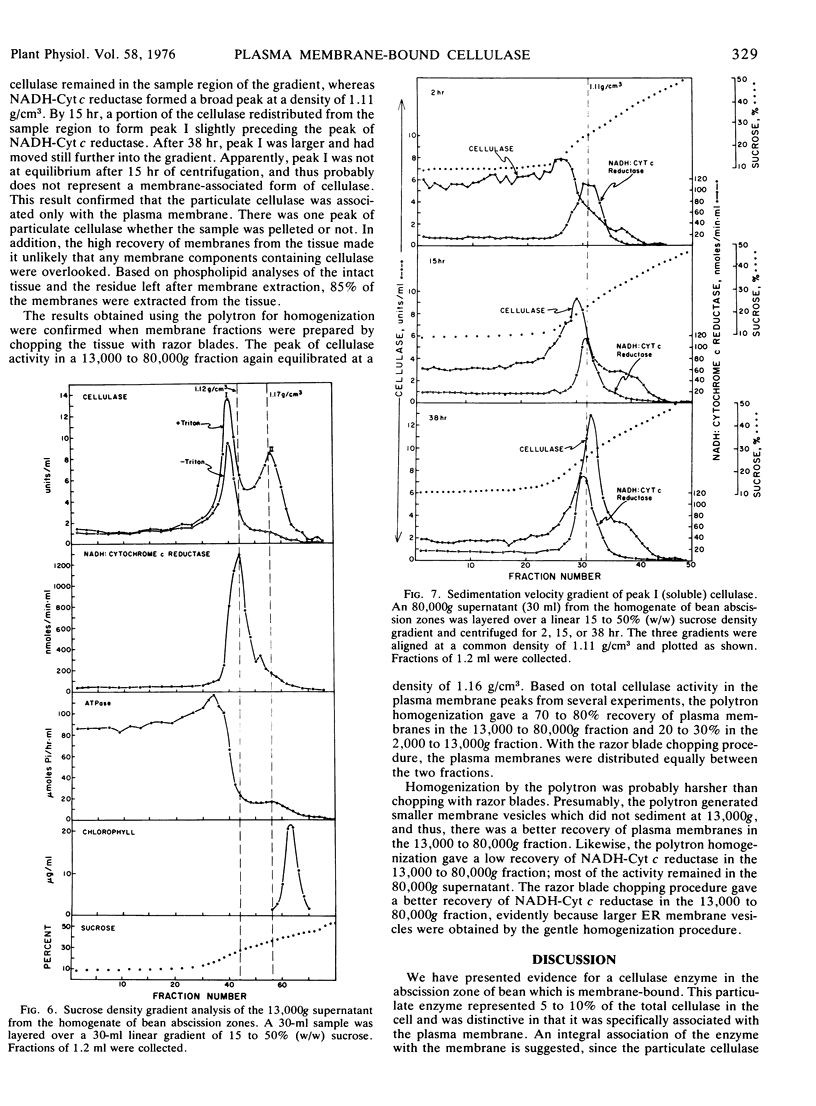
Membranes isolated from abscission zones of Phaseolus vulgaris L., cv. Red Kidney, contained cellulase activity. This particulate activity was enhanced 10- to 20-fold by treatment with Triton X-100. Sucrose density gradient analyses of cell fractions showed that the membranes with which cellulase was associated had a peak equilibrium density of 1.16 to 1.17 g/cm3 which coincided with that of ion-activated ATPase, a marker for plasma membranes. The membrane fraction having the highest cellulase activity also contained a high proportion of plasma membranes as shown by electron microscopy of sucrose density gradient fractions after staining by periodic acid-chromic acid-phosphotungstic acid. It was concluded that the particulate cellulase was associated with the plasma membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emmelot P., Bos C. J., van Hoeven R. P., van Blitterswijk W. J. Isolation of plasma membranes from rat and mouse livers and hepatomas. Methods Enzymol. 1974;31:75–90. doi: 10.1016/0076-6879(74)31008-7. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Wehrli E., Boehm C. Preparation and identification of yeast plasma membrane vesicles. Biochim Biophys Acta. 1974 Sep 23;363(3):295–310. doi: 10.1016/0005-2736(74)90070-4. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. N., Varner J. E. Synthesis of Cellulase during Abscission of Phaseolus vulgaris Leaf Explants. Plant Physiol. 1970 Aug;46(2):194–199. doi: 10.1104/pp.46.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkins A. E., Lewis L. N., Palmer R. L. Hormonally Induced Changes in the Stem and Petiole Anatomy and Cellulase Enzyme Patterns in Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):554–560. doi: 10.1104/pp.52.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Billmire E., Aaronson S. Isolation of the Ochromanas danica plasma membrane and identification of several membrane enzymes. Biochim Biophys Acta. 1974 Dec 24;373(3):347–355. doi: 10.1016/0005-2736(74)90014-5. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. D., Strong H. G. Cellulase and Abscission in the Red Kidney Bean (Phaseolus vulgaris). Plant Physiol. 1974 May;53(5):732–737. doi: 10.1104/pp.53.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Synergistically stimulated (Na+,K+)-adenosine triphosphatase from plasma membrane of a marine diatom. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4376–4380. doi: 10.1073/pnas.71.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsamer A. G., Wright P. L., Wetzel M. G., Korn E. D. Plasma and phagosome membranes of Acanthamoeba castellanii. J Cell Biol. 1971 Oct;51(1):193–215. doi: 10.1083/jcb.51.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]



