Abstract
Objective
Diagnostic laparoscopy is recommended for the pretherapeutic staging of gastric cancer to detect any unexpected or unconfirmed intra-abdominal metastasis. The aim of this study was to evaluate the role and indications of diagnostic laparoscopy in the detection of intra-abdominal metastasis.
Methods
Standard diagnostic laparoscopy with peritoneal cytology examination was performed prospectively on patients who were clinically diagnosed with primary local advanced gastric cancer (cT≥2M0). We calculated the rate of intra-abdominal metastases identified by diagnostic laparoscopy, and examined the relationship between peritoneal dissemination (P) and cytology results (CY). Split-sample method was applied to find clinical risk factors for intra-abdominal metastasis. Multivariate logistic regression analysis and receiver-operator characteristic (ROC) analysis were performed in training set to find out risk factors of intra-abdominal metastasis, and then validate it in testing set.
Results
Out of 249 cM0 patients, 51 (20.5%) patients with intra-abdominal metastasis were identified by diagnostic laparoscopy, including 20 (8.0%) P1CY1, 17 (6.8%) P0CY1 and 14 (5.6%) P1CY0 patients. In the training set, multivariate logistic regression analysis and ROC analysis showed that the depth of tumor invasion on computer tomography (CT) scan ≥21 mm and tumor-occupied ≥2 portions of stomach are predictive factors of metastasis. In the testing set, when diagnostic laparoscopy was performed on patients who had one or two of these risk factors, the sensitivity and positive predictive value for detecting intra-abdominal metastasis were 90.0% and 32.1%, respectively.
Conclusions
According to our results, depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis.
Keywords: Gastric cancer, diagnostic laparoscopy, peritoneal dissemination, intra-peritoneal free cancer cells, pretherapeutic staging
Introduction
Gastric cancer is the fourth leading cause of cancer deaths worldwide (1) and the third in China (2). Missed preoperative diagnosis of intra-abdominal metastases, including peritoneal dissemination and intraperitoneal free cancer cells, is among the leading cause of poor prognosis, surgical failure and futile laparotomy. Therefore, accurate preoperative staging, especially through improved diagnostic accuracy of peritoneal dissemination and intraperitoneal free cancer cells, is particularly important for alternative treatment strategies and prognostic evaluations.
As an alternative of laparotomy, diagnostic laparoscopy has been suggested to effectively detect peritoneal diseases and can be used to collect peritoneal lavage fluid for cytology examination with minimal invasion. However, diagnostic laparoscopy plays a currently undefined role in gastric cancer staging due to controversy regarding its indications. National Comprehensive Cancer Network (NCCN) guidelines recommend laparoscopic staging for patients with T3 and/or N+ if they are considered for surgical resection without preoperative therapy; European Society for Medical Oncology (ESMO) guidelines recommend laparoscopic staging for patients with IB-III gastric cancer; and Japanese Gastric Cancer Association (JGCA) guidelines mention only laparoscopy prior to neoadjuvant chemoradiotherapy (3-5). In China, most gastric cancer patients present with advanced disease stages; however, a consensus on the role of diagnostic laparoscopy still has not been established because of the lack of direct evidence (6).
The aim of this study was to provide an assessment of the detection rate of intra-abdominal metastasis using diagnostic laparoscopy in gastric cancer patients, and investigate the clinical risk factors of intra-abdominal metastasis before exploratory operation to determine the indications of diagnostic laparoscopy for patients with gastric cancer in China.
Materials and methods
Design of study
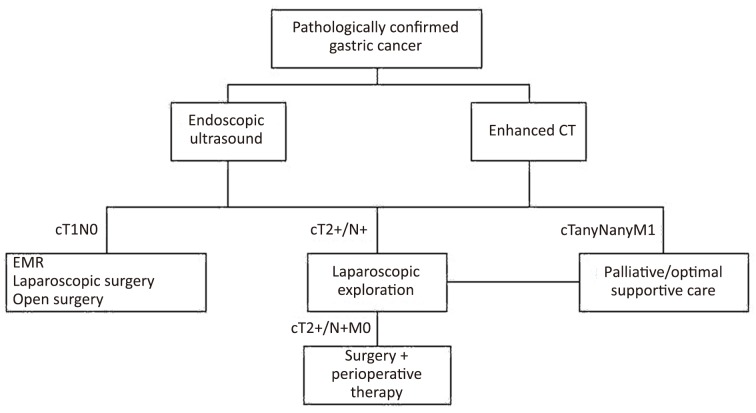
Between September 2011 and September 2013, a prospective study of pretherapeutic diagnostic laparoscopy was performed on consecutive gastric cancer patients in the Department of Gastrointestinal Surgery at the Peking University Cancer Hospital (Figure 1).
1.
Basic algorithm for selection and treatment of patients with locally advanced gastric cancer.
Patients meeting the inclusion criteria were enrolled, and the following data were recorded: basic information, including age, gender, height, body weight and weight loss; and clinical information, including results of endoscopy, computed tomography (CT), endoscopic ultrasound (EUS) and result of blood test. More specifically, Borrmann type, proportion of the tumor in relation to circumference of the stomach (<1/4, 1/4 to <1/2, 1/2 to <3/4, and ≥3/4) and portions of stomach (cardia, fundus, body and antrum) were determined by endoscopy; T stage, N stage, tumor depth, basal diameter and basal area were from EUS; and serosal invasion, enlarged lymph node, and tumor depth were measured by CT. Tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), CA72-4, and CA242] were determined by blood test.
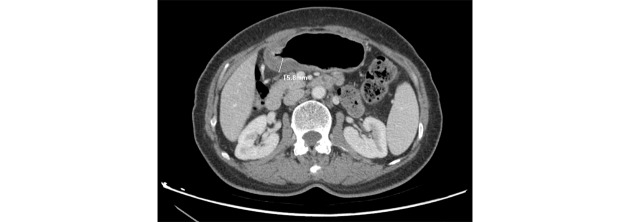
We adopted a CT criteria described by Jin Woong Kim to measure serosal invasion on CT. Specifically, when an irregular or nodular outer margin of the outer layer and/or a dense band-like perigastric fat infiltration was visualized, serosal invasion was suspected (7).
Depth of invasion was measured using CT slices as shown in Figure 2. To maintain consistency of degree of distension, a gas distension method was used during CT scan (8).
2.
Measurement of the depth of invasion on CT.
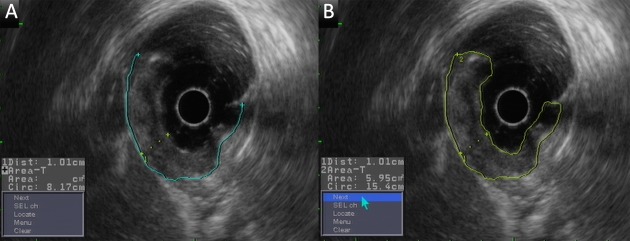
Measurement of the depth, the basal diameter and basal area are shown in Figure 3. Lymph nodes were considered positive for metastasis if they were equal to or larger than 8 mm in the short-axis diameter under EUS (9).
3.
Measurement of the depth, basal diameter and basal area using EUS. (A) Depth and basal diameter; (B) Basal area.
Ethics approval
This study is conducted in accordance with the “Declaration of Helsinki” and “Ethical Guidelines for Clinical Research”, and has been approved by the Institutional Review Boards of Peking University Cancer Hospital. Informed consent was obtained before surgery.
Inclusion criteria & exclusion criteria
The inclusion criteria included: 1) histologically confirmed diagnosis of primary gastric carcinoma; 2) abdominal enhanced spiral CT and EUS showed a clinical stage of cT2-4N0-3M0; 3) fit for radical gastrectomy or neo-adjuvant treatment; and 4) agree to have diagnostic laparoscopy.
The exclusion criteria included: 1) unfit for laparoscopic operation or general anesthesia (suspicious abdominal adhesions, severe cardiopulmonary disease, etc.); 2) underwent emergency surgery to relieve obstruction, bleeding or perforation; or 3) preoperative examination shows signs of metastasis, including peritoneal thickening, ascites, distant lymph node enlargement, etc.
Laparoscopic staging technique
Laparoscopy was performed under general anesthesia. The patient was placed in a supine position. A 10-mm disposable trocar (observing hole) was inserted into the sub-umbilicus, and a 30° telescope was used. Another 10-mm trocar and a 5-mm trocar (operating hole) were inserted into the right and left upper quadrants, respectively. Prior to any manipulation, 250 mL of warm normal saline was infused into the subphrenic space, subhepatic space, omentum, bilateral paracolic sulci and the pouch of Douglas. Care was taken to avoid direct contact of the irrigation with the primary tumor. At least 100 mL of fluid was aspirated from the subphrenic space, subhepatic space and pouch of Douglas. The fluid was immediately sent for centrifugation and cytological examination. Subsequently, a systematic inspection of the abdominal cavity was performed clockwise from the right quadrant. Any suspicious lesion was biopsied and sent for pathologic examination.
Assessment of diagnostic laparoscopy and cytology results
Patients without macroscopic peritoneal dissemination (P–) and with negative cytology results (CY–) were defined as having negative diagnostic laparoscopy results (DL–), which means there are no intra-abdominal metastases. And patients with peritoneal dissemination, which was confirmed by frozen pathology (P+) or free cancer cells in the peritoneal lavage (CY+), were defined as having positive diagnostic laparoscopy results (DL+).
Data analysis
To determine the clinical risk factors as predictors for gastric cancer patients with intra-abdominal metastasis before exploratory operation, a split-sample method was applied. The patient cohort was divided randomly into 2 groups: 3/4 for a training set and 1/4 for a testing set. In the training set, the clinical characteristics of the DL– and DL+ patients were compared using the independent samples group t-test and Chi-squared test. To determine the risk factors, all variables found to be significant by univariate analysis were assessed by binary logistic regression analysis (method: Backward Wald, probability for stepwise: 0.5 for entry, 1.0 for removal). The receiver operating characteristic (ROC) curve and area under the curve (AUC) analyses were used to determine sensitivity, specificity and corresponding cut-off values of each factor. In the testing set, all patients were classified according to their number of risk factors determined by training set, and the sensitivity and specificity of different combinations of factors were calculated to predict the metastasis. P<0.05 was considered statistically significant. All statistical analyses were carried out using the IBM SPSS Statistics (Version 20.0; IBM Corp., New York, USA).
Results
Patient characteristics
From September 2011 to September 2013, 249 patients with histologically confirmed gastric carcinoma underwent diagnostic laparoscopy with peritoneal cytology examination as a routine pretreatment workup, 70.7% of whom are males, with a median age of 57.6 (range: 29–81) years. On the basis of CT and EUS findings, 13 (5.2%) patients were found to have clinical stage I disease, 64 (25.7%) were stage II disease, and 172 (69.1%) were stage III disease. For T stage, 15 (6.0%) patients were T2, 65 (26.1%) were T3 and 169 (67.9%) were T4. As for N stage, 53 (21.3%) patients were N0, and 196 (78.7%) were N+.
Results of diagnostic laparoscopy
From the diagnostic laparoscopy findings, 14 (5.6%) of the 249 patients had visible peritoneal dissemination only, which was confirmed by frozen pathology, 17 (6.8%) had free cancer cells in the peritoneal lavage only and 20 (8.0%) had both metastases. In total, 51 (20.5%) patients were DL+ and 198 were DL–. Among the DL+ patients, 42 underwent hyperthermic intraperitoneal chemotherapy (HIPEC) followed by palliative chemotherapy after diagnostic laparoscopy, and 9 underwent palliative surgery. Among the DL– patients, 101 underwent radical D2 gastrectomy, and 97 underwent neoadjuvant chemotherapy.
Comparison of clinical characteristics
The split sample method was used, and the 249 patients were divided into two sets using random sampling: 3/4 (187 patients) as a training set for developing predictive factors of metastasis and 1/4 (62 patients) as a testing set for validating these factors.
The clinical characteristics were compared between the DL+ and DL– patients (Table 1). In the training set, 38 (20.3%) patients were DL+, and 149 patients were DL–. CA72-4 expression, proportion of the tumor in relation to the circumference of the stomach, tumor-occupied stomach portions, Borrmann type, depth of tumor invasion on CT and depth of tumor invasion on EUS were found to be significant in our univariate analyses. Next, all these factors were evaluated by multivariate analysis. Among these factors, multivariate analysis indicated that the depth of tumor invasion on CT scan and tumor-occupied stomach portions are predictive factors of metastasis (Table 2).
1.
Clinicopathologic features of DL+ and DL– patients (univariate analysis, training set)
| Clinicopathologic characteristics | DL+ (N=38) | DL– (N=149) | P | |
| Gender [n (%)] | 0.298 | |||
| Female (N=52) | 8 (15.4) | 44 (84.6) | ||
| Male (N=135) | 30 (22.2) | 105 (77.8) | ||
Age (year) ( ) )
|
54.9±11.2 | 58.1±10.1 | 0.090 | |
BMI (kg/m2) (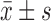 ) )
|
23.3±3.3 | 23.1±3.2 | 0.716 | |
Weight loss (kg) (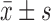 ) )
|
3.4±4.3 | 2.3±3.6 | 0.112 | |
| CEA [n (%)] | 0.389 | |||
| High (N=45) | 11 (24.4) | 34 (75.6) | ||
| Normal (N=135) | 25 (18.5) | 110 (81.5) | ||
| CA19-9 [n (%)] | 0.082 | |||
| High (N=24) | 8 (33.3) | 16 (66.7) | ||
| Normal (N=155) | 28 (18.1) | 127 (81.9) | ||
| CA72-4 [n (%)] | 0.046 | |||
| High (N=55) | 16 (29.1) | 39 (70.9) | ||
| Normal (N=124) | 20 (16.1) | 104 (83.9) | ||
| CA242 [n (%)] | 0.103 | |||
| High (N=24) | 8 (33.3) | 16 (66.7) | ||
| Normal (N=144) | 27 (18.8) | 117 (81.2) | ||
| Tumor-occupied walls of stomach [n (%)] | 0.010 | |||
| 1 (N=37) | 2 (5.4) | 35 (94.6) | ||
| 2 (N=44) | 11 (25.0) | 33 (75.0) | ||
| 3 (N=67) | 11 (16.4) | 56 (83.6) | ||
| 4 (N=37) | 13 (35.1) | 24 (64.9) | ||
| Tumor-occupied portions of stomach [n (%)] | <0.001 | |||
| 1 (N=86) | 10 (11.6) | 76 (88.4) | ||
| 2 (N=76) | 16 (21.1) | 60 (78.9) | ||
| 3 (N=22) | 9 (40.9) | 13 (59.1) | ||
| 4 (N=3) | 3 (100) | 0 (0) | ||
| T stage by EUS [n (%)] | 0.697 | |||
| T2 (N=12) | 2 (16.7) | 10 (83.3) | ||
| T3 (N=47) | 7 (14.9) | 40 (85.1) | ||
| T4 (N=102) | 21 (20.6) | 81 (79.4) | ||
| N stage by EUS [n (%)] | 0.298 | |||
| N0 (N=34) | 6 (17.6) | 28 (82.4) | ||
| N1 (N=46) | 7 (15.2) | 39 (84.8) | ||
| N2 (N=52) | 8 (15.4) | 44 (84.6) | ||
| N3 (N=29) | 9 (31.0) | 20 (69.0) | ||
| Serosal invasion on CT [n (%)] | 0.457 | |||
| Invasion (N=130) | 28 (21.5) | 102 (78.5) | ||
| Non-invasion (N=43) | 7 (16.3) | 36 (83.7) | ||
| Table 1 (continued) |
2.
Predictive factors of metastasis (multivariate analysis, training set)
| Predictive factors | P | OR | Wald | 95% CI |
| OR, odds ratio; 95% CI, 95% confidence interval; CT, computed tomography. | ||||
| Tumor-occupied portions of stomach | 0.021 | 2.178 | 9.830 | 1.124–4.219 |
| Depth of tumor invasion on CT | 0.005 | 1.126 | 12.275 | 1.037–1.222 |
From an ROC curve (Figure 4, 5 and Table 3), the highest Youden index (0.217 and 0.270, respectively) was obtained when the depth of tumor invasion on CT was cut off at 21 mm (AUC: 0.668, P<0.01) and tumor-occupying portions of the stomach were cut off between 1 and ≥2 (AUC: 0.676, P<0.01).
4.
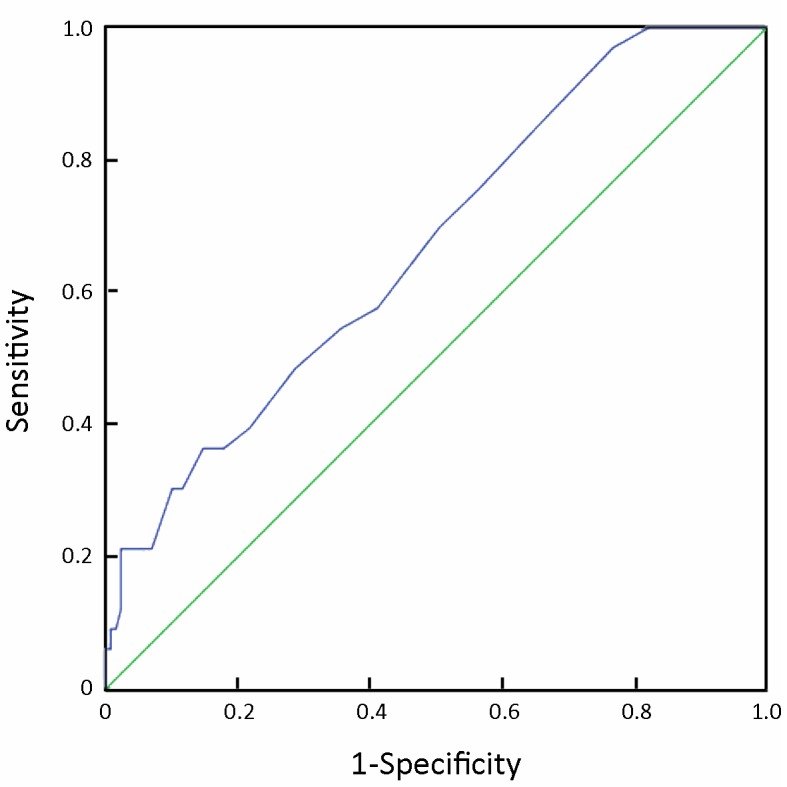
ROC for the prediction of intra-abdominal metastasis by the depth of tumor invasion found by CT scans.
5.
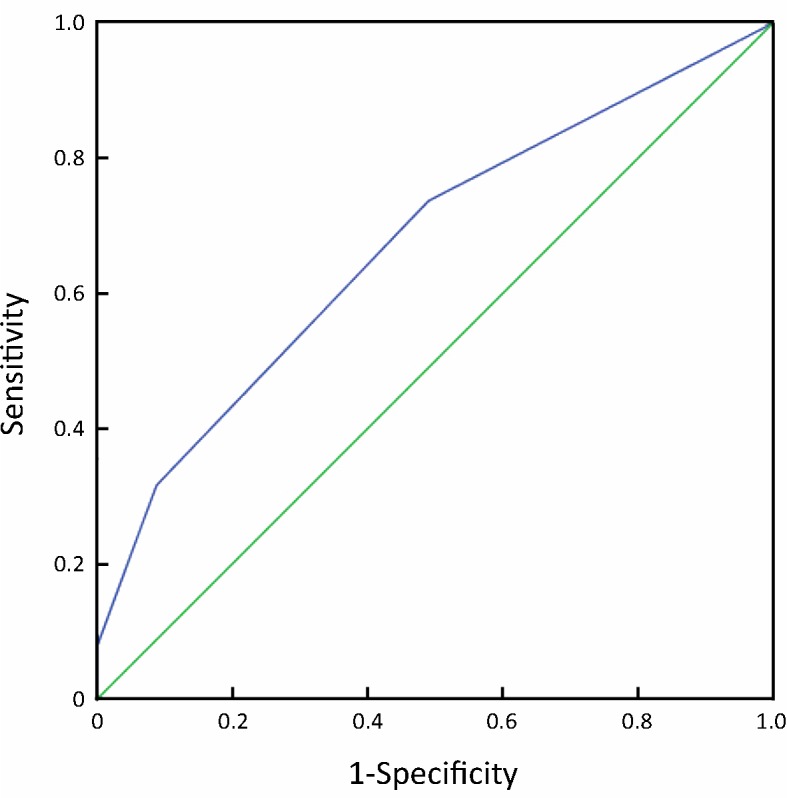
ROC for the prediction of intra-abdominal metastasis by the tumor occupying portions of stomach.
3.
Prediction of intra-abdominal metastasis by the depth of tumor invasion found by CT scans and the tumor-occupied portions of stomach (training set)
| Factors | Cut-off | Sensitivity (%) | Specificity (%) | YI |
| YI, Youden index. | ||||
| Tumor-occupied portions of stomach | 2 | 75.8 | 51.2 | 0.270 |
| Depth of tumor invasion on CT | 21 mm | 36.4 | 85.3 | 0.217 |
In the testing set, 55 patients (among 62 patients) with complete clinical data included 10 (18.2%) DL+ patients and 45 DL– patients. All these patients were classified according to their number of independent risk factors for a DL+ status: 27 had no risk factors, 22 had only one risk factor and 6 had two risk factors. When the patients were divided into groups by those with no risk factors and those with at least one risk factor, the sensitivity and positive predictive value (PPV) for detecting intra-abdominal metastasis were 90.0% and 32.1%, respectively. When the patients were divided into groups by those with no risk factors or only one risk factor and those with two risk factors, the sensitivity and PPV were 40.0% and 66.7%, respectively (Table 4).
4.
Indications for diagnostic laparoscopy based on the number of independent risk factors (testing set) (%)
| Predictive factors | Sensitivity | Specificity | PPV | NPV | Accuracy |
| PPV, positive predictive value; NPV, negative predictive value. | |||||
| 1–2 vs. 0 | 90.0 | 57.8 | 32.1 | 96.3 | 63.6 |
| 2 vs. 0–1 | 40.0 | 95.6 | 66.7 | 87.8 | 85.5 |
Discussion
The highest rates of gastric cancer in the world occur in Eastern Asia, and China accounts for approximately 40% of the worldwide gastric cancer burden. The modern treatment modality for gastric cancer involves comprehensive treatment guided by staging. Thus, the accuracy of clinical staging is crucial. In the present study, 20.5% of cM0 gastric cancer patients were identified to have peritoneal dissemination or free cancer cells by diagnostic laparoscopy. This finding suggests that pretherapeutic diagnostic laparoscopy with cytology examination should be strongly recommended for gastric cancer patients, especially those with related risk factors.
Sarela et al. (10) performed diagnostic laparoscopy on 657 patients with minimal symptoms and no definite M1 disease, and M1 disease was detected in 23% of patients with this method. In this study, only 65 patients were examined by high-quality spiral CT scans for staging. Although all the patients were examined by high-quality spiral CT scans in our study, the metastasis detection rate was not much different. Therefore, diagnostic laparoscopy cannot be replaced by high-quality imaging techniques. Kaiser et al. (11-13) also reported similar metastasis detection rates for resectable M0 gastric cancer patients (20.6%, 24.7% and 23.0%, respectively). It is widely accepted that peritoneal dissemination spreads from the tumor to the serosa, but in our 51 DL+ patients, we also found 2 cT2N0 patients who had free cancer cells, 3 cT3N0 patients who had free cancer cells and 2 cT3N0 patients who had visible peritoneal dissemination.
Compared with laparotomy, laparoscopy has various advantages. Tsuchida et al. (14) and Osorio et al. (13) reviewed patients who underwent diagnostic laparoscopy and laparotomy in the same period, respectively, and showed that there was no significant difference in the rate of intra-abdominal metastasis (45.2% vs. 35.5%, P=0.299; 20.6% vs. 20.8%, P=0.977). Hao et al. (15) demonstrated that the collection of peritoneal lavage fluid for cytology during laparoscopy has the same value as its collection during laparotomy. Osorio et al. (13) compared the results of patients who underwent either diagnostic laparoscopy or laparotomy and were not submitted to any additional surgical procedure due to intra-abdominal spread. The patients who underwent diagnostic laparoscopy had a lower morbidity (11.8% vs. 50.0%), lower mortality (0% vs. 16.7%), shorter hospital stay (6.3 d vs. 15.3 d), greater chance for recovery until fit for chemotherapy (64.7% vs. 33.3%) and longer survival (11.6 months vs. 4.8 months). Consequently, diagnostic laparoscopy and laparotomy exploration have similar contributions to the assessment of intra-abdominal metastasis and peritoneal lavage cytology. However, in diagnostic laparoscopy, the entire abdominal cavity can be magnified and easily accessed. Thus, it is easier to find any tiny peritoneal nodules that are located in the subphrenic space or pouch of Douglas’, areas that are difficult to evaluate even by laparotomy.
However, a low detection rate is a problem for diagnostic laparoscopy. From our present results, almost 80% of cases would be expected to have a negative examination. Researchers are trying to identify proper indications to narrow the potential candidates for diagnostic laparoscopy. Sarela et al. (10) reported that tumor location [gastroesophageal junction (GEJ) or whole stomach] and a lymphadenopathy of ≥1 cm were independent, significant risk factors for M1, and M1 was not detected in any patient with neither risk factor. Tsuchida et al. (14) reported that tumor location involving three stomach portions; macroscopic types 3, 4 or 5; and positive lymph node metastasis to all three factors are significantly correlated with either peritoneal metastasis or positive cytology. Our study also found similar risk factors: tumor-occupied ≥2 portions of stomach and depth of tumor invasion on CT ≥21 mm (measurements of stomach wall thickness were made when the stomach was well distended during the CT scan). Kurita et al. (16) also showed similar results in their study of 236 gastric cancer patients. In our testing set, only one M1 disease was detected in patients with neither risk factor. For the patients who had at least one of these two identified independent risk factors, the sensitivity, PPV and negative predictive value were 90.0%, 32.1% and 96.3%, respectively. According to these results, if we performed diagnostic laparoscopy with cytology examination for the selected patients according to the risk factors, the detection rate would be increased by up to 10%, and only approximately 2% of patients would be missed in preoperative diagnosis of intra-abdominal metastases. However, we continue to recommend diagnostic laparoscopy with cytology examination for all advanced gastric cancer patients.
Another critical problem is the lack of an accepted treatment protocol after diagnostic laparoscopy. We now have a multimodal therapy plan that includes neoadjuvant chemotherapy, neoadjuvant chemoradiotherapy and HIPEC. Therefore, the request for improvement in the accuracy of treatment has been met. In our study, we chose HIPEC and systemic chemotherapy to treat the patients with peritoneal dissemination or free cancer cells in the peritoneal lavage. Seven of the 10 patients showed a negative metastasis after this preoperative treatment and then underwent gastrectomy. Nakagawa et al. (17) reported that preoperative chemotherapy induced the downstaging of gastric cancer in 11 (61.1%) of the 18 patients in their study, with positive cytology from diagnostic laparoscopy. Several studies (17-19) used diagnostic laparoscopy as a staging procedure prior to considering patients for neoadjuvant chemotherapy or chemoradiation. The selection of patients, proper treatment regimens, combination treatments with or without intraperitoneal chemotherapy and the type of surgery after chemotherapy remain controversial. Cytoreductive surgery (CRS) and HIPEC are indicated for gastric cancer with peritoneal disease that is completely or significantly resectable (20). Staging laparoscopy is very useful in detecting unsuspected peritoneal seeding and in differentiating between localized peritoneal seeding and disseminated carcinomatosis. In Japan and Korea, CRS and HIPEC are used with palliative or curative intent and for prophylactic treatment in gastric cancer patients (21). However, the treatment of carcinomatosis from gastric cancer by peritonectomy and HIPEC has globally demonstrated worse long-term results than the treatment of peritoneal carcinomatosis from other cancers (22).
Conclusions
Our preliminary data suggest that diagnostic laparoscopy is necessary for the clinical diagnosis of gastric cancer patients. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis.
Acknowledgements
This work was supported by grants supporting the research program of early diagnosis, standardized treatment and therapy effect evaluation of gastric cancer (No. D141100000414004) from Beijing Ministry of Science and Technology.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res. 2016;28:1–11. doi: 10.3978/j.issn.1000-9604.2016.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–23. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Xu D, Bu Z. Chinese version of NCCN Clinical Practice Guidelines in Oncology officially authorized by NCCN. Chin J Cancer Res. 2016;28:144–5. doi: 10.3978/j.issn.1000-9604.2016.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi57-63.
- 6.Li Z, Ji J. Application of laparoscopy in the diagnosis and treatment of gastric cancer. Ann Transl Med. 2015;3:126. doi: 10.3978/j.issn.2305-5839.2015.03.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JW, Shin SS, Heo SH, et al. Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual. Eur Radiol. 2012;22:654–62. doi: 10.1007/s00330-011-2283-3. [DOI] [PubMed] [Google Scholar]
- 8.Park HS, Lee JM, Kim SH, et al. Three-dimensional MDCT for preoperative local staging of gastric cancer using gas and water distention methods: a retrospective cohort study. AJR Am J Roentgenol. 2010;195:1316–23. doi: 10.2214/AJR.10.4320. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010;25:512–8. doi: 10.1111/j.1440-1746.2009.06106.x. [DOI] [PubMed] [Google Scholar]
- 10.Sarela AI, Lefkowitz R, Brennan MF, et al. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg. 2006;191:134–8. doi: 10.1016/j.amjsurg.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser GM, Sotiropoulos GC, Frühauf NR, et al. Value of staging laparoscopy for multimodal therapy planning in esophago-gastric cancer. Int Surg. 2007;92:128–32. [PubMed] [Google Scholar]
- 12.Nath J, Moorthy K, Taniere P, et al. Peritoneal lavage cytology in patients with oesophagogastric adenocarcinoma. Br J Surg. 2008;95:721–6. doi: 10.1002/bjs.6107. [DOI] [PubMed] [Google Scholar]
- 13.Osorio J, Rodríguez-Santiago J, Muñoz E, et al. Outcome of unresected gastric cancer after laparoscopic diagnosis of peritoneal carcinomatosis. Clin Transl Oncol. 2008;10:294–7. doi: 10.1007/s12094-008-0200-2. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchida K, Yoshikawa T, Tsuburaya A, et al. Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J Surg. 2011;35:2703–9. doi: 10.1007/s00268-011-1290-5. [DOI] [PubMed] [Google Scholar]
- 15.Hao YX, Yu PW, Qian F. Changes of peritoneal free gastric cancer cells and its significance in patients after laparoscopic radical gastrectomy. Zhonghua Wai Ke Za Zhi (in Chinese) 2008;46:1784–9. [PubMed] [Google Scholar]
- 16.Kurita N, Shimada M, Utsunomiya T, et al. Predictive factors of peritoneal metastasis in gastric cancer. Hepatogastroenterology. 2010;57:980–3. [PubMed] [Google Scholar]
- 17.Nakagawa S, Nashimoto A, Yabusaki H. Role of staging laparoscopy with peritoneal lavage cytology in the treatment of locally advanced gastric cancer. Gastric Cancer. 2007;10:29–34. doi: 10.1007/s10120-006-0406-3. [DOI] [PubMed] [Google Scholar]
- 18.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol. 2008;15:2684–91. doi: 10.1245/s10434-008-0055-3. [DOI] [PubMed] [Google Scholar]
- 19.Power DG, Schattner MA, Gerdes H, et al. Endoscopic ultrasound can improve the selection for laparoscopy in patients with localized gastric cancer. J Am Coll Surg. 2009;208:173–8. doi: 10.1016/j.jamcollsurg.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Roviello F, Caruso S, Marrelli D, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol. 2011;20:e38–54. doi: 10.1016/j.suronc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219–28. doi: 10.1016/S1470-2045(04)01425-1. [DOI] [PubMed] [Google Scholar]
- 22.Stewart JH 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol 2005;12:765-77.