Abstract
Objective
No standard postoperative adjuvant chemotherapy has ever been established in node-positive esophageal squamous cell carcinoma (ESCC). This is a study to explore the effect of postoperative paclitaxel (PTX) and cisplatin (DDP) in lymph node-positive, completely resected thoracic ESCC patients.
Methods
We conducted a prospective phase II trial. Patients had pathologically node-positive thoracic ESCC with negative margins. Outcomes of disease-free survival (DFS) and overall survival (OS) were compared with a matched historical control cohort. The postoperative chemotherapy regimen consisted of 4 to 6 cycles of PTX 150 mg/m2 administered intravenously on d 1 followed by DDP 50 mg/m2 on d 2 every 14 d.
Results
Forty-three patients were accrued from December 2007 to May 2012 at Cancer Hospital of Chinese Academy of Medical Sciences for adjuvant chemotherapy. The historical control group consisted of 80 patients who received complete resection but no adjuvant chemotherapy during the same period of time. Of the 43 patients with adjuvant chemotherapy, 37 (86.0%) patients completed 4 to 6 cycles of chemotherapy. The 3-year DFS rates were 56.3% in the adjuvant group and 34.6% in the control group (P=0.006). The 3-year OS rates were 55.0% in the adjuvant group and 37.5% in the control group (P=0.013). Multivariate analysis revealed that postoperative chemotherapy was the significant predictor for improved OS (P=0.005).
Conclusions
Biweekly adjuvant PTX and DDP might improve 3-year DFS and OS in lymph node-positive, curatively resected thoracic ESCC patients. These conclusions warrant further study in randomized phase III clinical trials.
Keywords: Esophageal cancer, adjuvant chemotherapy, surgery, paclitaxel, cisplatin
Introduction
Esophageal cancer is a highly aggressive malignancy with a poor overall outcome. For locoregional disease, surgical resection remains the mainstay of treatment. Nevertheless, the clinical outcome of surgery alone was unsatisfactory because esophageal carcinoma is often far advanced at the time of diagnosis due to the late symptoms manifestation. The 5-year survival rate after radical esophagectomy is modest at about 40% (1). Unfortunately, the majority of patients experienced disease recurrence or progressed to distant metastases. The patients with regional lymph node metastases have worse outcome than those without lymph node metastases (2). Those node-positive patients have been thought to be a systemic disease (3). Many efforts have been made to explore more effective treatment, especially multi-modality in nature.
As of today, the use of adjuvant chemotherapy for this indication remains investigational. Esophageal squamous cell carcinoma (ESCC) is generally regarded as a different entity from the adenocarcinoma of esophagus or gastro-esophageal junction. The survival benefit of postoperative adjuvant chemotherapy has been demonstrated and widely accepted as a standard therapy in the adenocarcinoma of esophagus or gastro-esophageal junction (4,5). However, the role of adjuvant chemotherapy in ESCC patients remains undefined. There are a few randomized trials on ESCC patients evaluated postoperative adjuvant chemotherapy with vindesine or 5-fluorouracil (5-FU) and surgery compared with surgery alone (6-8); However, no improvement in survival with adjuvant chemotherapy in these patients.
Paclitaxel (PTX) is a newer agent compared to 5-FU and regarded as one of the most active agents in the treatment of esophageal cancer. Our previous study of biweekly PTX and cisplatin (DDP) regimen showed an encouraging overall response rate of 56.5% in recurrent or metastatic ESCC patients (9). We therefore designed a prospective study to evaluate the impacts on disease-free survival (DFS) and overall survival (OS) of postoperative adjuvant chemotherapy with a combination of PTX and DDP in patients with curative resected node-positive thoracic ESCC and compared it with the historical control group.
Materials and methods
Patients
This study was a prospective phase II trial. Eligibility criteria included: age ≥18 years, ability to give informed consent; histological proof of thoracic ESCC with negative proximal and distal margins (R0 resection); node-positive and pathologic stage M0; Eastern Cooperative Oncology Group (ECOG) performance status 0–1; and no prior chemotherapy or concurrent radiation therapy. Patients were enrolled 4 to 10 weeks after surgery. Before protocol entry, patients needed to be accessed by computed tomography (CT) of the chest and abdomen to make sure that there was no evidence of tumor recurrence. Adequate organ function was required in 2 weeks of registration and was defined as: white blood cell account ≥3.5×109/L, absolute neutrophil account ≥1.5×109/L, platelet ≥100×109/L, hemoglobin ≥10 g/L, serum creatinine within normal institutional limit, creatinine clearance ≥60 mL/min, and aspartate aminotransferase (AST) and bilirubin <2 times of upper normal institutional limits. Patients were also required to be absence of clinically significant hearing loss or symptomatic peripheral neuropathy during initial examination. The study was approved by the Ethic Committee of Cancer Hospital, Chinese Academy of Medical Sciences (CAMS). All patients provided written informed consent before participation in the study. This study was registered with ClinicalTrials.Gov as NCT02133612.
Treatment
The adjuvant chemotherapy regimen consisted of PTX 150 mg/m2 intravenously over 3 h on d 1, followed by DDP 50 mg/m2 intravenously on d 2 every 14 d for 4 to 6 cycles. The first cycle was administrated 4 to 10 weeks after surgery. Appropriate use of anti-emetics and hydration was recommended in our protocol.
Dose modification and follow-up
Toxicities were accessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. In addition, absolute neutrophil account ≥1.0×109/L, platelets ≥100×109/L, and normal liver and renal function were necessary for the administration of next cycle of chemotherapy.
Dose modification based on grade 4 hematologic toxicities of previous cycle was allowed. The protocol allowed the dose of PTX to be reduced by 10 mg/m2 and a reduction of DDP dose by 20%. PTX would be terminated if grade 3 sensory or motor neuropathy was developed. Chemotherapy would be withheld until toxicity resolved to eligible criteria. Chemotherapy was terminated early in patients who had tumor recurrence, developed intolerable adverse effects, wished to stop, or had not recovered to eligible criteria when chemotherapy had been withheld for over 14 d. Patients were followed every 3 months after the final cycle of treatment for 2 years, then every 6 months afterwards. Tumor recurrence was defined as biopsy-proven recurrence or definite evidence of progression by CT.
Matched historical controls
Records of patients who underwent complete esophagectomy with the same surgical approach but did not accept the postoperative chemotherapy from Dec 2007 to May 2012 were extracted from database of Cancer Hospital, CAMS. Then four strata were defined by gender and the following age categories (at the start of enrollment): <55 years old and ≥55 years old; control subjects were randomly drawn from each stratum to achieve a 2:1 ratio for control subjects to chemotherapy case subjects. Therefore, 40 chemotherapy cases and 80 controls were included in the final analysis. All the patients who received esophagectomy in Cancer Hospital, CAMS were asked to follow-up every 3 months after the final cycle of treatment for 2 years, then every 6 months afterwards.
Statistical analysis
The primary endpoint of our study was 3-year OS and the secondary endpoint was DFS. DFS was defined as the time from the date of surgery to the date of first evidence of recurrence or death of any cause. OS was defined as the time from the date of surgery to death or the date of last follow-up. The 3-year OS was calculated by the Kaplan-Meier method, and groups were compared with the Log-rank test. A two-tailed P<0.05 was considered statistically significant. Pearson χ2 and the Fisher’s exact test were used for comparison of categorical variables. Multivariate Cox regression analyses were performed to identify prognostic factors for recurrence and survival. All statistical analyses were carried out by SPSS software (Version 17.0; SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Forty-three patients were accrued at the Cancer Hospital, CAMS from December 2007 to May 2012. One patient died on d 2 of cycle 1 of upper digestive hemorrhage, and two patients refused the first cycle of the treatment with DDP after grade 2 allergic reaction occurred on d 1 during PTX infusion. This analysis was performed on February 17, 2016, and based on 40 patients who completed at least one cycle of postoperative chemotherapy. The clinical characteristics of the enrolled patients are shown inTable 1. The historical control group consisted of 80 patients. All patients in both arms had ESCC and pathologically confirmed regional lymph node metastases. Both groups had similar pathologic characteristics in tumor status, location, and differentiation. The median follow-up for living patients was 75.2 months for the adjuvant chemotherapy group and 77.3 months for the control group, respectively.
1.
Characteristics of the eligible patients
| Characteristics | n (%) | P | |
| Surgery + chemotherapy (N=40) | Surgery alone (N=80) | ||
| ECOG, Eastern Cooperative Oncology Group; LVI, lymphvascular invasion. | |||
| Age (year) | 0.698 | ||
| <55 | 20 (50.0) | 37 (46.3) | |
| ≥55 | 20 (50.0) | 43 (53.8) | |
| Gender | 1.000 | ||
| Male:Female | 36:4 | 72:8 | |
| Performance status | 1.000 | ||
| ECOG 0–1 | 40 (100) | 80 (100) | |
| Location | 0.685 | ||
| Upper | 3 (7.5) | 3 (3.8) | |
| Middle | 15 (37.5) | 32 (40.0) | |
| Lower | 22 (55.0) | 45 (56.2) | |
| T status | 1.000 | ||
| T1 | 2 (5.0) | 4 (5.0) | |
| T2 | 3 (7.5) | 7 (8.8) | |
| T3 | 35 (87.5) | 69 (86.2) | |
| N category | 0.558 | ||
| N1 | 18 (45.0) | 44 (55.0) | |
| N2 | 18 (45.0) | 28 (35.0) | |
| N3 | 4 (10.0) | 8 (10.0) | |
| LVI | 16 (40.0) | 19 (23.8) | 0.065 |
| Tumor stage | 0.773 | ||
| IIB | 1 (2.5) | 5 (6.3) | |
| IIIA | 21 (52.5) | 45 (56.2) | |
| IIIB | 14 (35.0) | 22 (27.5) | |
| IIIC | 4 (10.0) | 8 (10.0) | |
| Differentiation | 0.302 | ||
| Well | 4 (10.0) | 17 (21.3) | |
| Moderate | 22 (55.0) | 37 (46.2) | |
| Poor | 14 (35.0) | 26 (32.5) | |
| Lymphadenectomy | 0.685 | ||
| Two-field | 37 (92.5) | 76 (95.0) | |
| Three-field | 3 (7.5) | 4 (5.0) | |
Treatment summary
Thirty-seven patients (86%) completed 4 to 6 cycles of chemotherapy. The median number of cycles administered was 5.Table 2 provides the grade 3/4 toxicities. Of the total 43 patients, 32 (74%) developed grade 3 or 4 toxicity with leucopenia or neutropenia (31 patients), and nausea/vomiting (7 patients), and diarrhea (3 patients) as the most common toxicities. One patient died on d 2 of cycle 1 of upper digestive hemorrhage.
2.
Adverse events graded according to National Cancer Institute CTCAE version 3.0 (N=43)
| Adverse events* | n (%) | |
| Grade 3 | Grade 4 | |
| CTCAE, Common Terminology Criteria for Adverse Events; *, One patient died on d 2 of cycle 1 of upper digestive hemo-rrhage. | ||
| Leukopenia/neutropenia | 22 (51.2) | 9 (20.9) |
| Anemia | 1 (2.3) | 0 |
| Thrombocytopenia | 0 | 0 |
| Neurologic | 0 | 0 |
| Metabolic | 0 | 0 |
| Nausea/vomiting | 7 (16.3) | 0 |
| Arthralgia/myalgia | 0 | 0 |
| Anorexia | 2 (4.7) | 0 |
| Infection | 1 (2.3) | 0 |
| Febrile neutropenia | 1 (2.3) | 0 |
| Diarrhea | 3 (7.0) | 0 |
| Weight loss | 0 | 0 |
| Fatigue | 1 (2.3) | 0 |
| Allergic reaction | 0 | 0 |
DFS and OS
At last follow-up, 20 of 40 patients in the adjuvant group have died. Of these 20 patients, 17 patients died of causes related to ESCC recurrence or metastasis, 1 patient died of pneumonia, 1 patient died of esophageal fistula without evidence of tumor recurrence, and 1 patient died of suicide because of esophageal stricture. One patient in the adjuvant group is alive with disease at last follow-up and received chemotherapy and targeted therapy. The other 19 patients in the adjuvant group were alive without evidence of disease. In the control group, 58 patients eventually relapsed. Sites of first failure in 76 patients are shown inTable 3. Distant metastasis was predominant cause as well as local recurrence in both groups.
3.
Patterns of failure
| Site of first recurrence | n (%) | |
| Adjuvant (N=18) | Control (N=58) | |
| Locoregional lymph node | 6 (33.3) | 24 (41.4) |
| Distant | 3 (16.7) | 19 (32.8) |
| Both local and distant | 8 (44.4) | 13 (22.4) |
| Unknown | 1 (5.6) | 2 (3.4) |
| Distant total | 11 (61.1) | 32 (55.2) |
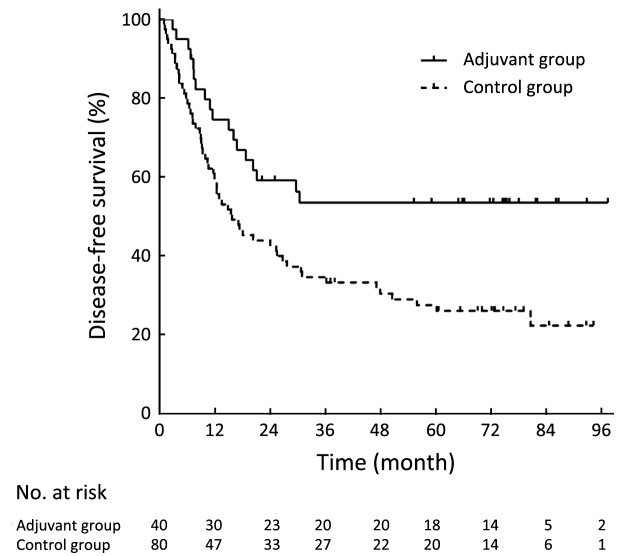
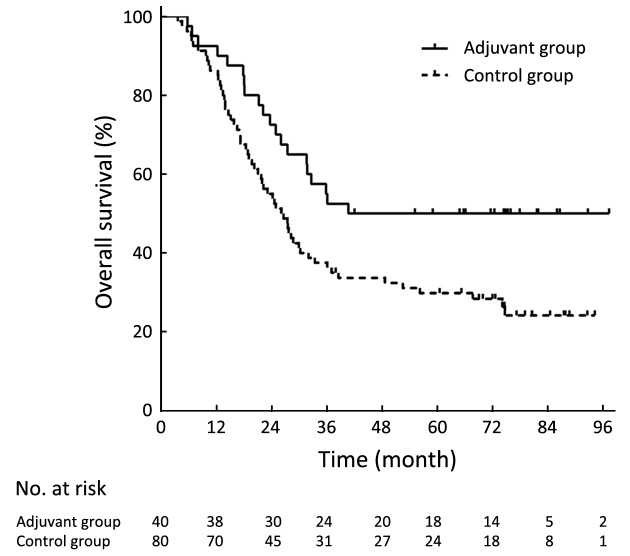
The DFS was significantly longer in the adjuvant group than in the control group (P=0.006,Figure 1). The 3-year DFS rates were 56.3% in the adjuvant group and 34.6% in the control group. The 5-year DFS rates were 56.3% in the adjuvant group and 27.5% in the control group. The OS was significantly longer in the adjuvant group than in the control group (P=0.013,Figure 2). The 3-year OS rates were 55.0% in the adjuvant group and 37.5% in the control group. The 5-year OS rates were 50.0% in the adjuvant group and 29.8% in the control group.
1.
Kaplan-Meier curves of disease-free survival of the adjuvant group and the control group.
2.
Kaplan-Meier curves of overall survival of the adjuvant group and the control group.
The significant predictive factor for tumor recurrence on multivariate Cox analysis was adjuvant chemotherapy (P=0.004). Age, T status, and N category were not influential on the DFS in the analysis. In the multivariate Cox analysis for survival, involvement of less than 3 locoregional lymph nodes (P=0.008) and adjuvant chemotherapy (P=0.005) were significantly associated with improved survival (Table 4). Age, T status and N category were again not influential on the OS.
4.
Multivariate analysis of prognostic factors for survival and tumor recurrence (N=120)
| Variables | Survival | Recurrence | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| 95% CI, 95% confidence interval; HR, hazard ratio. | |||||
| Age (years) | |||||
| <55 | 1 | 1 | |||
| ≥55 | 1.339 (0.853–2.102) | 0.205 | 0.926 (0.585–1.467) | 0.743 | |
| T status | |||||
| T1+T2 | 1 | 1 | |||
| T3 | 1.577 (0.769–3.234) | 0.214 | 1.101 (0.543–2.230) | 0.790 | |
| N category | |||||
| N1 | 1 | 1 | |||
| N2+N3 | 1.866 (1.177–2.960) | 0.008 | 1.482 (0.922–2.384) | 0.105 | |
| Differentiation | |||||
| Well | 1 | 1 | |||
| Moderate | 1.252 (0.671–2.336) | 0.479 | 1.045 (0.566–1.929) | 0.889 | |
| Poor | 1.287 (0.673–2.464) | 0.446 | 1.075 (0.555–2.083) | 0.831 | |
| Adjuvant chemotherapy | |||||
| No | 1 | 1 | |||
| Yes | 0.479 (0.285–0.803) | 0.005 | 0.448 (0.260–0.773) | 0.004 | |
Discussion
We conducted a prospective phase II trial to assess the effect of adjuvant PTX and DDP on the survival of patients with R0 resected, node-positive ESCC. Our results revealed a statistically significant benefit compared with historical controls who underwent similar R0 resection. To our knowledge, this is the first trial to show survival benefit of postoperative chemotherapy compared with historical controls in the same time period. In general, the majority of patients was able to tolerate 4 to 6 cycles of chemotherapy and might be recommended as adjuvant chemotherapy regimen in the future.
It remains unclear whether perioperative chemotherapy has a positive impact on OS in ESCC patients treated with radical esophagectomy. To our knowledge, there are four large randomized trials that have provided information on perioperative adjuvant chemotherapy and survival (10-13). One of these trials showed an increased OS by additional perioperative chemotherapy, whereas the three other studies showed no such conclusion. Medical Research Council Oesophageal Cancer Working Group (MRC) randomly allocated 802 resectable esophageal cancer patients into the neoadjuvant group and the surgery alone group, and found two cycles of neoadjuvant regimen of DDP and 5-FU every 21 d plus surgery improved median OS compared with surgery alone, regardless of ESCC or adenocarcinoma (10,11). In contrast, the INT0113 trial revealed that there was no difference in OS for patients receiving perioperative chemotherapy compared with the surgery only group (12,13). The conflicting results between these two trials might be due to the difference with respect to tolerance of chemotherapy and inter-treatment interval among the study subjects. Only 71% patients finished all 3 preoperative cycles, and 38% of the patients who were stable or responsive to chemotherapy finished 2 cycles of postoperative chemotherapy according to the schedule, which was much lower than 90% of the MRC trial. Moreover, 9 patients died of chemotherapy-related adverse events in the INT0113 trial, which might be responsible for its negative results. Furthermore, the chemotherapy interval of the INT0113 trial was 7 d longer than that of the MRC trial. The other two trials revealed no significant improvement in 5-year OS with 2 cycles of DDP and vindesine or 5-FU as adjuvant regimen (6-8). While, several retrospective studies have demonstrated that postoperative adjuvant chemotherapy with DDP plus 5-FU or vindesine or taxane improves survival for certain small fraction of ESCC patients (14-16).
We believe there may be two reasons which could potentially explain better prognosis with adjuvant chemotherapy after radical surgery. First, we chose the optimal chemotherapeutic agents with the biweekly combination of PTX and DDP. PTX was a newer drug which had been proven to be effective in ESCC. However, the most majority of previous studies used 5-FU and DDP as adjuvant regimens which were suboptimal.
Second, the improvement of survival was possibly related to the adequate dosage and longer duration of chemotherapy delivered in present study. The median number of chemotherapy received in our study was 5 cycles. Previous prospective trials on ESCC adjuvant chemotherapy used only 2 to 3 chemotherapy cycles, which is suboptimal duration of chemotherapy, and were challenged by inadequate dosage of chemotherapy (6-8).
In present study, the adjuvant chemotherapy was generally well tolerated by most patients with an acceptable toxicity profile. Although 32 (74%) patients developed grade 3 to 4 adverse events with leukopenia/neutropenia being the most common (72.1%), most of these toxicities were manageable, and only one patient experienced febrile neutropenia during treatment.
The study has a major limitation. It is a non-randomized study and conducted in one single center. Moreover, the limited number of patients reduced the reliability of our results. The non-uniform follow-up intensity between the adjuvant chemotherapy group and the control group may weaken the effect of adjuvant chemotherapy considering a lower frequency of follow-up in the control group. Thus, it was difficult to draw a definite conclusion about the optimal treatment based on the retrospective nature of our study, and a prospective phase III randomized clinical trial in lymph node-positive resected ESCC to compare the effects of different adjuvant therapies with surgery alone to further validate these findings is warranted.
Conclusions
Postoperative adjuvant chemotherapy with the biweekly combination of PTX and DDP was feasible and might improve 3-year DFS and OS rate compared with surgery alone. The findings of our study should be validated by further randomized controlled trials.
Acknowledgements
This study was partially supported by the grant from Beijing Medical Award Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Peyre CG, Hagen JA, DeMeester SR, , et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. <DOI: 10.1097/SLA.0b013e318188c474> <PMID: 18936567>] [DOI] [PubMed] [Google Scholar]
- Xiao ZF, Yang ZY, Miao YJ, , et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys. 2005;62:82–90. doi: 10.1016/j.ijrobp.2004.08.046. [Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82-90. <DOI: 10.1016/j.ijrobp.2004.08.046> <PMID: 15850906>] [DOI] [PubMed] [Google Scholar]
- Heroor A, Fujita H, Sueyoshi S, , et al. Adjuvant chemotherapy after radical resection of squamous cell carcinoma in the thoracic esophagus: who benefits? A retrospective study. Dig Surg. 2003;20:229–35. doi: 10.1159/000070390. [Heroor A, Fujita H, Sueyoshi S, et al. Adjuvant chemotherapy after radical resection of squamous cell carcinoma in the thoracic esophagus: who benefits? A retrospective study. Dig Surg 2003;20:229-35. <DOI: 70390> <PMID: 12759503>] [DOI] [PubMed] [Google Scholar]
- Sakuramoto S, Sasako M, Yamaguchi T, , et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. <DOI: 10.1056/NEJMoa072252> <PMID: 17978289>] [DOI] [PubMed] [Google Scholar]
- Bang YJ, Kim YW, Yang HK, , et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. doi: 10.1016/S0140-6736(11)61873-4. [Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. <DOI: 10.1016/S0140-6736(11)61873-4> <PMID: 22226517>] [DOI] [PubMed] [Google Scholar]
- Ando N, Iizuka T, Ide H, , et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study — JCOG9204. J Clin Oncol. 2003;21:4592–6. doi: 10.1200/JCO.2003.12.095. [Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study——JCOG9204. J Clin Oncol 2003;21:4592-6. <DOI: 10.1200/JCO.2003.12.095> <PMID: 14673047>] [DOI] [PubMed] [Google Scholar]
- Ando N, Iizuka T, Kakegawa T, , et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg. 1997;114:205–9. doi: 10.1016/S0022-5223(97)70146-6. [Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg 1997;114:205-9. <DOI: 10.1016/S0022-5223(97)70146-6> <PMID: 9270637>] [DOI] [PubMed] [Google Scholar]
- Lee J, Lee KE, Im YH, , et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Ann Thorac Surg. 2005;80:1170–5. doi: 10.1016/j.athoracsur.2005.03.058. [Lee J, Lee KE, Im YH, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2005;80:1170-5. <DOI: 10.1016/j.athoracsur.2005.03.058> <PMID: 16181835>] [DOI] [PubMed] [Google Scholar]
- Huang J, Zhou Y, Zhang H, , et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol. 2013;30:343. doi: 10.1007/s12032-012-0343-4. [Huang J, Zhou Y, Zhang H, et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol 2013;30:343. <DOI: 10.1007/s12032-012-0343-4> <PMID: 23263828>] [DOI] [PubMed] [Google Scholar]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–33. doi: 10.1016/S0140-6736(02)08651-8. [Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. <DOI: 10.1016/S0140-6736(02)08651-8> <PMID: 12049861>] [DOI] [PubMed] [Google Scholar]
- Allum WH, Stenning SP, Bancewicz J, , et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. doi: 10.1200/JCO.2009.22.2083. [Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. <DOI:10.1200/JCO.2009.22.2083> <PMID: 19770374>] [DOI] [PubMed] [Google Scholar]
- Kelsen DP, Ginsberg R, Pajak TF, , et al. Chemo-therapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–84. doi: 10.1056/NEJM199812313392704. [Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. <DOI: 10.1056/NEJM199812313392704> <PMID: 9869669>] [DOI] [PubMed] [Google Scholar]
- Kelsen DP, Winter KA, Gunderson LL, , et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–25. doi: 10.1200/JCO.2006.10.4760. [Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol 2007;25:3719-25. <DOI: 10.1200/JCO.2006.10.4760> <PMID: 17704421>] [DOI] [PubMed] [Google Scholar]
- Heroor A, Fujita H, Sueyoshi S, , et al. Adjuvant chemotherapy after radical resection of squamous cell carcinoma in the thoracic esophagus: who benefits? A retrospective study. Dig Surg. 2003;20:229–35. doi: 10.1159/000070390. [Heroor A, Fujita H, Sueyoshi S, et al. Adjuvant chemotherapy after radical resection of squamous cell carcinoma in the thoracic esophagus: who benefits? A retrospective study. Dig Surg 2003;20:229-35. <DOI: 70390> <PMID: 12759503>] [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang YW, Chen ZW, , et al. Adjuvant chemotherapy of cisplatin, 5-fluorouracil and leucovorin for complete resectable esophageal cancer: a case-matched cohort study in east China. Dis Esophagus. 2008;21:207–13. doi: 10.1111/j.1442-2050.2007.00748.x. [Zhang J, Zhang YW, Chen ZW, et al. Adjuvant chemotherapy of cisplatin, 5-fluorouracil and leucovorin for complete resectable esophageal cancer: a case-matched cohort study in east China. Dis Esophagus 2008;21:207-13. <DOI: 10.1111/j.1442-2050.2007.00748.x> <PMID: 18430100>] [DOI] [PubMed] [Google Scholar]
- Lyu X, Huang J, Mao Y, , et al. Adjuvant chemo-therapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol. 2014;110:864–8. doi: 10.1002/jso.23716. [Lyu X, Huang J, Mao Y, et al. Adjuvant chemotherapy after esophagectomy: is there a role in the treatment of the lymph node positive thoracic esophageal squamous cell carcinoma? J Surg Oncol 2014;110:864-8. <DOI: 10.1002/jso.23716> <PMID: 24976079>] [DOI] [PubMed] [Google Scholar]