Abstract
Introduction
The National Lipid Association Statin Intolerance (SI) Panel recognized the need for better understanding of the patient SI experience.
Objective
The objective of this research was to develop a patient-reported outcome (PRO) questionnaire to assess a patient’s experience with SI.
Methods
Questionnaire development was informed via a series of research activities: literature review, concept elicitation, item generation, and content evaluation. Following the literature review and concept elicitation, a draft questionnaire was constructed and subsequently modified based on feedback from therapeutic area experts and patients via cognitive debriefing interviews.
Results
Muscle-related symptoms were the most commonly reported symptoms associated with SI in the literature review (35 of 41 articles reviewed [85%]) and in semi-structured interviews with experts (n = 5 [100%]) and patients (n = 17 of 20 [85.0%]). Physical and other impacts of SI symptoms on daily activities were also frequently reported. A 17-item draft questionnaire was created, and cognitive debriefing with experts (n = 5) and patients (n = 15) was conducted. Overall, the items, response options, and instructions were comprehensible and positively reviewed; minor changes resulted in the 15-item Statin Experience Assessment Questionnaire (SEAQ)©. Using a 30-day recall period, the SEAQ© assesses the severity and impact of six SI symptoms (muscle ache, muscle pain, muscle cramps, muscle weakness, tiredness, and joint pain) on an 11-point numeric scale. Statin discontinuation and likelihood of discontinuation due to symptoms are assessed and scored on a yes/no and five-point verbal response scale, respectively.
Conclusion
The SEAQ© is a novel content-valid PRO questionnaire that assesses patient SI experience and fosters dialogue about SI between patients and providers.
Electronic supplementary material
The online version of this article (doi:10.1007/s40271-016-0211-y) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Statins are widely prescribed and generally well tolerated for the treatment of hypercholesterolemia. However, statin intolerance has an estimated prevalence of up to 25% in the population. |
| The National Lipid Association Statin Intolerance Panel has emphasized statin intolerance as best understood from a patient-centric perspective. Furthermore, better communication between patients and providers regarding statin intolerance is needed. No validated tools that assess the patient experience with statin use currently exist. |
| This article describes the development of the content-valid Statin Experience Assessment Questionnaire© with the goal of identifying statin intolerance in clinical settings and fostering communication about statin intolerance between patients and providers. The use of this questionnaire will allow shared learning between patients and providers in identifying and evaluating statin intolerance. |
Introduction
Hypercholesterolemia is a major risk factor for cardiovascular disease (CVD), which accounts for nearly one in three deaths in the USA [1]. The estimated annual costs associated with CVD in the USA are more than $US320 billion (approximately $US196 billion in direct costs and $US124 billion in indirect costs). Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors, are recommended by current cholesterol management guidelines as the initial drug treatment of choice for individuals with elevated cholesterol levels and/or cardiovascular risk for whom lifestyle change alone is insufficient [2–6].
Despite the general tolerability and widespread use of statins, they can be associated with untoward side effects, including muscle symptoms, gastrointestinal symptoms, headache, and liver abnormalities [7, 8]. Statin intolerance (SI) can be associated with some of these side effects. It is estimated from randomized controlled trials, patient surveys, and retrospective studies that muscle-related symptoms occur in 5–25% of patients being treated with statins [9–15]. Furthermore, primarily as a result of muscle-related symptoms, SI is a major cause of nonadherence, dose reduction, and discontinuation of statins [16–18] and may contribute to the high cost of CVD and affect clinical outcomes [19]. Thus, there is a need for a better understanding of the patient with SI [20].
In 2014, the National Lipid Association (NLA) SI Panel recognized this need for a better understanding of the SI experience, best defined in the context of a patient-centric perspective, as well as the need for appropriately developed SI scales that can assess the phenomenon and bothersome impacts of SI directly from the perspective of the patient [17]. The viewpoint is consistent with the American College of Cardiology (ACC)/American Heart Association (AHA) Task Force on Performance Measures, which emphasized the importance of “shared accountability” among patients and providers and the impact this could have on treatment decisions and ultimately clinical outcomes [21]. Therefore, tools are needed to assess SI from the patient perspective, but—according to current literature—no such suitable measures exist. The objective of the current research activities was to develop a patient-reported outcome (PRO) questionnaire for use in real-world settings to evaluate patient-centric attributes of SI. The tool is intended to help foster communication between patients and providers and to construct a means by which the complaints about and patient burden of SI may be quantified. In addition, the questionnaire should aid in a better understanding and documentation of the experiences of patients with SI and allow the systematic collection of patient-centric data in research settings.
Methods
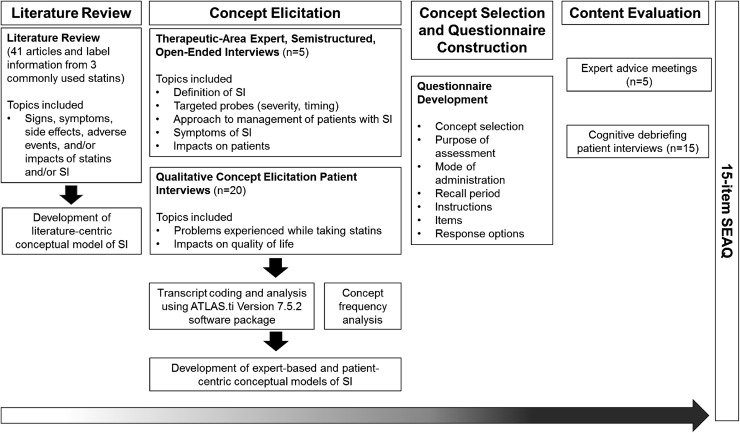
To identify and describe the symptoms and impacts that characterize SI, an empirical literature review of SI, advice meetings with SI experts, and interviews with patients who are statin intolerant were conducted. Results from these activities informed the selection of concepts for measurement and the construction of the PRO questionnaire. The questionnaire content was then assessed among clinical experts and patients with SI (Fig. 1).
Fig. 1.
Overall methodology schematic. SEAQ© Statin Experience Assessment Questionnaire, SI statin intolerance
Literature Review
Peer-reviewed literature regarding SI was limited to English-language studies published between 2006 and 4 November 2014. The search was conducted in MEDLINE, Embase, and PsycINFO using the OvidSP platform. Search queries included statin intolerance and these related terms: statin-intolerance, statin-induced, and statin therapy. Additionally, terms for signs, symptoms, side effects, impacts, and adverse events were used to narrow search results. The search was supplemented with articles identified by the members of the research team or manual review of reference lists that were not captured in the initial search. Relevant articles with a primary focus on signs, symptoms, side effects, adverse events, and/or impacts of statins and/or SI were identified and retrieved. Articles were excluded if they (1) primarily focused on the pathogenesis, genetics, or molecular biology of SI without discussion of signs, symptoms, side effects, adverse events, and/or impacts of statins and/or SI; (2) solely discussed statins used in combination with other non-statin drugs; or (3) solely discussed case studies of three or fewer patients. Key concepts associated with SI were extracted and informed the development of an SI conceptual model as well as documentation describing those concepts and justifying their relevance to the condition. The ‘Warnings and Precautions’ and ‘Adverse Reactions’ sections of the product labels for three commonly used drugs for the treatment of hypercholesterolemia, that is, atorvastatin (Lipitor), ezetimibe/simvastatin (Vytorin), and ezetimibe (Zetia), were reviewed for side effects. The targeted literature review was conducted early in the research process to document any untoward effects of lipid-modifying therapies assumed to be effective. All potentially effective lipid-modifying therapies were included in an effort to understand and document any similarities among or differences between therapies.
Concept Elicitation
Patient Interviews
Patients were recruited by providers who were specialists in internal medicine (n = 3), cardiology (n = 2), or primary care (n = 2). Individual concept-elicitation interviews (CEIs) were conducted using a semi-structured interview guide to explore the concepts relevant and important to the patients who experienced SI. The guide served as a map rather than a verbatim script to be referenced during the interviews to capture complete and meaningful information related to the research questions while maintaining spontaneity. Patients were asked a series of open-ended questions about their experience with statins and associated impacts on quality of life. Open-ended follow-up questions were used to clarify concepts (e.g., concept severity, frequency, and duration) and to encourage elicitation of all pertinent concepts associated with SI. This method of inquiry avoids the use of leading questions that may bias patients’ responses and allows the experience to be described in the patient’s own language. Interviewers were trained in good interviewing practices, National Institutes of Health Participant Protection, sponsor adverse event reporting, and internal data protection. Mock interviews were conducted with the interviewers to ensure good interviewing practices and to identify any issues. The final sample size was justified by achievement of saturation (i.e., the stage in the data assembly process when no new relevant information is obtained) [22–25]. Patients aged ≥18 years, having primary hypercholesterolemia (heterozygous familial hypercholesterolemia [FH] or non-FH) with moderate, high, or very high cardiovascular risk, and having the inability to tolerate one or more statin (i.e., modified statin dose level or frequency, recommended new statin, or discontinued statin) because of objectionable symptoms (real or perceived) within the 3 months prior to screening were included in the study. FH diagnosis and cardiovascular risk level were identified by the provider via a yes/no response and check box option, respectively. Patients with a diagnosis of fibromyalgia or a history of the following conditions were excluded from the study: severe neuropathic pain; rheumatological disease associated with symptoms that may be confounded by symptoms of SI (e.g., rheumatoid arthritis); myalgia or myopathy that began or increased during treatment with lipid-modifying therapy other than statin therapy and stopped when the lipid-modifying therapy was discontinued; myopathy, other than statin-associated myopathy; rhabdomyolysis (defined as evidence of organ damage with creatine kinase >10,000 IU/l); or homozygous FH. Interviews lasted approximately 1 h and were audio-recorded, transcribed verbatim by an independent transcription company, and analyzed as described subsequently. Before the study was conducted, all study documents were submitted to an ethics review board for consideration, and approval to execute the interviews was received from Copernicus Group Independent Review Board (CGIRB) on 3 February 2015.
Expert Advice Meetings
Individual expert advice meetings were conducted with physicians and nurse practitioners who were experienced with treating patients with SI and who were identified from peer-reviewed publications in cardiovascular and SI research. A semi-structured open-ended expert advice meeting guide was developed to reference during the interviews conducted between 1 December 2014 and 5 January 2015. As with the patient interviews, interviews were conducted by trained interviewers, and the guide served as a map rather than a verbatim script to encourage the spontaneous collection of relevant and complete information related to the research questions. Each expert was asked to define SI in his or her own words and given the opportunity to spontaneously describe the concepts that characterize SI. Each meeting lasted approximately 1 h and was audio-recorded, transcribed verbatim by an independent transcription company, and analyzed.
Concept Selection and Questionnaire Construction
The goals of the concept selection and questionnaire construction were to evaluate and select the primary SI-associated concepts for measurement based on the results from the literature review and concept-elicitation activities and to subsequently construct the first draft of the Statin Experience Assessment Questionnaire (SEAQ)©, including specification of the mode of administration and recall period and development of the questionnaire instructions, items, and response options. The SEAQ© draft was initiated during a concept-selection and item-generation meeting of the questionnaire developers and subsequently finalized upon consensus. Informed by the harmonized results of the literature search, expert advice meetings, and patient interviews, concepts that best characterized the relevant symptom and impacts of SI were selected as measurement targets for the SEAQ©. The SEAQ© was developed after concept selection with the following aspects defined based on relevant information gathered from the concept-elicitation activities, as well as measurement-development best practices: purpose of assessment, mode of administration, recall period, instructions, items, and response options.
Content Evaluation
The purpose of the content evaluation was to assess the content of the SEAQ© among clinical experts and patients with SI in ways that were acceptable to patients and could generate results that were useful to both patients and providers. To achieve this goal, one-on-one meetings with experts (n = 5) were conducted to evaluate the relevance, comprehensiveness, and suitability of the SEAQ© and to modify the questionnaire, as necessary, based on these results. Next, cognitive debriefing interviews (CDIs) with 15 patients with SI were conducted to evaluate the SEAQ© with respect to their ability to read, understand, and complete the questionnaire (note that these were different patients from those who participated in the patient CEIs). Results from each of these activities informed subsequent modifications to the SEAQ©, ultimately leading to the final tool.
During the expert advice meetings (conducted between 17 April 2015 and 1 May 2015), experts were provided the draft SEAQ© and asked open-ended questions to explore the main research questions using a semi-structured expert advice meeting guide. Verbatim interview transcriptions were coded and analyzed. Interim modifications to the SEAQ© based on expert feedback were made before the debriefing interviews with patients.
Individual patient CDIs were conducted following interim modifications using a semi-structured cognitive debriefing interview guide. Patients were included in this phase of the study if they met the inclusion criteria described previously for the concept-elicitation phase of the study. Interviews were audio-recorded, transcribed verbatim, and analyzed. After feedback from expert advice meetings and patient CDIs was received, the SEAQ© was modified to generate the final version. Before interviews, all study documents were submitted to an ethics review board for consideration. Approval to conduct the interviews was received from CGIRB on 8 April 2015.
Qualitative Data Analyses
Verbatim transcripts were entered into ATLAS.ti Version 7.5.2 to facilitate the organization and analysis of qualitative data [26]. Transcript text germane to the research questions was identified, highlighted, and matched to a code from a codebook that best characterized the data. The codebook, a comprehensive list of all of the codes used to characterize important segments of transcript text, was used as a guide to organize the content and meaning of actual subject language. Several researchers or coders performed the coding by identifying transcript text in which a subject expressed an SI-related experience (i.e., a concept). When the data could not be matched to an existing code, a new code was created. The semi-structured CEI guide served as the basis for the preliminary coding scheme, which was updated as the coders analyzed the transcripts and added or modified codes. Codes added or modified were shared with all coders to maintain consistency with the scheme. A senior member of the project team reviewed the coding of every transcript to minimize variability across coders and to ensure inter-coder reliability. After completion of coding, data were pooled and qualitatively analyzed for common themes.
To facilitate coding in the concept-elicitation phase, the composition of each individual code followed this basic structure: domain::root concept::descriptor. ‘Domain’ conveyed the overarching general concept into which a specific root concept fell (e.g., symptom or impact). ‘Root concept’ was the unique characteristic of SI described by the expert or patient that was unlike any other concept (e.g., muscle pain). The ‘descriptor’ was intended to characterize, clarify, or otherwise describe some aspect of the root concept (e.g., a descriptor code may offer detail such as severity, frequency, duration, or location). Concept frequency was determined by counting the number of unique experts or patients who mentioned the concept at least once during an interview.
For the content-evaluation phase, a codebook organized feedback on the questionnaire. Coding was performed as described previously for the CEIs. The preliminary coding scheme was based on the semi-structured expert advice meeting guide or patient interview guide and was updated as transcripts were analyzed. Responses were compared and aggregated around themes associated with particular segments of the questionnaire (i.e., instructions, items, response options, and recall period) and more general topics such as the utility of the measure and overall format. A semi-quantitative analysis entailed counting the number of unique expert responses captured under a theme. Content analysis of patient responses consisted of evaluating patient interpretation of the instructions, items, and response options against their intended meaning.
Results
Literature Review
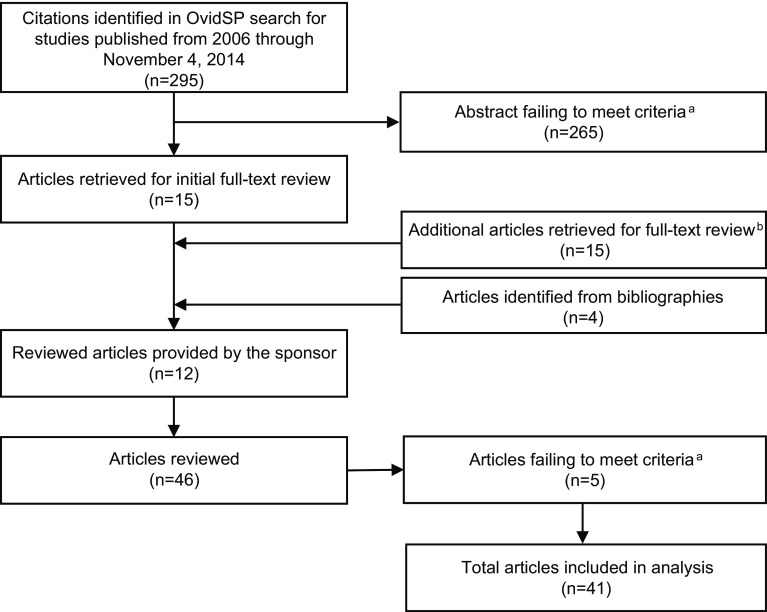
The literature review included 41 articles (19 review articles and 22 primary research articles) [9, 11, 15, 17, 27–63] (Fig. 2) and labels from three commonly prescribed lipid-modifying drugs [64–66]. Of the 41 articles reviewed, only four provided a working definition of SI [17, 51, 52, 63]. The literature described SI broadly as being any symptom significant enough to warrant a change in treatment. All three drug labels and 35 of the 41 articles reviewed reported muscle-related symptoms. SI concepts reported in the literature and labels included myalgia, myositis, myopathy, rhabdomyolysis, muscle atrophy, diminished muscle coordination, tendon pain/tendonitis, alopecia, renal impairment, liver enzyme abnormality, sleep disturbances, indigestion, headache, blisters, and nausea (Table 1 in the Electronic Supplementary Material [ESM]). The concepts identified in the literature review were organized into two conceptual models: one depicting SI (Fig. 1a in the ESM) and one depicting the side effects and adverse events associated with statins, as concepts specifically in the context of SI were often difficult to discern (Fig. 1b in the ESM). Conceptual models illustrate the relationship between a specified condition and its proximal to distal health outcomes [67]. These outcomes can be either unidimensional and have a direct relationship to the condition or multidimensional and indirectly relate to the condition. These models are not intended to be causal; arrows imply a dominant causal pathway, but a lack of arrows does not imply the absence of a relationship. The ultimate goal of this research was to generate a harmonized conceptual model incorporating the literature review and concept-elicitation activities.
Fig. 2.
Literature search flow diagram. aArticles were included if they focused on the signs, symptoms, side effects, adverse events, and/or impacts of statins, and/or statin intolerance and excluded if they focused on pathogenesis, genetics, or molecular biology of statin intolerance, or solely discussed statins used in combination with other drugs, in a non-adult population (i.e., aged <18 years), and/or in case studies. bFollowing review of statin intolerance–related articles, an additional 15 articles that primarily focused on the side effects or adverse events of statins were selected from the initial list of 295 abstracts for full-text review
Concept Elicitation
Five experts (four physicians and one nurse practitioner) from diverse geographical areas in the USA and with a reported patient treatment experience of 19–29 years agreed to participate (Table 2 in the ESM). Each expert provided a unique definition of SI, and four (80.0%) reported that SI is different from experiencing side effects with statins. A total of 13 concepts reflecting the patient experience with SI were identified: muscle pain (n = 5 [100.0%]), muscle weakness (n = 4 [80.0%]), and forgetfulness (n = 2 [40.0%]), with burping, constipation, diarrhea, fatigue, gas, headache, indigestion, muscle cramps, reflux, and upset stomach being reported by one expert each. Experts frequently reported impacts on physical activity and daily living (e.g., inability to get out of bed or chair [n = 3 (60.0%)], difficulty walking [n = 3 (60%)], and impact on general daily activities [n = 3 (60.0%)]). The concepts generated from these meetings were organized into a conceptual model (Fig. 2 in the ESM).
Table 1 describes the characteristics of the 20 patients who participated in CEIs, including demographics, general health, and statin therapeutic history. Patients were nearly evenly divided on whether they were familiar with the term ‘statin intolerance’ (55.0% familiar vs. 45.0% unfamiliar). The majority of patients who were familiar with the term (n = 7 [63.6%]) reported that SI was related to experiencing side effects. The saturation analysis of SI-related symptom data concluded that saturation was achieved (i.e., no new symptoms were elicited during the final quartile of interviews; Table 3 in the ESM). Patients reported 37 impact-level SI concepts across the following ten domains: emotional (n = 15 [75.0%]), activities of daily living (n = 13 [65.0%]), physical (n = 11 [55.0%]), social (n = 7 [35.0%]), sleep (n = 6 [30.0%]), work (n = 3 [15.0%]), cognitive (n = 2 [10.0%]), leisure (n = 2 [10.0%]), attire (n = 1 [5.0%]), and identity (n = 1 [5.0%]). Generated concepts were organized in a conceptual model (Fig. 3 in the ESM).
Table 1.
Provider- and patient-reported demographic and health information of patients who participated in concept-elicitation interviews and cognitive debriefing interviews
| Characteristic | CEIs (N = 20) | CDIs (N = 15) |
|---|---|---|
| Age, years | ||
| Range | 55.9–78.9 | 48.2–74.2 |
| Mean ± SD | 65.6 ± 7.4 | 62.9 ± 9.1 |
| Sex, female | 13 (65.0) | 9 (60.0) |
| CV risk | ||
| Moderate | 7 (35.0) | 5 (33.3) |
| High | 8 (40.0) | 5 (33.3) |
| Very high | 5 (25.0) | 5 (33.3) |
| Currently taking a statin | 17 (85.0) | 13 (86.7) |
| No lactose intolerance | 20 (100.0) | 14 (93.3) |
| No vitamin D deficiency | 19 (95.0) | 13 (86.7) |
| Hypothyroidism | ||
| Yes (currently controlled) | 3 (15.0) | 1 (6.7) |
| No | 17 (85.0) | 14 (93.3) |
| Health in general | ||
| Excellent | 0 (0.0) | NR |
| Very good | 2 (10.0) | 1 (6.7) |
| Good | 10 (50.0) | 7 (46.7) |
| Fair | 6 (30.0) | 5 (33.3) |
| Poor | 2 (10.0) | 2 (13.3) |
| Patient-reported statin medication historya | ||
| Type(s) of statin medication had been changed | 11 (55.0) | NR |
| Statin medication had been discontinued | 5 (25.0) | NR |
| Dosage(s) of statin medication had been changed | 4 (20.0) | NR |
| Statin medication dose/type had not been changed | 2 (10.0) | NR |
| Medications other than statinsa | ||
| Antihypertensive medications | 6 (30.0) | 12 (80.0) |
| Diabetes medications | 2 (10.0) | 3 (20.0) |
Data are presented as n (%) unless otherwise indicated
CDI cognitive debriefing interview, CEI concept-elicitation interview, CV cardiovascular, NR not reported, SD standard deviation
aCounts not mutually exclusive
SEAQ© Development
Harmonized concepts from the literature review and the concept elicitations (Table 2) were utilized to generate a 17-item draft questionnaire (Fig. 4 in the ESM). One set of items assesses the level of severity for each symptom, and the second set assesses each symptom’s interference in daily life. To assess severity and interference, 11-point numeric rating scales (NRSs) were selected. The Symptom Severity Scale uses an NRS ranging from 0 (none) to 10 (as bad as you can imagine), and the Symptom Interference Scale ranges from 0 (not at all) to 10 (extremely). A recall period of ‘the past 30 days’ was deemed clinically appropriate to capture the patients’ experiences between visits with their providers, which typically occur about 1 month after initiating statin treatment and gradually decrease over time.
Table 2.
Harmonization of results from concept-elicitation phase
| Reported by three perspectives | Reported by two perspectives | Reported by patients only | Reported by experts only | Reported by literature only |
|---|---|---|---|---|
| Muscle cramps Muscle weakness Muscle ache Muscle pain Headache Nausea Tiredness Joint pain or joint stiffnessa |
Muscle spasmb
Body achec Indigestion Forgetfulness Diarrhea Runny nosed |
Jitteriness Lightheaded Swelling |
Constipation Gas |
Diminished muscle coordination Tendon pain/tendonitis Alopecia Blisters |
aDescribed as joint pain by patients and in the literature. Expert-reported ‘muscle pain’ around the joints
bDescribed similarly by patients as muscle cramps; however, patients who reported this symptom indicated the two were distinct based on duration
cPatient-reported ‘body ache’. Expert-reported ‘muscle ache’ sometimes described by patients as ‘body ache’
dPatient-reported ‘runny nose’. Sinusitis reported as a side effect or adverse event in the product label for ezetimibe
Content Evaluation
The initial 17-item draft SEAQ© was debriefed with the same experts who participated in the concept elicitation. Expert feedback received after review of the draft SEAQ© confirmed the relevance of the muscle-related items in the questionnaire (n = 5 [100.0%]); however, two experts (40.0%) disagreed on the relevance of non–muscle-related items. All experts agreed on the severity and interference assessment of symptoms, and all but one expert (80.0%) indicated that the 30-day recall period was appropriate. Experts also made suggestions regarding the instructions, NRS, overall format, and additional information to be collected from the questionnaire. As a result of the feedback, two additional items were added to the SEAQ© during an interim modification before conducting CDIs with patients: one item asking patients whether they stopped taking their statin because of symptoms (using a dichotomous yes/no response scale) and another item asking patients how likely they would be to stop taking their statin because of symptoms (rated on a five-point verbal response scale).
The patients who participated in the CDIs were unique from those who participated in the CEIs, but their characteristics were similar (Table 1). A summary of results from the patient CDIs is included in Table 4 in the ESM. All patients (n = 15) reported that the SEAQ© was easy to read and answer. All patients for whom data were collected (n ≥ 13 [100.0%]) interpreted the instructions and response scales as intended; data were not available for patients who were not asked a specific question and/or who provided a response that was not sufficient for analysis. At least 93% of patients for whom data were collected interpreted each item as intended. The majority of patients found the muscle-related items (as well as joint pain and tiredness items) relevant to their experience with SI (ranging from n = 10 [66.7%] to n = 15 [100.0%] patients), whereas headache and nausea were experienced by less than half of the patients for whom data were collected (n = 6 [40.0%] and n = 2 [13.3%], respectively). The removal of nausea and headache from the SEAQ© as target measurement concepts was the only change based directly on patient feedback. All experts (n = 5 [100.0%]) and patients (n = 15 [100.0%]) indicated that the SEAQ© would aid in facilitating patient–provider communication.
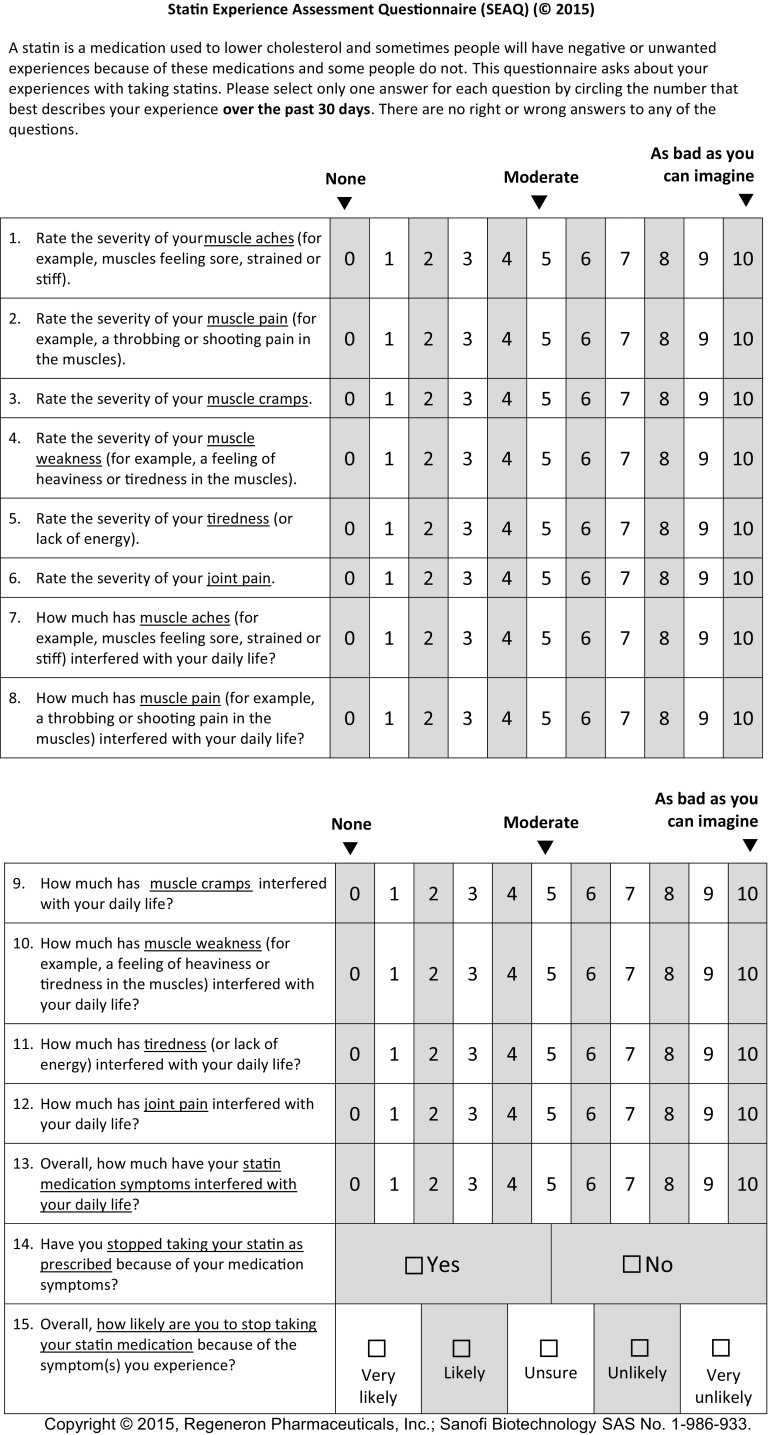
Based on expert feedback and CDIs with patients, the following modifications were made to the SEAQ© instructional text: indication that some people have no negative or unwanted symptoms (60.0% of experts agreed); adjustment to the NRS to include a middle anchor to represent the severity/interference of moderate symptoms (80.0% of experts agreed); reordering of muscle-related symptoms from least invasive (based on one expert recommendation); and removal of nausea and headache from the questionnaire as target concepts based on irrelevance to SI according to some experts (n = 2 [40.0%]) and the majority of patients (headaches and nausea were not relevant to 7 [53.8%] and 12 [80.0%] patients, respectively). Figure 3 presents the final 15-item SEAQ©. After finalization of the SEAQ©, a conceptual framework was constructed to depict the relationship between the concepts and subsequent items in the instrument (Fig. 4) [68].
Fig. 3.
Final 15-item Statin Experience Assessment Questionnaire©
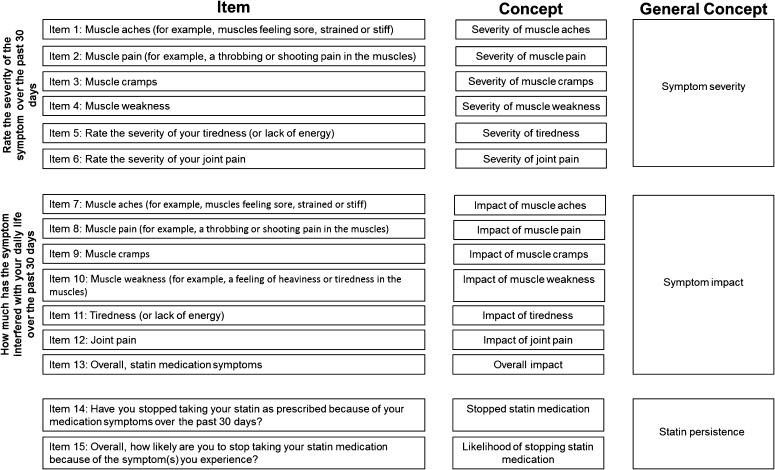
Fig. 4.
Statin Experience Assessment Questionnaire© conceptual framework. The left column (‘Item’) lists the specific items from the SEAQ©, the middle column (‘Concept’) lists the specific concept of measurement as related to the item, and the right column (‘General Concept’) is the targeted measurement domain. SEAQ Statin Experience Assessment Questionnaire©
Scoring of the SEAQ© is based on the conceptual framework, which hypothesized a symptom severity scale (items 1–6), a symptom interference scale (items 7–12), and three additional items assessing ‘overall impact’ (item 13), whether the respondent stopped taking statins as prescribed (item 14), and intention to stop taking statins (item 15). A symptom severity scale score is created by summing and averaging responses to items 1 through 6, and a symptom interference scale score by summing and averaging responses from items 7 through 12. Additionally, a combined total score can be created by averaging the scores of all items from both the symptom severity and the symptom interference scales (items 1–12). Finally, the items assessing overall impact (item 13) and whether the respondent stopped taking statins as prescribed (item 14) or intends to stop taking statins (item 15) are scored independently. For the dichotomously scored item 14, we recommend a 0 (no) and 1 (yes) scoring method. Item 15 may be scored from 0 (very likely) to 4 (very unlikely).
Discussion
The primary purpose of the development of the content-valid SEAQ© is to facilitate clinically meaningful communication between patients and their providers to improve the understanding of SI and its associated patient experience. To date, no validated scales accurately assess the patient SI experience in the clinic. Although new and proposed tools for providers are becoming available, they do not allow for direct input from the patient, an important component of SI diagnosis and management. The statin myalgia clinical index (SMCI) score proposed by the NLA provides weighted values based on the distribution of muscle complaints, information on the temporal pattern of onset and improvement after withdrawal from statins, and reoccurrence on rechallenge [69]. Additionally, the ACC has implemented an SI app to help guide providers through the process of identification, management, and treatment of patients reporting muscle-related symptoms while taking statins [70]. There is the potential to combine the SEAQ© with specific symptom evaluation tools such as the SMCI as a way to use both clinical and patient-centric approaches to assess the SI experience.
The SEAQ© was developed following best practices established by the US FDA and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) PRO Good Research Practices Task Force [24, 68]. Best practice guidance from the FDA for developing PRO measures designates that content validity be supported by evidence from qualitative studies demonstrating that the items in a questionnaire are appropriate and comprehensive relative to the intended measurement concept, population, and application [68]. Patient input to item generation and evaluation of comprehension of the questionnaire also contribute to content validity. Patients with SI provided input that was an integral part of the SEAQ© development. Evidence from the patient CDIs indicates that the SEAQ© was easy to complete, comprehensive, and interpreted as intended. All patients who participated confirmed that the resulting SEAQ© would aid in the dialogue with their providers. The concept-elicitation activities for the SEAQ© development demonstrated saturation, which indicates that these results are robust and reliable. Research establishes that qualitative data should be gathered to the point of saturation to ensure that the items in a PRO questionnaire represent the ‘universe of content’ for a given concept [24, 25, 71–74]. According to the ISPOR PRO Task Force, saturation should be documented when developing a new instrument [24].
The resulting SEAQ© emphasizes that the patient SI experience is multifaceted and primarily characterized in the context of muscle pain and weakness. Removal of headache and nausea from the draft SEAQ© aligned with the proposed SI definitions from the NLA, International Lipid Expert Panel, and European Atherosclerosis Society, which primarily focus on muscle-related symptoms and recognize these symptoms as the main reason for discontinuation of therapy [17, 18, 75]. The SEAQ© also underscores that SI is best assessed from the patient perspective. This is consistent with the recommendations of the NLA SI Panel, which highlights the importance of collaboration between patients and providers through effective communication for the management of SI. According to the ACC/AHA Task Force on Performance Measures, active engagement of patients in self-care, defined by their ability to perform activities necessary to attain, preserve, and encourage best health, leads to greater success in achieving treatment goals. For optimal shared accountability with their providers, patients would ideally be aware of symptoms, discuss any symptoms with their providers, become educated about the condition, execute a treatment plan, and follow up to assess outcomes.
The methodology utilized for the development of the SEAQ© had several strengths. First, by obtaining data via research interviews that allowed spontaneous responses, experts’ definitions and characterizations of SI were not limited to prespecified concepts about the topics. Second, the nature of the qualitative research allowed for collection of more in-depth data and deeper understanding of the experts’ perspectives of SI. The experts who were interviewed provided detailed input and are likely to partner with patients to utilize the final SEAQ© for a better understanding of the patient-centric SI experience. Third, items in the questionnaire were rated on an 11-point NRS, suggested by researchers and measurement development specialists because of its relative advantages in minimization of missing data, patient preference, and ease of recording and implementation in research settings [76]. Finally, the research activities followed the guidelines provided by the FDA and ISPOR for the development of PRO questionnaires.
There were also limitations in the activities leading to the generation of the SEAQ©. First, because of the small sample size of experts and patients, additional research would be necessary to gain further consensus on clinical perspectives related to SI and to ensure that results are generalizable. Second, the recall period was set to 30 days because the questionnaire was intended to document patient experiences between visits with their providers. This would preclude the assessment of SI among patients who are no longer taking statins but who had experienced symptoms characteristic of SI in the past (i.e., longer than 30 days prior to assessment). In an effort to minimize the burden on study participants, limited demographic and health information was collected. In particular, detailed information regarding the history of statin use and associated statin-related problems was lacking. The limited demographic information collected could impair the utility of the SEAQ© in the general population. Specifically, the lack of information regarding education and reading levels of those patients who participated in the CDIs makes it difficult to determine whether a broader group of patients might find the questionnaire comprehensible and easy to read. The last limitation is the lack of a patient review of the final SEAQ©. However, changes made to the questionnaire following patient CDIs were minor. In other words, the most substantial changes to the SEAQ© were made after the expert advice meetings and, therefore, were evaluated by patients. Thus, we are confident that the current version of the SEAQ© will be easily comprehended by future patients completing the questionnaire.
As a measure of SI, the SEAQ© is a content-valid PRO questionnaire that has been developed and documented in accordance with measurement-development best practices. The SEAQ© can be utilized in clinical practice for shared learning of the patient SI experience, for facilitating communication between patients and providers about patient health status, and potentially for informing treatment decisions regarding the use of statins. In a research setting, the SEAQ© can be used as a means to gather patient-centric data. Future research is needed to quantitatively evaluate the psychometric properties and interpretability of the scores and to assess the impact of the SEAQ© on treatment decision making.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Writing and editorial support for this manuscript was provided by MicroMass Communications, Inc., Cary, NC, USA, with funding from Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA, and Sanofi US, Bridgewater, NJ, USA.
Author contributions
Each author contributed to the conception and design of the study, acquisition, analysis, or interpretation of the data. In addition, authors were involved in drafting and revising the manuscript critically for important intellectual content and provided final approval for the version submitted.
Compliance with Ethical Standards
Funding
Support for this study was provided by Regeneron Pharmaceuticals, Inc., and Sanofi US. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this publication.
Conflict of interest
T. A. Jacobson is a consultant for Amarin, Amgen, AstraZeneca, Merck, Sanofi US, and Regeneron Pharmaceuticals, Inc. S.V. Edelman is a consultant for Sanofi US and Merck. N. Galipeau and A.L. Shields were consultants for Sanofi US at the time this research was conducted. U.G. Mallya and A. Koren are employed by and have ownership interest in Sanofi US. M.H. Davidson is a consultant for Sanofi US and Amgen.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29(2):151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 3.European Association for Cardiovascular Prevention & Rehabilitation ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9(2):129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–934. doi:10.1016/j.jacc.2013.11.002. [DOI] [PubMed]
- 6.Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, et al. Treatment B) drug therapy: executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan—2012 version. J Atheroscler Thromb. 2013;20(12):850–860. doi: 10.5551/jat.19166. [DOI] [PubMed] [Google Scholar]
- 7.Finegold JA, Manisty CH, Goldacre B, Barron AJ, Francis DP. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol. 2014;21(4):464–474. doi: 10.1177/2047487314525531. [DOI] [PubMed] [Google Scholar]
- 8.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97(8A):89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patient—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 10.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23(8):1182–1186. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6(3):208–215. doi: 10.1016/j.jacl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 12.El-Salem K, Ababneh B, Rudnicki S, Malkawi A, Alrefai A, Khader Y, et al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44(6):877–881. doi: 10.1002/mus.22205. [DOI] [PubMed] [Google Scholar]
- 13.Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther. 2007;29(8):1761–1770. doi: 10.1016/j.clinthera.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes Care. 2013;36:S325–S330. doi: 10.2337/dcS13-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyton JR, Bays HE, Grundy SM, Jacobson TA. An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol. 2014;8(3 suppl):S72–S81. doi: 10.1016/j.jacl.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez RJ, Graham JH, Evans MA, Mallya UG, Panaccio MP, Steinhubl SR. Clinical and economic consequences of statin intolerance in the U.S.: Results from an integrated health system [abstract]. Circ Cardiovasc Qual Outcomes. 2015;8(Suppl 2):A146.
- 20.Arca M, Pigna G. Treating statin-intolerant patients. Diabetes Metab Syndr Obes. 2011;4:155–166. doi: 10.2147/DMSO.S11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson ED, Ho PM, Barton M, Beam C, Burgess LH, Casey DE, Jr, et al. ACC/AHA/AACVPR/AAFP/ANA concepts for clinician-patient shared accountability in performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2014;64(20):2133–2145. doi: 10.1016/j.jacc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18:59–82. doi: 10.1177/1525822X05279903. [DOI] [Google Scholar]
- 23.Lamoureux RE, Shields A, Stokes J, Yaworsky A, Galipeau N. How many subjects are enough for symptom-focused concept elicitation studies? A retrospective analysis of saturation across twenty-six studies [abstract] Value Health. 2015;18(3):A33. doi: 10.1016/j.jval.2015.03.198. [DOI] [Google Scholar]
- 24.Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–977. doi: 10.1016/j.jval.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12(8):1075–1083. doi: 10.1111/j.1524-4733.2009.00603.x. [DOI] [PubMed] [Google Scholar]
- 26.Friese S. ATLAS.ti 7 User Manual. Berlin: ATLAS.ti Scientific Software Development GmbH; 2013. [Google Scholar]
- 27.Abdulrazzaq HA, Sulaiman SAS. Prediction of renal impairment induced by statin therapy in cardiac outpatients. Int J Pharm Pharm Sci. 2012;4(Suppl 1):371–373. [Google Scholar]
- 28.Abdulrazzaq HA, Sulaiman SAS. Neurological adverse drug reactions and statin therapy. Int J Pharm Pharm Sci. 2012;4:446–449. [Google Scholar]
- 29.Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119(5):400–409. doi: 10.1016/j.amjmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol. 2007;100(3):554–555. doi: 10.1016/j.amjcard.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 31.Baykal D. An update on statin safety with an emphasis on differences. LipidSpin. 2013;11:5–8. [Google Scholar]
- 32.Bays H. Statin safety: an overview and assessment of the data—2005. Am J Cardiol. 2006;97(8A):6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Blaier O, Lishner M, Elis A. Managing statin-induced muscle toxicity in a lipid clinic. J Clin Pharm Ther. 2011;36(3):336–341. doi: 10.1111/j.1365-2710.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 34.Cham S, Evans MA, Denenberg JO, Golomb BA. Statin-associated muscle-related adverse effects: a case series of 354 patients. Pharmacotherapy. 2010;30(6):541–553. doi: 10.1592/phco.30.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chee YJ, Chan HHV, Tan NC. Understanding patients’ perspective of statin therapy: can we design a better approach to the management of dyslipidaemia? A literature review. Singap Med J. 2014;55(8):416–421. doi: 10.11622/smedj.2014099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho L, Rocco M, Colquhoun D, Sullivan D, Rosenson RS, Dent R, et al. Design and rationale of the GAUSS-2 study trial: a double-blind, ezetimibe-controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy. Clin Cardiol. 2014;37(3):131–139. doi: 10.1002/clc.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colivicchi F, Tubaro M, Santini M. Clinical implications of switching from intensive to moderate statin therapy after acute coronary syndromes. Int J Cardiol. 2011;152(1):56–60. doi: 10.1016/j.ijcard.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. Am J Physiol Cell Physiol. 2006;291(6):C1208–C1212. doi: 10.1152/ajpcell.00226.2006. [DOI] [PubMed] [Google Scholar]
- 39.Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95(5):2015–2022. doi: 10.1210/jc.2009-2689. [DOI] [PubMed] [Google Scholar]
- 40.Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29(7):800–811. doi: 10.1592/phco.29.7.800. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald K, Redmond E, Harbor C. Statin-induced myopathy. Glob Adv Health Med. 2012;1(2):32–36. doi: 10.7453/gahmj.2012.1.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedrich DA. Vitamin D deficiency and statin intolerance. LipidSpin. 2013;11:21–22. [Google Scholar]
- 43.Fujimoto M, Hosomi K, Takada M. Statin-associated lower urinary tract symptoms: data mining of the public version of the FDA adverse event reporting system, FAERS. Int J Clin Pharmacol Ther. 2014;52(4):259–266. doi: 10.5414/CP202033. [DOI] [PubMed] [Google Scholar]
- 44.Grundy SM. Statin discontinuation and intolerance: the challenge of lifelong therapy [editorial] Ann Intern Med. 2013;158(7):562–563. doi: 10.7326/0003-4819-158-7-201304020-00010. [DOI] [PubMed] [Google Scholar]
- 45.Harris LJ, Thapa R, Brown M, Pabbathi S, Childress RD, Heimberg M, et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol. 2011;5(4):299–307. doi: 10.1016/j.jacl.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Harrison TN, Derose SF, Cheetham TC, Chiu V, Vansomphone SS, Green K, et al. Primary nonadherence to statin therapy: patients’ perceptions. Am J Manag Care. 2013;19(4):e133–e139. [PubMed] [Google Scholar]
- 47.Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol. 2011;27(2):146–151. doi: 10.1016/j.cjca.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Li JH, Joy SV, Haga SB, Orlando LA, Kraus WE, Ginsburg GS, et al. Genetically guided statin therapy on statin perceptions, adherence, and cholesterol lowering: a pilot implementation study in primary care patients. J Pers Med. 2014;4(2):147–162. doi: 10.3390/jpm4020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15(1):291. doi: 10.1007/s11883-012-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mareedu RK, Modhia FM, Kanin EI, Linneman JG, Kitchner T, McCarty CA, et al. Use of an electronic medical record to characterize cases of intermediate statin-induced muscle toxicity. Prev Cardiol. 2009;12(2):88–94. doi: 10.1111/j.1751-7141.2009.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matteucci E, Giampietro O. Statin intolerance: why and what to do—with a focus on diabetic people. Curr Med Chem. 2013;20(11):1397–1408. doi: 10.2174/0929867311320110004. [DOI] [PubMed] [Google Scholar]
- 52.Nair RK, Karadi RL, Kirpatrick ES. Managing patients with ‘statin intolerance’: a retrospective study. Br J Cardiol. 2008;15(3):158–160. [Google Scholar]
- 53.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40(3):567–572. doi: 10.1016/S0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 54.Roberto G, Biagi C, Montanaro N, Koci A, Moretti U, Motola D. Statin-associated gynecomastia: evidence coming from the Italian spontaneous ADR reporting database and literature. Eur J Clin Pharmacol. 2012;68(6):1007–1011. doi: 10.1007/s00228-012-1218-5. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis. 2013;23(9):871–875. doi: 10.1016/j.numecd.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Sakaeda T, Kadoyama K, Okuno Y. Statin-associated muscular and renal adverse events: data mining of the public version of the FDA adverse event reporting system. PLoS One. 2011;6(12):e28124. doi: 10.1371/journal.pone.0028124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shannon JA, John SM, Parihar HS, Allen SN, Ferrara JJ. A clinical review of statin-associated myopathy. J Pharm Technol. 2013;29(5):219–230. doi: 10.1177/8755122513500915. [DOI] [Google Scholar]
- 58.Silva M, Matthews ML, Jarvis C, Nolan NM, Belliveau P, Malloy M, et al. Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clin Ther. 2007;29(2):253–260. doi: 10.1016/j.clinthera.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Tompkins R, Schwartzbard A, Gianos E, Fisher E, Weintraub H. A current approach to statin intolerance. Clin Pharmacol Ther. 2014;96(1):74–80. doi: 10.1038/clpt.2014.84. [DOI] [PubMed] [Google Scholar]
- 60.Tuccori M, Lapi F, Testi A, Coli D, Moretti U, Vannacci A, et al. Statin-associated psychiatric adverse events: a case/non-case evaluation of an Italian database of spontaneous adverse drug reaction reporting. Drug Saf. 2008;31(12):1115–1123. doi: 10.2165/0002018-200831120-00007. [DOI] [PubMed] [Google Scholar]
- 61.Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol. 2013;7(5):472–483. doi: 10.1016/j.jacl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Welding JM, Ragheb B. Evidence-based approach to the use of CoQ10 to deal with statin intolerance. LipidSpin. 2013;11(4):13–14. [Google Scholar]
- 63.Zysek V, Spoon J, Kopecky S. Mayo Clinic: management of patients with statin intolerance. Clin Lipidol. 2013;8(5):541–549. doi: 10.2217/clp.13.54. [DOI] [Google Scholar]
- 64.Zetia [package insert]. Whitehouse Station: Merck and Co; 2013.
- 65.Lipitor [package insert]. New York: Pfizer; 2015.
- 66.Vytorin [package insert]. Whitehouse Station: Merck and Co; 2015.
- 67.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- 68.US Department of Health and Human Services, Food and Drug Administration. Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed 19 May 2016.
- 69.Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The National Lipid Association’s Muscle Safety Expert Panel: An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3 Suppl):S58–71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 70.American College of Cardiology. Statin intolerance [mobile application]. http://www.acc.org/StatinIntoleranceApp. Accessed 19 May 2016.
- 71.Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res. 2009;18(9):1263–1278. doi: 10.1007/s11136-009-9540-9. [DOI] [PubMed] [Google Scholar]
- 72.Burke LB, Kennedy DL, Miskala PH, Papadopoulos EJ, Trentacosti AM. The use of patient-reported outcome measures in the evaluation of medical products for regulatory approval. Clin Pharmacol Ther. 2008;84(2):281–283. doi: 10.1038/clpt.2008.128. [DOI] [PubMed] [Google Scholar]
- 73.Kerr C, Nixon A, Wild D. Assessing and demonstrating data saturation in qualitative inquiry supporting patient-reported outcomes research. Expert Rev Pharmacoecon Outcomes Res. 2010;10(3):269–281. doi: 10.1586/erp.10.30. [DOI] [PubMed] [Google Scholar]
- 74.Lasch KE, Marquis P, Vigneux M, Abetz L, Arnould B, Bayliss M, et al. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res. 2010;19(8):1087–1096. doi: 10.1007/s11136-010-9677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K, et al. Statin intolerance—an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci. 2015;11(1):1–23. doi: 10.5114/aoms.2015.49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.