Abstract
Oncolytic virotherapy is an emerging immunotherapeutic modality for cancer treatment. Oncolytic viruses with genetic modifications can further enhance the oncolytic effects on tumor cells and stimulate antitumor immunity. The oncolytic vaccinia viruses JX-594-GFP+/hGM-CSF (JX-GFP) and TG6002 are genetically modified by secreting granulocyte-macrophage colony-stimulating factor (GM-CSF) or transforming 5-fluorocytosine (5-FC) into 5-fluorouracil (5-FU). We compared their properties to kill tumor cells and induce an immunogenic type of cell death in a human melanoma cell model using SK29-MEL melanoma cells. Their influence on human immune cells, specifically regarding the activation of dendritic cells (DCs) and the interaction with the autologous cytotoxic T lymphocyte (CTL) clone, was investigated. Melanoma cells were infected with either JX-GFP or TG6002 alone or in combination with 5-FC and 5-FU. The influence of viral infection on cell viability followed a time- and multiplicity of infection dependent manner. Combination of virus treatment with 5-FU resulted in stronger reduction of cell viability. TG6002 in combination with 5-FC did not significantly strengthen the reduction of cell viability in this setting. Expression of calreticulin and high mobility group 1 protein (HMGB1), markers of immunogenic cell death (ICD), could be detected after viral infection. Accordingly, DC maturation was noted after viral oncolysis. DCs presented stronger expression of activation and maturation markers. The autologous CTL clone IVSB expressed the activation marker CD69, but viral treatment failed to enhance cytotoxicity marker. In summary, vaccinia viruses JX-GFP and TG6002 lyse melanoma cells and induce additional immunostimulatory effects to promote antitumor immune response. Further investigation in vivo is needed to consolidate the data.
Keywords: oncolytic virus, vaccinia virus, immunogenic cell death, dendritic cells, cytotoxic T lymphocytes, immunotherapy
Introduction
An effective antitumor immune response is important for progression- and metastatic-free survival in cancer patients. Therapeutic agents, which cause an immunogenic type of cell death, correlate with better prognosis and overall survival (OS).1–3 Several studies indicated that a long-term cure of cancer might be immune driven.4–7 A higher amount of tumor-infiltrating lymphocytes in the microenvironment correlates with a better outcome.8 Oncolytic viruses offer an approach for immunotherapy.9–11 Genetically engineered viruses, encoding for genes, which induce immunogenic cell killing of tumor cells, became of further interest over the past years to optimize the oncolytic activity and thereby improve antitumor immune response.12–16 The field of oncolytic immunotherapy has expanded dramatically.17 More than 10 different viral species have entered clinical trials. Recently, a phase 3 pivotal trial of T-VEC (Imlygic®, talimogene laherparepvec, Amgen, Thousand Oaks, CA, USA), an oncolytic herpes virus expressing granulocyte-macrophage colony-stimulating factor (GM-CSF), in patients with advanced melanoma met its primary end point.18 The overall response rate (ORR) and the median OS were higher in the T-VEC arm compared to those in the GM-CSF arm (ORR: 26.4% vs 5.7%; OS: 23.3 months vs 18.9 months; hazard ratio [HR]: 0.79 and P=0.051). This led the US Food and Drug Administration (FDA) to approve Imlygic®, the first FDA-approved oncolytic virus therapy, at the end of October 2015, for the treatment of melanoma lesions in the skin and lymph nodes and subsequently to the European Medicines Agency (EMA) approval.
Pexa-Vec (pexastimogene devacirepvec, JX-594) is an oncolytic and immunotherapeutic vaccinia virus engineered to express GM-CSF. Pexa-Vec mechanisms of actions include tumor cell infection and lysis,19–21 antitumor immune response induction22,23 and acute vascular disruption.24 Pexa-Vec is derived from the commonly used Wyeth vaccine strain (Dryvax®; Wyeth Laboratories, Dallas, TX, USA). In a randomized phase 2 study (HEP007, NCT00554372) in advanced first-line hepatocellular carcinoma (HCC), a significant improvement in OS (HR 0.39, P=0.02) was observed with Pexa-Vec at 109 pfu (14.1 m) vs 108 pfu (6.7 m).22
Pexa-Vec has now entered a randomized controlled phase 3 trial in advanced first-line HCC (NCT02562755), comparing the administration of Pexa-Vec and sorafenib to sorafenib alone, with OS as the primary end point.
The oncolytic vaccinia viruses JX-GFP and TG6002 were used in the studies described here. JX-GFP is derived from JX-594 and modified by insertion of a gene cassette in the thymidine kinase (TK) locus expressing green fluorescent protein (GFP) and human granulocyte-macrophage colony-stimulating factor (hGM-CSF). Inactivation of vaccinia TK attenuates JX-GFP in normal cells, which have lower levels of cellular TK. In contrast, in cancer cells, endogenous TK is typically overexpressed so that the virus can continue to replicate.14,20,25 TG6002 is derived from the Copenhagen strain and is deleted of two genes (TK and ribonucleotide reductase) and expresses the suicide gene FCU1.26 FCU1 encodes a bifunctional chimeric protein that catalyzes the direct conversion of 5-fluorocytosine (5-FC), a nontoxic antifungal agent, into the toxic metabolites 5-fluorouracil (5-FU) and 5-fluorouridine-5′monophosphate (5-FUMP), thus bypassing the natural resistance of certain human tumor cells to 5-FU and reducing systemic toxicity.26–28
JX-GFP and TG6002 were analyzed for their ability to induce viral oncolysis in a human melanoma in vitro cell model using SK29-MEL melanoma cells and antigen-specific, corresponding cytotoxic T lymphocytes (CTLs).29–31 Combination with 5-FC was indicated to test the encoded transgene and analyze additional cytotoxic effects compared to a direct combination with 5-FU. Furthermore, immunogenic parameters induced by oncolytic cell death were studied. Finally, we sought to specify the influence of virally induced tumor cell lysates (TCLs) on the activation and maturation of dendritic cells (DCs) and CTLs.
Materials and methods
Human melanoma cell lines, human immune cells and viruses
Human melanoma cell line SK29-MEL-1 and its HLA-A2 loss clone SK29-MEL-1.22 (both cell lines were gifts of T. Woelfel’s group, University Medical Center Mainz30–32) were propagated in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco® Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in 5% CO2 atmosphere. For coculture experiments, Roswell Park Memorial Institute Medium (RPMI; Gibco®, CA, USA) was used. Both culture mediums were supplemented with 10% fetal calf serum (FCS; PAA Laboratories GmbH, Coelbe, Germany) and 1% penicillin/streptomycin (Gibco®, Thermo Fisher Scientific).
Melanoma cell clone SK29-MEL-1 is derived from human patient SK29 with metastatic melanoma.31 The SK29-MEL-1.22 cell line is a selected HLA-A2 loss (A2−) variant of HLA-A2-positive (A2) SK29-MEL-1 cells.31 An HLA-A2-restricted CTL clone named IVSB recognizing the tyrosinase peptide 369–376, is derived from an autologous mixed lymphoid tumor cell culture (MLTC) of the SK29 model.32 CTL clones were maintained in long-term culture as previously described.30
Monocytes were isolated by adherence from HLA-A2-positive human buffy coats from healthy blood donors through the Department of Transfusion Medicine, University Medical Center Mainz (Mainz, Germany). Monocytes were treated with 500 U interleukin (IL)-4 (ImmunoTools, Friesoythe, Germany) and 500 U GM-CSF (Berlex; Bayer Healthcare Pharmaceuticals, Leverkusen, Germany) for 6 d to obtain immature dendritic cells (iDCs). A cytokine cocktail containing tumor necrosis factor (TNF)-α, IL-6, IL-1-β (all Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and PGE2 (Sigma-Aldrich Chemie GmbH, Munich, Germany) led to maturation of DCs (mature dendritic cells [mDCs]).
Vaccinia virus JX-GFP was previously described.13,14,20,25,33 Attenuated recombinant TG6002 vaccinia virus was derived from the Copenhagen strain. TG6002 was deleted of the TK and ribonucleotide reductase genes and expressed the suicide gene FCU1. JX-GFP and TG6002 were propagated and titrated on chicken embryo fibroblasts as previously described.26
Approval by the ethics commission of the State Chamber of physicians of Rhineland-Palatinate regarding the research procedure (use of samples from tumor and blood bank) and the gene technology forms has been obtained from University Mainz prior to the start of the experiments.
Coculture model
For coculture experiments, SK29-MEL-1 or SK29-MEL-1.22 cells were seeded in six-well plates and treated with viruses or chemotherapeutics as described further. DCs were isolated as described and seeded in six-well plates in ratio 5:1 with SK29-MEL-1 or SK29-MEL-1.22 cells and cultivated together for 3 d.
Autologous CTLs (IVSB) were cocultured for 1 d or 3 d in a six-well plate with TCL-incubated DCs, DCs alone or TCLs alone at a ratio of 1:5.
Viral infection of human melanoma cells (JX-GFP, TG6002)
A total of 105 melanoma cells per well were cultivated for 1 d in six-well plates before virus infection. For infection with JX-GFP or TG6002, cell medium was removed and an infection was performed with a range of multiplicity of infections (MOIs) for both viruses (0.01 to MOI 0.0001), for different incubation times (24–48 h). Initial incubation period was 2 h, and an appropriate amount of fresh medium was supplemented to the cells. Where indicated, 5-FC and 5-FU were added after 24 h or 48 h of infection, and a supplementary incubation period of 4 or 5 d with the combined treatment was performed. Concentrations of 5-FC were chosen twice the concentrations of 5-FU corresponding to an estimated transformation rate of 50% by the FCU1 gene in TG6002. To compare the effect of 5-FC in both viruses, JX-GFP was also combined with 5-FC.
The experiments were performed with a total incubation period of 48 h for JX-GFP or TG6002 and an additional period of 5 d for the combined treatment. Consequently, 7 d after infection, supernatants were collected and cells were detached for experiments as described previously.
Chemotherapeutics
Twenty-four hours and 48 h after virus infection, 5-FC (InvivoGen Europe, Toulouse, France) (100 µg/mL) or 5-FU (Pharmacy of the University Medical Center Mainz, Mainz, Germany) (50 µg/mL) were added. Doxorubicin (Pharmacy of the University Medical Center Mainz) is described as a drug that induces immunogenic cell death (ICD),34 and a concentration of 2 µM was added 24 h before performing tests to perform a positive control.
Luminescence assays
For 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromid (MTT) viability assay, cells were seeded in 96-well plates (Sigma, München, Germany). For single treatment in MTT assay, serial dilutions of cytostatic drugs or a dilution of MOIs were generated and added to the different cell lines. MTT was added and after dissolving, the produced purple formazan with sodium dodecyl sulfate (SDS) absorption was measured at 562 nm by spectrophotometer (enzyme-linked immunosorbent assay [ELISA] Reader; Bio-Tek Instruments, Bad Friedrichshall, Germany).
The ATP assay (Enliten® ATP Assay; Promega Corporation, Madison, WI, USA) was performed as described by the manufacturer. Ninety-six-well plates (Greiner Bio-One, Frickenhausen, Germany) were filled with 50 µL of supernatants, which were collected by aspirating medium from the coculture. Luminescence was measured with Apliskan® (Thermo Fisher Scientific, Vantaa, Finland).
Flow cytometry
The type of cell death was analyzed with the apoptosis detection kit BD with addition of APC-labeled Annexin V (both from BD Biosciences Pharmingen, Heidelberg, Germany). The analysis was performed as recommended by the manufacturer and as previously described.35
Flow cytometry was used for quantification of the percentage of calreticulin located at the cell surface. To analyze the amount of calreticulin at the day of cocultivation, flow cytometry was performed 7 d after treatment (48 h viral infection following 5 d of 5-FC or 5-FU). Adherent cells were dissociated with PBS/EDTA and collected together with cells floating in the medium by centrifugation (500× g, 5 min). After washing with PBS +5% FCS, primary antibody (Anti-calreticulin Antibody, clone 16B11.1; Merck Millipore, Darmstadt, Germany) was added. After 1 h, cells were washed, and secondary antibody (PE or FITC goat anti-mouse IgG [minimal x-reactivity] antibody, BioLegend®, San Diego, CA, USA) was added and incubated for 1 h. Cells were gated on Annexin-positive, propidium iodide (PI)-negative cells for further analysis on surface calreticulin expression.
In DC coculture experiments with tumor lysates, cells were harvested and stained with anti-CD80 APC, -CD83 PE and -CD86 PE antibodies (BD Biosciences Pharmingen). The expression of these receptors on DCs was analyzed on a FACSCalibur flow cytometry system (BD Biosciences). Fluorescence was measured with a minimum of 15,000 events per sample. Data analysis was performed using the Cell Quest Pro software (BD Biosciences).
ELISA
Supernatants of cocultivated cells were collected before harvesting and stored at −80°C. High mobility group 1 protein (HMGB1) immunoassay was performed as described by the manufacturer.36 Absorption was measured at 562 nm by spectrophotometer (ELISA Reader; Bio-Tek Instruments). Interferon (IFN)-γ assay was performed as per protocol of ELISA kit (eBioscience, Frankfurt, Germany). The plates were read in the spectrophotometer at 450 nm, and values of 570 nm were subtracted to diminish background noise.
Statistical analysis
Data were analyzed for statistical significance using Prism software (GraphPad). Significance was tested using unpaired Student’s t-test or Kruskall–Wallis test as indicated; P<0.05 was considered to be significant. The P-values and standard deviations were calculated from at least two or more independent experiments.
Results
JX-GFP and TG6002 induced oncolytic cell death
SK29-MEL-1 and its HLA-loss clone SK29-MEL-1.22 were infected with different MOIs of viruses, and different concentrations of 5-FC and 5-FU were added to evaluate the susceptibility of the melanoma cells to these agents. MTT viability assays were performed after different incubation periods. The effect of 5-FU was compared to that of 5-FC (Figures 1 and 2).
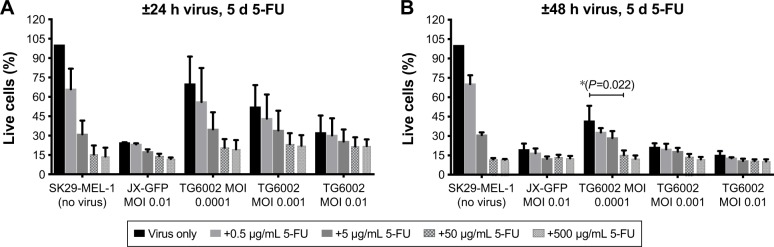
Figure 1.
Influence of JX-GFP and TG6002±5-FU on SK29-MEL-1 cell viability.
Notes: The effects of JX-GFP and TG6002 on the viability were measured by MTT assay, and graphs show the percentage of living cells after virus infection vs untreated cell control (=100% viability). (A) Cells were treated with 5-FU for 5 d in a dilution period of 0.5 to 500 µg/mL; or cells were infected with JX-GFP with a MOI of 0.01 for 24 h and 5-FU was added for 5 d in a concentration of 0.5 to 500 µg/mL; or cells were infected with TG6002 with MOIs from 0.01 to 0.0001 for 24 h and 5-FU was added for 4 d or 5 d in a concentration of 0.5 to 500 µg/mL. (B) Cells were treated with 5-FU for 5 d in a dilution period of 0.5 to 500 µg/mL; or cells were infected with JX-GFP with a MOI of 0.01 for 48 h and 5-FU was added for 5 d in a concentration of 0.5 to 500 µg/mL; or cells were infected with TG6002 with MOIs from 0.01 to 0.0001 for 48 h and 5-FU was added for 4 d or 5 d in a concentration of 0.5 to 500 µg/mL. Data are shown for at least two independent experiments. *P≤0.05.
Abbreviations: 5-FU, 5-fluoruoracil; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid; d, day; MOI, multiplicity of infection; h, hour.
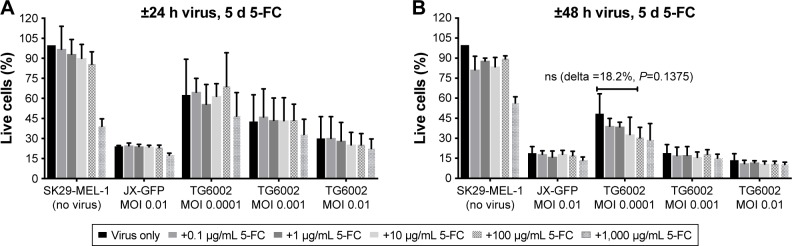
Figure 2.
Influence of JX-GFP and TG6002±5-FC on SK29-MEL-1 cell viability.
Notes: The effects of JX-GFP and TG6002 on the viability were measured by MTT assay, and graphs show the percentage of living cells after virus infection vs untreated cell control (=100% viability). (A) Cells were treated with 5-FU for 5 d in a dilution period of 0.1 to 1,000 µg/mL; or cells were infected with JX-GFP with a MOI of 0.01 for 24 h and 5-FC was added for 5 d in a concentration of 0.1 to 1,000 µg/mL; or cells were infected with TG6002 with MOIs from 0.01 to 0.0001 for 24 h and 5-FC was added for 4 d or 5 d in a concentration of 0.1 to 1,000 µg/mL. (B) Cells were treated with 5-FC for 5 d in a dilution period of 0.1 to 1,000 µg/mL; or cells were infected with JX-GFP with a MOI of 0.01 for 48 h and 5-FC was added for 5 d in a concentration of 0.1 to 1,000 µg/mL; or cells were infected with TG6002 with MOIs from 0.01 to 0.0001 for 48 h and 5-FC was added for 4 d or 5 d in a concentration of 0.5 to 500 µg/mL. Data are shown for at least two independent experiments.
Abbreviations: 5-FC, 5-Fluorcytosin; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromid; d, day; MOI, multiplicity of infection; h, hour; ns, nonsignificant.
Both viruses induced a reduction of viability, in a time-and concentration-dependent manner (Figures 1A and B and 2A and B). Combination of both viruses with 5-FU caused a stronger reduction of cell viability than the virus (Figure 1A and B). A longer incubation period and also higher MOIs resulted in a stronger reduction of viable cells in an almost linear relationship and compared to 5-FU alone (Figure 1A and B).
5-FC alone showed no significant influence on cell viability below a concentration of 1,000 µg/mL (Figure 2A and B). 5-FC in combination with JX-GFP did not show a synergistic effect below a concentration of 1,000 µg/mL of 5-FC (Figure 2A and B). Combination therapy of 5-FC and TG6002 showed a stronger reduction of cell viability compared to TG6002 alone (Figure 2A and B). Viability reduction was not as strong as directly combined with 5-FU in all cases. For example, a treatment with TG6002 MOI 0.0001 for 48 h followed by 5-d treatment with 5-FU 50 µg/mL resulted in a cell viability of 15.3% (delta to TG6002 alone =26.2%, Figure 1B) compared to the 5-FC in the same setting (28.8%, delta to TG6002 alone =18.2%, Figure 2B). Following a time- and concentration-dependent manner with regard to the viral toxicity, the overall most effective reduction could be seen for MOI 0.01 and high-dose 5-FC (10.5% viability, Figure 2B) and 5-FU (10.3% viability, Figure 1B). To study the transgenic design of TG6002 and the additional effect of its encoded enzyme FCU1, the setting with the highest delta in combined treatment with 5-FC was chosen. Although not statistically significant, this was shown after an incubation period of 48 h with TG6002, an MOI of 0.0001 and a treatment with 5-FC for 5 d (Figure 2B). This setting showed a difference in cell viability of 18.2% between TG6002 and TG6002+100 µg/mL 5-FC-treated cells in case of SK29-MEL-1 (Figure 2B). Thus, these conditions were chosen for following experiments and compared to the combination with 50 µg/mL 5-FU, considering ~50% conversion rate of TG6002 of 5-FC into 5-FU.
Similar results were observed for the HLA-A2 loss clone melanoma cells SK29-MEL-1.22 (data not shown). Further treatment schedules of 24 h or 48 h of viral infection and 4 d of treatment with 5-FC and 5-FU were tested and showed similar results (data not shown).
Type of cell death, apoptosis and necrosis
To define the type of cell death which is induced by the vaccinia viruses, we performed an apoptosis assay with FACS staining of phosphatidylserine via Annexin V and DNA via PI. Early apoptotic cells express phosphatidylserine, while the DNA of necrotic cells could be stained with PI and a combined signal of both could be associated with late apoptosis. 5-FC and 5-FU treatments were added in the experiment to analyze the combination treatment.
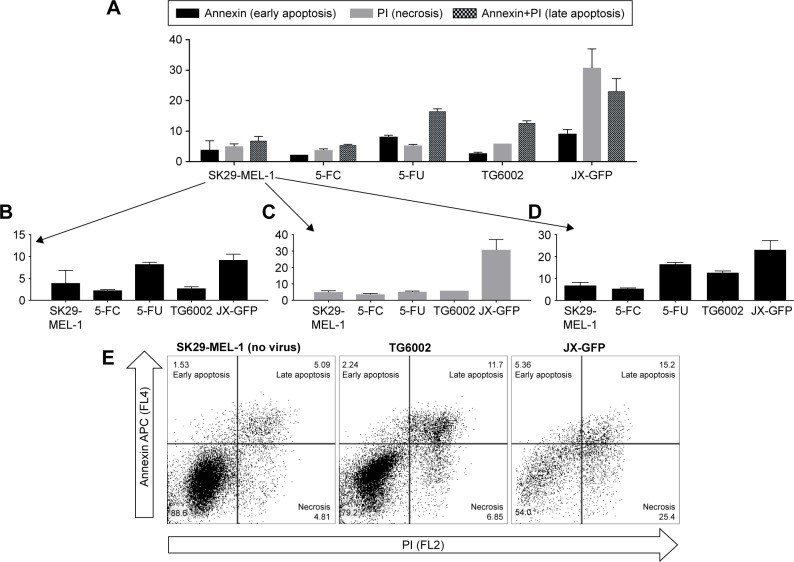
Cells infected with JX-GFP showed an increased signal for PI in both cell lines, characterizing a more necrotic type of cell death (Figure 3A and C). The data of TG6002 infected cells were different in SK29-MEL-1 and SK29-MEL-1.22. TG6002-infected SK29-MEL-1 cells showed the highest signal for late apoptosis (Figure 3D and E), SK29-MEL-1.22 showed a stronger signal in necrosis, compared to that for late apoptosis (data not shown).
Figure 3.
Type of cell death induced by viral oncolysis of JX-GFP and TG6002 in SK29-MEL-1.
Notes: Cell death was analyzed by flow cytometry. Cells were infected with JX-GFP or TG6002 using an MOI of 0.0001 and combined with 5 d of treatment with 100 µg/mL 5-FC or 50 µg/mL 5-FU. Cells were harvested and stained with Annexin V and propidium iodide and measured via flow cytometry. Data are shown for at least two independent experiments. (A) Overview of all cells. (B) Annexin V positive representing cells in early apoptosis. (C) Propidium iodide-positive cells representing cells undergoing necrosis. (D) Annexin V and propidium iodide-positive cells representing cells in late apoptosis. (E) Representative dot plots of untreated SK29-MEL-1 and TG6002 or JX-GFP-treated cells using quadrant gating. X-axis = propidium iodide (PI) and Y-axis = Annexin APC labeled.
Abbreviations: MOI, multiplicity of infection; d, day; 5-FC, 5-fluorcytosin; 5-FU, 5-fluoruoracil.
5-FU treatment alone induced the strongest signal for late apoptosis in both cell lines. The signal of Annexin V was higher than that of PI, indicating a higher proportion of early apoptotic cells in comparison to necrotic cells (Figure 3B and C). 5-FC-treated cells did not show any difference compared to the untreated control cells (Figure 3B–D). Compared with 5-FU, all virus-treated cells had the lowest signal for Annexin V alone, representing the stage of early apoptosis (Figure 3A and B).
ICD
To the best of our knowledge, the direct effect of virally induced cell death on expression and release of immunogenic markers has not been analyzed for JX-GFP and TG6002 in human melanoma cells. To determine a direct effect on immune cells beside stimulation by TCL, we measured the ICD markers HMGB1 and ATP in supernatants after viral infection and checked the cell surface of infected melanoma cells for calreticulin expression.34,37–41 Again, the combination of viral infection with 5-FC and 5-FU was performed in the analysis. Treatment with doxorubicin was chosen as a positive control, because doxorubicin was previously described as an inducer of ICD.34
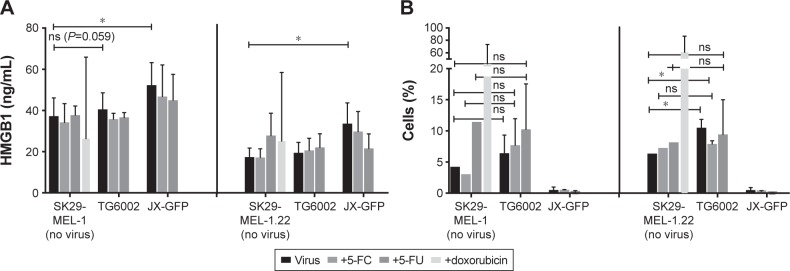
JX-GFP-infected cells presented an increased concentration of HMGB1 in supernatants of both cell lines, compared to the controls. There was no significant difference between controls and TG6002-treated cells, although we observed a higher HMGB1 release upon TG6002 incubation, which was close to significance (P=0.059) (Figure 4A).
Figure 4.
Immunogenic cell death markers were evaluated by ELISA and flow cytometry analysis.
Notes: (A) Supernatants of infected cells were collected after 7 d of treatment (48 h ± virus ±5 d of 5-FC or 5-FU) and stored in the refrigerator at −80°C until performing HMGB1-ELISA. HMGB1 concentration in supernatants after virally induced oncolysis and/or treatment with 5-FC, 5-FU or doxorubicin was measured via ELISA assay performed per protocol readout was done by ELISA microplate reader. (B) Adherent cells were detached after 7 d of treatment (48 h ± virus ±5 d of 5-FC or 5-FU), stained and analyzed by flow cytometry to detect calreticulin expression. Cells were stained simultaneously with Annexin V and PI. Graphs show percentage of calreticulin-expressing cells of the Annexin-positive and PI-negative cell population, respective cells in early apoptosis. *P≤0.05.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; d, day; h, hour; 5-FC, 5-fluorcytosin; 5-FU, 5-fluoruoracil; HMGB1, high mobility group 1 protein; PI, propidium iodide; ns, nonsignificant.
Cells were gated on Annexin V-positive and PI-negative cells to determine calreticulin surface expression. TG6002-infected cells expressed more calreticulin compared to untreated cells but the treatment did not reach a statistically significant difference compared to the untreated control in case of SK29-MEL-1 (Figure 4B, left part). The combination of viral infection and either 5-FC or 5-FU did further increase the expression of calreticulin in case of SK29-MEL-1 cells but did not reach statistical significance too (Figure 4B, left part). SK29-MEL-1.22 treated with TG6002 and the combined treatment with TG6002 and 5FC showed statistically higher expression of surface calreticulin compared to untreated cell control (Figure 4B, right part). JX-GFP-infected cells did not show significant differences in the expression of calreticulin but even a lower expression of calreticulin. Cells treated with 5-FU alone displayed a higher expression of calreticulin. Aiming to study the expression of calreticulin at the point of cocultivation with DCs, flow cytometry was performed 7 d after treatment (48 h of viral infection following 5 d of 5-FC or 5-FU). Thus, a higher calreticulin expression, mostly described in early apoptosis of cells, might be measurable at earlier time points after viral infection.
ATP levels were very low in all experimental settings (10−8 mole ATP). There were no differences in ATP levels between the virally infected cells or the combination with 5-FC and 5-FU compared to untreated human melanoma cells (data not shown). These observations are in accordance with previously published results on TG6002 in mouse models with renal carcinoma with no increase in the ATP levels after virally induced cell death.28
Coculture with DCs (iDCs)
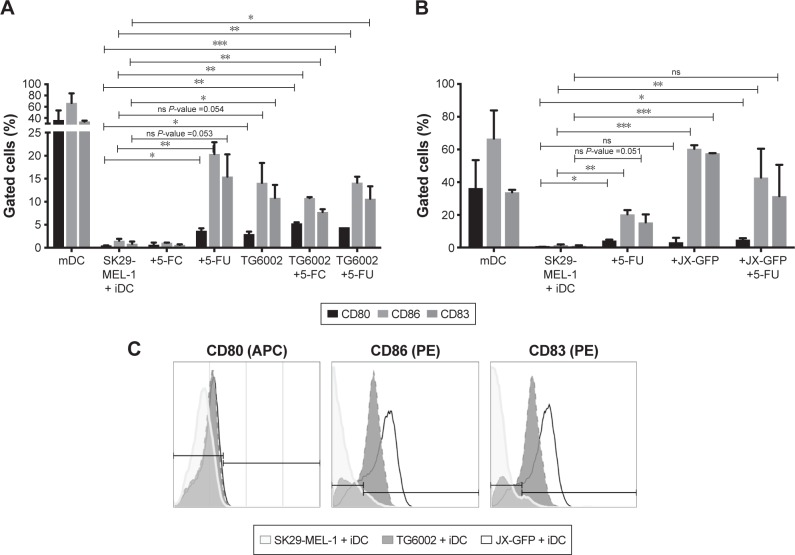
The effect of virally induced TCL on human immune cells was analyzed in coculture experiments with human DCs by flow cytometry analysis of maturation markers.
Lysates induced by both viruses increased the expression of maturation markers on DCs. While the induction of maturation markers by TG6002 was less strong (Figure 5A), DCs in coculture with TCLs induced by oncolytic vaccinia virus JX-GFP showed increased expression of all maturation markers compared to the untreated cell control. Especially CD83 and CD86 showed a high expression (Figure 5B).
Figure 5.
Coculture of virally and/or chemotherapy-induced TCLs with iDCs.
Notes: Flow cytometry analyses of maturation marker CD80, CD83 and CD86 were performed. Maturation of iDCs was driven by a cytokine cocktail. Thus, derived mature dendritic cells (mDCs) were as positive control. Virally and chemotherapy-induced TCLs were cocultivated with iDCs for 3 d. (A) Cells treated with 5-FC, 5-FU, TG6002, TG6002+5-FC or TG6002+5-FU. (B) Cells treated with 5-FC, 5-FU, JX-GFP or JX-GFP +5-FU. (C) Representative histograms from flow cytometry analysis. *P≤0.05; **P≤0.01; ***P≤0.001.
Abbreviations: TCL, tumor cell lysate; mDC, mature dendritic cells; iDCs, immature dendritic cells; d, day; 5-FC, 5-fluorcytosin; 5-FU, 5-fluoruoracil; ns, nonsignificant; APC, allophycocyanin; PE phycoerythrin.
Treatment with 5-FU enhanced the maturation of DCs, which showed an increased expression of CD83 and CD86 (Figure 5A and B). 5-FC-treated cocultures did not show a difference in the expression of maturation markers (Figure 5A).
The combination of virally and chemotherapy-induced TCLs did not further enhance the maturation of DCs. On the contrary, there was a trend to lower the expressions of maturation marker on DCs in case of the combined treatment (Figure 5A and B). The highest expression of CD80, CD83 and CD86 were derived by cytokine cocktail-induced maturation of DCs and could not be reached by virally or drug-induced TCLs (Figure 5A and B).
Coculture with CTLs
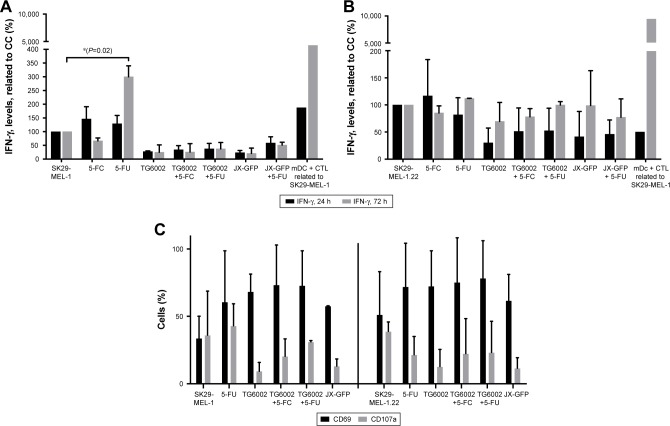
We wanted to increase the activation of CTLs by stimulation with TCL, either directly or via cross presentation with matured DCs. Thus, we determined the effect of virally induced TCL in our human melanoma model by coculture experiments with infected melanoma cells, iDCs and CTLs.
JX-GFP- or TG6002-induced TCLs in coculture with CTL, either alone or in combination with 5-FC or 5-FU, did not induce an increase in IFN-γ secretion, presumed as an activation marker and sign of cytotoxic activity of CTL (Figure 6A). Treatment with 5-FU alone resulted in increased levels of IFN-γ production after 72 h for SK29-MEL-1 cells (Figure 6A) but not for SK29-MEL-1.22 cell line (Figure 6B).
Figure 6.
Activation of CTLs after coculture of virally or drug-induced TCLs with iDCs and CTLs for 24 and 72 h was analyzed by IFN-γ ELISA and flow cytometry.
Notes: (A) Activation of CTL was analyzed by IFN-γ ELISA. Data of cocultivation of SK29-MEL-1 melanoma cells are shown. (B) Activation of CTL was analyzed by IFN-γ ELISA. Data of cocultivation of HLA-loss clone SK29-MEL-1.22 melanoma cells are shown. (C) Cocultivation of virally or drug-induced TCLs with iDCs and CTLs was performed. Activation markers CD69 and CD107a were stained, and flow cytometry analyzes were performed. Left: coculture with SK29-MEL-1 melanoma cells and right: coculture with SK29-MEL-1.22 melanoma cell clone. *P≤0.05.
Abbreviations: h, hour; CC, cell control (untreated cells); CTLs, cytotoxic T lymphocytes; TCL, tumor cell lysates; IFN, interferon; ELISA, enzyme-linked immunosorbent assay; iDCs, immature dendritic cells; mDC, mature dendritic cells; 5-FU, 5-fluoruoracil; 5-FC, 5-fluorcytosin.
Furthermore, we tested the expression of early activation markers CD69 and CD107a on CTLs after 24 h. CD69 acts as a costimulatory molecule for T-cell activation and proliferation42 and CD107a (LAMP-1) is a marker for degranulation and activated CD8+ T cells.43 CD69 expression was increased in all treated cell lines.42 No significant increase of CD107a in all settings was observed (Figure 6C).
Discussion
Genetically modified oncolytic viruses can gain additional properties of naturally oncolytic viruses by improving the tolerability and also the effectiveness of oncolytic virotherapy. For JX-GFP and TG6002, the disruption of the TK locus creates specificity for viral replication in tumor cells. The expression of GM-CSF in case of JX-GFP adds an additional cytokine to further stimulate the local immune infiltration.13,14,24,33,44 In case of TG6002, the expression of the FCU1 suicide gene, which transforms 5-FC into 5-FU, causes a local toxic effect on tumor cells but avoids systemic side effects.26,27,45 We could confirm the value of the specific additional features of both viral strains partly in our human melanoma model.
TG6002 in combination with 5-FC led to reduction of viability in melanoma cells compared to either agent alone, as demonstrated by MTT viability assays. The additional effect of a combined treatment with 5-FC was not statistically significant in this human melanoma cell model but could be enhanced by time and concentration. The synergistic effect of 5-FC was more obvious for low MOIs of the respective virus and a long incubation period of the combined treatment. 5-FC alone did diminish the viability, but at a high concentration (1 mg/mL). This might be due to a direct toxic effect on the tumor cell lines at this high concentration.46 As expected, combined treatment of cells with JX-GFP and 5-FC did not result in a cytotoxic synergistic effect. A concentration of 1,000 µg/mL of 5-FC in combination with JX-GFP caused a reduced viability, which reflects the direct toxic effect of 5-FC. A treatment with 5-FU resulted in stronger reduction of cell viability overall. In human, a higher toxicity of 5-FU might also cause more side effects. Combined treatment with 5-FC avoids systemic side effects while being similarly efficient on the local tumor site. But negative effects of a combination therapy must be considered, too, eg, an inhibition of viral replication due to additional chemotherapy, which was shown for herpes simplex virus-1 in combination with 5-FU, CPT-11 or MTX.47
Under the experimental in vitro conditions used here, we conclude that at low MOI of the virus TG6002, a synergistic effect in the presence of 5-FC can be observed. In this context, Foloppe et al26 could show a benefit for the combination therapy of virus and 5-FC in a colorectal cancer xenograft model in vivo. In an immune-competent syngenic orthotopic renal carcinoma model, Fend et al28 did not see an effect on survival but demonstrated a higher in situ cytotoxicity for the combination therapy. Ottolino-Perry et al showed that a combination therapy of an oncolytic herpes simplex virus is effective, not just for a combination with chemotherapy but also with radiotherapy. Furthermore, they suggested that a combination therapy with biologics or other immunothera-pies should be further explored.48
Vaccinia viruses induce a combined cell death of apoptosis and necrosis. Especially JX-GFP induces a high amount of necrotic cells. TG6002-induced cell death did not differ much between necrosis and late apoptosis, so both types of cell death seem to be important but in both cases, early apoptosis was not the leading type of cell death in this setting. The type of cell death is thought to be important for the immunogenicity and the influence on immune cells in the tumor microenvironment. Especially necrosis and a mixed cell death of apoptosis and necrosis are described to be more immunogenic than apoptosis alone.49–52 Guo et al described a genetically engineered vaccinia virus with deletion in antiapoptotic genes SPI-1 and SPI-2 to gain a selective replication in cancer cells and less pathogenicity. This vaccinia virus induced also a mixed cell death with a relevant part of necrotic cell death both in normal and in cancer cells.53 Former studies from our group with parvovirus H-1 (H-1PV) showed a more apoptotic cell death.54 Overall the type of cell death seems to be important for the immune response. Other oncolytic viruses such as Newcastle disease virus or a transgenic adenovirus showed a mixed cell death of apoptosis and necrosis, in some cases referred to as necroptosis, which presented to be the right way to induce a proper immune response.12,16,55,56
Both oncolytic vaccinia viruses JX-GFP and TG6002 induced parts of ICD with a trend to higher expression of calreticulin in case of TG6002 and higher amounts of extracellular HMGB1 in case of JX-GFP. In a mouse model with renal cell carcinoma, higher HMGB1 levels could be detected after infection with TG6002.28 Also the SPI-1- and SPI-2-deleted vaccinia virus induced a release of HMGB1 in the late infection phase of 48 h after infection, reflecting a necrotic type of cell death.53 All virally treated cells showed lower amounts of ATP in the supernatants of the cells. This might be due to a consumption of ATP during the viral replication.57 Additional immunogenic effects can be caused by HMGB1 and calreticulin, so that the virally induced cell death has influence on the immune infiltration in the tumor microenvironment to attract immune cells and stimulate them.37,38,40,50,58,59 There are two different types of ICD induction described. Principally, oncolytic viruses seem to fit to type II ICD inducers, which selectively target the ER. The virus can induce immunogenic apoptosis by directly altering ER homeostasis and triggering ER stress.60 In our melanoma model, both viruses were not able to induce a full picture of ICD. But our studies show that a mixed type of cell death seems to be promising to result in a proper immune response. A combination therapy or further improvement in genetic engineered viruses might be a solution to overcome this issue.61
JX-GFP-induced TCLs caused a strong induction of maturation of DCs. The additional expression of GM-CSF might drive this observation because GM-CSF promotes the growth of DCs and stabilizes their maturation. mDCs are important for antigen presentation and activation of CTLs. Necrotic cells are described to gain maturation of DCs.62–67 JX-GFP-induced CTL increased the expression of maturation marker probably due to the necrotic type of cell death and additional expression of GM-CSF. TG6002 induced more apoptotic cell death and lacks GM-CSF expression, resulting in less maturation. Still, a process of maturation in TG6002-induced TCL was detectable but might lack the potential to induce a response, strong enough to activate effector cells. A possible explanation is that human DCs support the ability of vaccinia virus infection and replication, indicating that vaccinia viruses abortively infect DCs, block their maturation and induce delayed apoptosis and so evade the immune response.68 JX-GFP and TG6002 are based on different strains of vaccinia viruses, which might cause a different susceptibility to induce maturation. Our experimental setting tries to overcome a replicative infection of DCs but viable viral particles cannot not be fully excluded. Especially in an in vivo setting, infection and inhibition of DCs in the tumor microenvironment must be considered. It is shown that splenic DCs from vaccinia virus-infected mice expressed elevated levels of MHC class I and costimulatory molecules on their cell surface. However, a vaccinia virus infection resulted in the downregulation of MHC class II expression and the impairment of antigen presentation to CD4+ T cells by DCs.69 Yates and Alexander-Miller70 showed that in mice vaccinia viruses cannot mature DCs but previously matured DCs exposed to vaccinia virus can generate a vaccinia virus-specific CD8+ T-cell response, providing a potential mechanism by which direct infection results in T-cell activation in vivo. Considering this, an activation and maturation of DCs by TCLs followed by a stimulation due to a JX-GFP reinfection of matured DCs is to discuss as a possible sequence of events. The expression of GM-CSF can stabilize this process. Another study showed a strong immune response after injection of B16 melanoma cells infected with a GM-CSF-expressing vaccinia virus. This vaccination approach resulted in tumor shrinkage and inhibition of lung metastasis.71
Coculture with CTLs caused an induction of IFN-γ, as an important activation marker of CTLs, in the treatment with 5-FU-induced TCLs but not after infection with JX-GFP and TG6002. Although JX-GFP could induce an increased DC activation, an activation of CTL was not observed. A study using dying cells induced by an adenovirus to vaccinate animals generated a specific CD8+ T-cell responses only if they died by apoptotic cell death.72 JX-GFP and TG6002 induced a mixed type of cell death. Also the cross presentation of tumor antigens via DCs in case of the HLA-A2-negative cell clone SK29-MEL-1.22 did not cause an increased activation of CTLs after treatment with the viruses. GM-CSF released by JX-GFP might be responsible for an additional stimulatory effect on DCs but could not stabilize the cross presentation to CTLs. Overall IFN-γ levels were higher in case of a longer incubation period of 72 h compared to 24 h in stimulating treatment setting with 5-FU, suggesting that a longer contact of TCL, DCs and CTL caused a progressive induction of T cells with increasing release of IFN-γ.
CD69 expression was increased in all treated cell lines. One of the earliest cell surface antigens expressed by T cells following activation is CD69. Once expressed, CD69 acts as a costimulatory molecule for T-cell activation and proliferation.42,73 CD107a did not show an increase in its expression. CD107a (LAMP-1) is a marker for degranulation and activated CD8+ T cells,43 known for its instability and very short expression in the early activation phase.74 We can conclude that a stimulation of CTLs with virally induced TCLs is possible. Nevertheless, there might be approaches to gain a stronger effect on CTL activation. In our in vitro model, a response and activation of cytotoxic CD8+ T cells could not be detected. Further in vivo studies will be needed to identify the innate and adaptive effector cells in the tumor microenvironment after oncolytic virotherapy. Nevertheless, oncolytic vaccinia viruses and oncolytic viruses in general seem to improve the conditions in the tumor micromilieu and increase the chance for an anticancer immune response especially in combination therapies.75
In this context, combination of oncoloytic viruses with checkpoint inhibitors seems to be a promising approach, as tested in different studies, for example, for the approved herpes simplex virus T-VEC (Imlygic®18,76) in combination with ipilimumab for metastatic melanoma.76,77 Several additional studies have reported synergistic effects of oncolytic viruses with checkpoint blockade. A genetically modified measles virus with expression of anti-CTLA-4 or anti-PD1 antibodies showed an improvement in the antitumor immune response.78 Combination studies in murine melanoma and NSCLC models with coxsackievirus A21 (CVA21) and checkpoint inhibitor antibodies (anti-PD-1 and anti-CTLA-4) demonstrated significantly increased antitumor activity of the combination vs either agent.79
Rojas et al tested vaccinia viruses in combination with anti-PD1 antibodies in mouse models. The authors conclude that the interaction between immune checkpoint inhibitors and oncolytic virotherapy was found to be complex, with correct selection of viral strain, antibody and timing of the combination being critical for synergistic effects. Furthermore, some combinations produced antagonistic effects and loss of therapeutic activity.80 Also in our case of combining chemotherapy and oncolytic virotherapy, not every setting could strengthen the antitumor effects. Further analysis will be needed to fully exhaust the potential of oncolytic immunovirotherapy.
Combination therapy might be the breakthrough by releasing antigens by different mechanisms of chemotherapy, oncolysis and stimulating the immune system by checkpoint inhibitors. Oncolytic vaccinia viruses JX-GFP and TG6002 showed promising results to partially induce an immunogenic type of cell death and also to stimulate DCs as a central part of the adaptive immune system, thus supporting the use of oncolytic vaccinia viruses for further clinical development.
Acknowledgments
Aspects of this article are part of the doctoral thesis of J Klein, V Engel and L Geberzahn. The authors especially thank Professor Dr PR Galle (First Department of Internal Medicine, University Medical Center Mainz), Dr CJ Breitbach (Sillajen Biotherapeutics, San Francisco, CA, USA) and P Schaefer as outstanding technicians in our laboratory and for their contributions to the research.
Footnotes
Disclosure
M Lusky, P Erbs and X Preville were employees of Trans-gene when the work was performed. Transgene is a member of the Institut Merieux Group, a publicly traded French biopharmaceutical company. The authors report no other conflicts of interest in this work.
References
- 1.Fahmueller YN, Nagel D, Hoffmann RT, et al. Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse indicate response to radioembolization therapy and prognosis in colorectal cancer patients. Int J Cancer. 2013;132(10):2349–2358. doi: 10.1002/ijc.27894. [DOI] [PubMed] [Google Scholar]
- 2.Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 3.Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol. 2012;2:88. doi: 10.3389/fonc.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott D, Lebbe C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–1064. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13(12):1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Moehler M, Goepfert K, Heinrich B, et al. Oncolytic virotherapy as emerging immunotherapeutic modality: potential of parvovirus h-1. Front Oncol. 2014;4:92. doi: 10.3389/fonc.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich B, Goepfert K, Delic M, Galle PR, Moehler M. Influence of the oncolytic parvovirus H-1, CTLA-4 antibody tremelimumab and cytostatic drugs on the human immune system in a human in vitro model of colorectal cancer cells. Onco Targets Ther. 2013;6:1119–1127. doi: 10.2147/OTT.S49371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett DL, Liu Z, Sathaiah M, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12(1):103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koks CA, Garg AD, Ehrhardt M, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2015;136(5):E313–E325. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 13.Breitbach CJ, Parato K, Burke J, Hwang TH, Bell JC, Kirn DH. Pexa-Vec double agent engineered vaccinia: oncolytic and active immunotherapeutic. Curr Opin Virol. 2015;13:49–54. doi: 10.1016/j.coviro.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Breitbach CJ, Thorne SH, Bell JC, Kirn DH. Targeted and armed oncolytic poxviruses for cancer: the lead example of JX-594. Curr Pharm Biotechnol. 2012;13(9):1768–1772. doi: 10.2174/138920112800958922. [DOI] [PubMed] [Google Scholar]
- 15.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 16.Guo ZS, Liu Z, Bartlett DL. Oncolytic Immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol. 2014;4:74. doi: 10.3389/fonc.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 18.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene laher-parepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 19.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Oh JY, Park BH, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14(3):361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9(6):533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 22.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MK, Breitbach CJ, Moon A, et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med. 2013;5(185):185ra163. doi: 10.1126/scitranslmed.3005361. [DOI] [PubMed] [Google Scholar]
- 24.Breitbach CJ, Arulanandam R, De Silva N, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73(4):1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 25.Parato KA, Breitbach CJ, Le Boeuf F, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–758. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foloppe J, Kintz J, Futin N, et al. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15(20):1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 27.Erbs P, Regulier E, Kintz J, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60(14):3813–3822. [PubMed] [Google Scholar]
- 28.Fend L, Remy-Ziller C, Foloppe J, et al. Oncolytic virotherapy with an armed vaccinia virus in an orthotopic model of renal carcinoma is associated with modification of the tumor microenvironment. Oncoimmunology. 2015;5(2):e1080414. doi: 10.1080/2162402X.2015.1080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moehler MH, Zeidler M, Wilsberg V, et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum Gene Ther. 2005;16(8):996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- 30.Wolfel T, Hauer M, Klehmann E, et al. Analysis of antigens recognized on human melanoma cells by A2-restricted cytolytic T lymphocytes (CTL) Int J Cancer. 1993;55(2):237–244. doi: 10.1002/ijc.2910550212. [DOI] [PubMed] [Google Scholar]
- 31.Wolfel T, Klehmann E, Muller C, Schutt KH, Meyer zum Buschenfelde KH, Knuth A. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med. 1989;170(3):797–810. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfel T, Van Pel A, Brichard V, et al. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24(3):759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 33.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18(11):1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 36.Lehner J, Wittwer C, Fersching D, Siegele B, Holdenrieder S, Stoetzer OJ. Methodological and preanalytical evaluation of an HMGB1 immunoassay. Anticancer Res. 2012;32(5):2059–2062. [PubMed] [Google Scholar]
- 37.Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21(1):79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 39.Kepp O, Tesniere A, Schlemmer F, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14(4):364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 40.Panaretakis T, Kepp O, Brockmeier U, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28(5):578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12(5):456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 43.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol. 2009;254(2):149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Lusky M, Erbs P, Foloppe J, Acres RB. Oncolytic vaccinia virus: a silver bullet? Expert Rev Vaccines. 2010;9(12):1353–1356. doi: 10.1586/erv.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erbs P, Findeli A, Kintz J, et al. Modified vaccinia virus Ankara as a vector for suicide gene therapy. Cancer Gene Ther. 2008;15(1):18–28. doi: 10.1038/sj.cgt.7701098. [DOI] [PubMed] [Google Scholar]
- 46.Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46(2):171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 47.Kulu Y, Kawasaki H, Donahue JM, et al. Concurrent chemotherapy inhibits herpes simplex virus-1 replication and oncolysis. Cancer Gene Ther. 2013;20(2):133–140. doi: 10.1038/cgt.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ottolino-Perry K, Diallo J-S, Lichty BD, Bell JC, Andrea McCart J. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18(2):251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green DR, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanden Berghe T, Kalai M, Denecker G, Meeus A, Saelens X, Vandenabeele P. Necrosis is associated with IL-6 production but apoptosis is not. Cell Signal. 2006;18(3):328–335. doi: 10.1016/j.cellsig.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 53.Guo ZS, Naik A, O’Malley ME, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65(21):9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- 54.Moehler M, Blechacz B, Weiskopf N, et al. Effective infection, apoptotic cell killing and gene transfer of human hepatoma cells but not primary hepatocytes by parvovirus H1 and derived vectors. Cancer Gene Ther. 2001;8(3):158–167. doi: 10.1038/sj.cgt.7700288. [DOI] [PubMed] [Google Scholar]
- 55.Saga K, Kaneda Y. Oncolytic Sendai virus-based virotherapy for cancer: recent advances. Oncolytic Virother. 2015;4:141–147. doi: 10.2147/OV.S66419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H, Xiao T, He L, Ji H, Liu XY. Interferon-beta-armed oncolytic adenovirus induces both apoptosis and necroptosis in cancer cells. Chin J Biochem Biophys. 2012;44(9):737–745. doi: 10.1093/abbs/gms060. [DOI] [PubMed] [Google Scholar]
- 57.Angelova AL, Grekova SP, Heller A, et al. Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol. 2014;88(10):5263–5276. doi: 10.1128/JVI.03688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31(5):1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 60.Workenhe ST, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res. 2013;1(5):309–319. doi: 10.1158/2326-6066.CIR-13-0059-T. [DOI] [PubMed] [Google Scholar]
- 61.Simpson GR, Relph K, Harrington K, Melcher A, Pandha H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother. 2016;5:1–13. doi: 10.2147/OV.S66083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9(1):10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 63.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388(6644):782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 64.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 65.Chan CW, Housseau F. The ‘kiss of death’ by dendritic cells to cancer cells. Cell Death Differ. 2008;15(1):58–69. doi: 10.1038/sj.cdd.4402235. [DOI] [PubMed] [Google Scholar]
- 66.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12(11):1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 67.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 68.Engelmayer J, Larsson M, Subklewe M, et al. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163(12):6762–6768. [PubMed] [Google Scholar]
- 69.Yao Y, Li P, Singh P, et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007;246(2):92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yates NL, Alexander-Miller MA. Vaccinia virus infection of mature dendritic cells results in activation of virus-specific naive CD8+ T cells: a potential mechanism for direct presentation. Virology. 2007;359(2):349–361. doi: 10.1016/j.virol.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin H, Chatterjee SK. Cancer gene therapy using tumor cells infected with recombinant vaccinia virus expressing GM-CSF. Hum Gene Ther. 1996;7(15):1853–1860. doi: 10.1089/hum.1996.7.15-1853. [DOI] [PubMed] [Google Scholar]
- 72.Boozari B, Mundt B, Woller N, et al. Antitumoural immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut. 2010;59(10):1416–1426. doi: 10.1136/gut.2009.196519. [DOI] [PubMed] [Google Scholar]
- 73.Lindsey WB, Lowdell MW, Marti GE, et al. CD69 expression as an index of T-cell function: assay standardization, validation and use in monitoring immune recovery. Cytotherapy. 2007;9(2):123–132. doi: 10.1080/14653240601182838. [DOI] [PubMed] [Google Scholar]
- 74.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 75.Rajani K, Parrish C, Kottke T, et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2016;24(1):166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andtbacka RHI, Collichio FA, Amatruda T, et al. OPTiM: a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2013;31 abstrLBA9008. [Google Scholar]
- 77.Puzanov I, Milhem M, Andtbacka R, et al. Phase 1 results of a phase 1b/2, multicenter, open-label trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) vs ipi alone in previously untreated, unresected stage IIIB-IV melanoma. J Immunother Cancer. 2013;1(suppl 1):84. [Google Scholar]
- 78.Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan Quah M, Wong Y, Andtbacka R, Au G, Shafren DR. Abstract 2341: Elevated immune activity following an anticancer combination therapy of a novel oncolytic immunotherapeutic agent, CAVATAK (Coxsackievirus A21), and immune checkpoint blockade. Cancer research. 2016;76(14 Supplement):2341. [Google Scholar]
- 80.Rojas JJ, Sampath P, Hou W, Thorne SH. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin Cancer Res. 2015;21(24):5543–5551. doi: 10.1158/1078-0432.CCR-14-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]